Potato Resistant Starch Type 1 Promotes Obesity Linked with Modified Gut Microbiota in High-Fat Diet-Fed Mice
Abstract
1. Introduction
2. Results
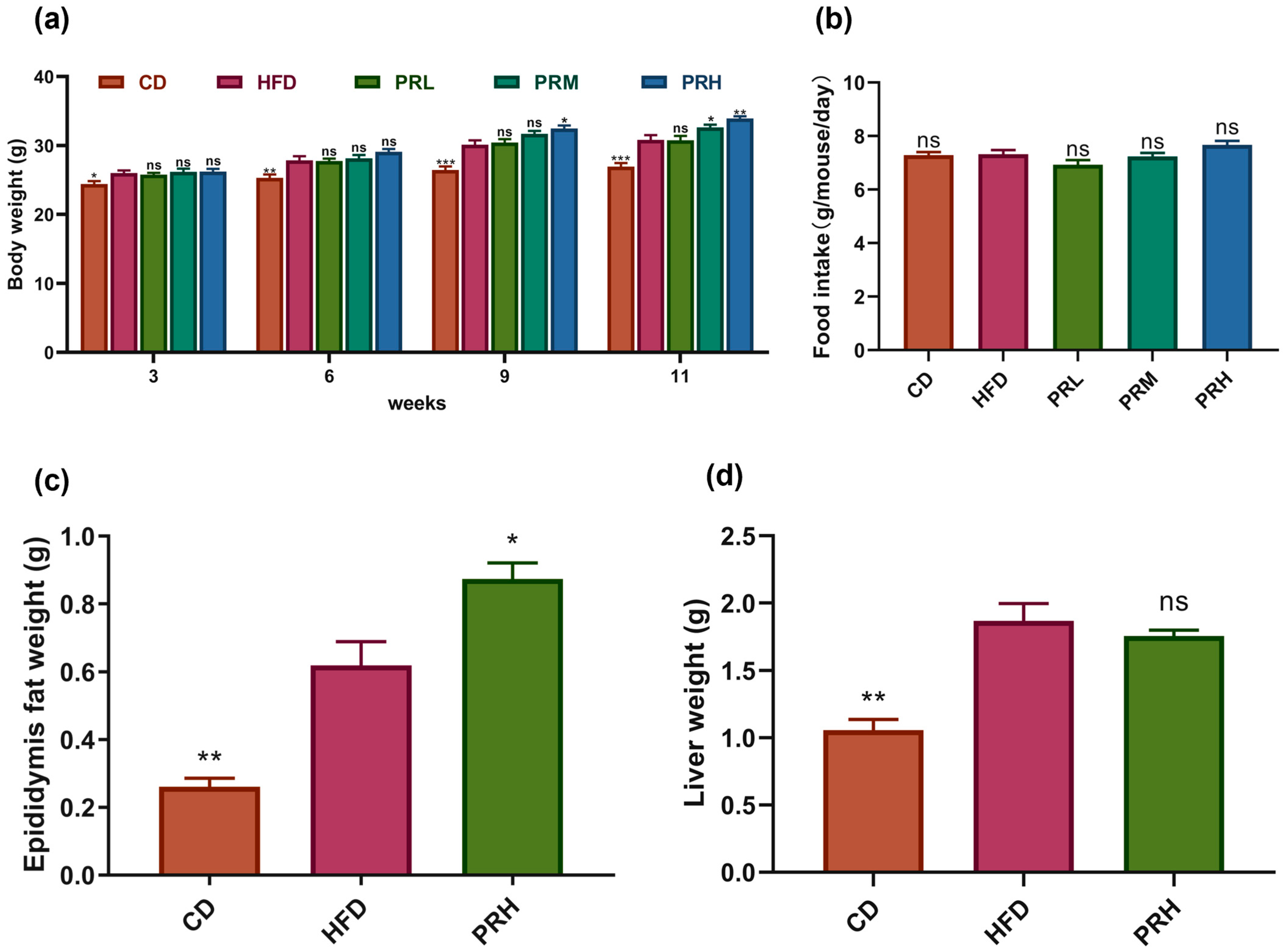
2.1. Effect of PRS1 on Body Weight, Tissue Weight, and Food Intake
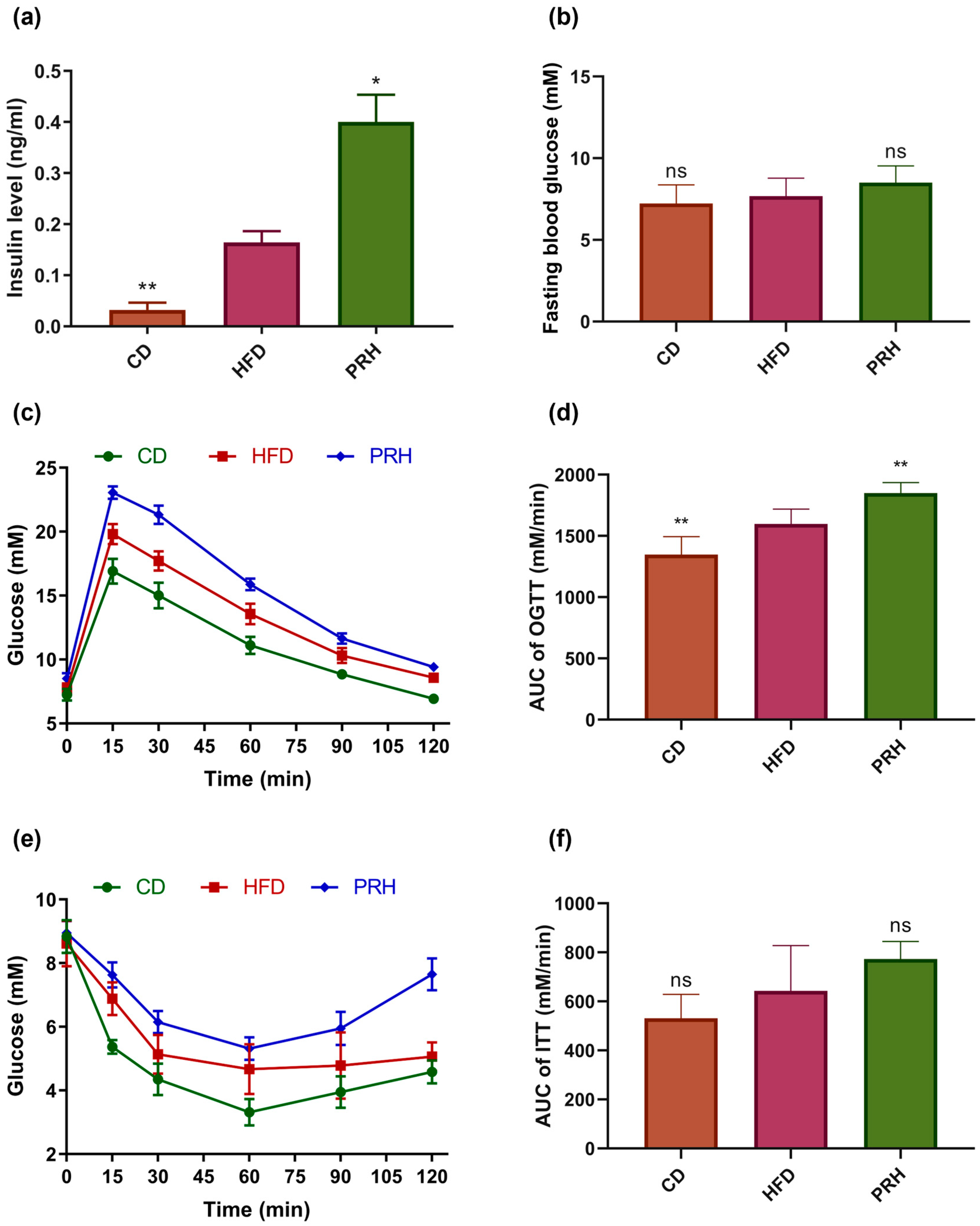
2.2. Effect of PRS1 on Glucose Metabolism in HFD-Fed Mice
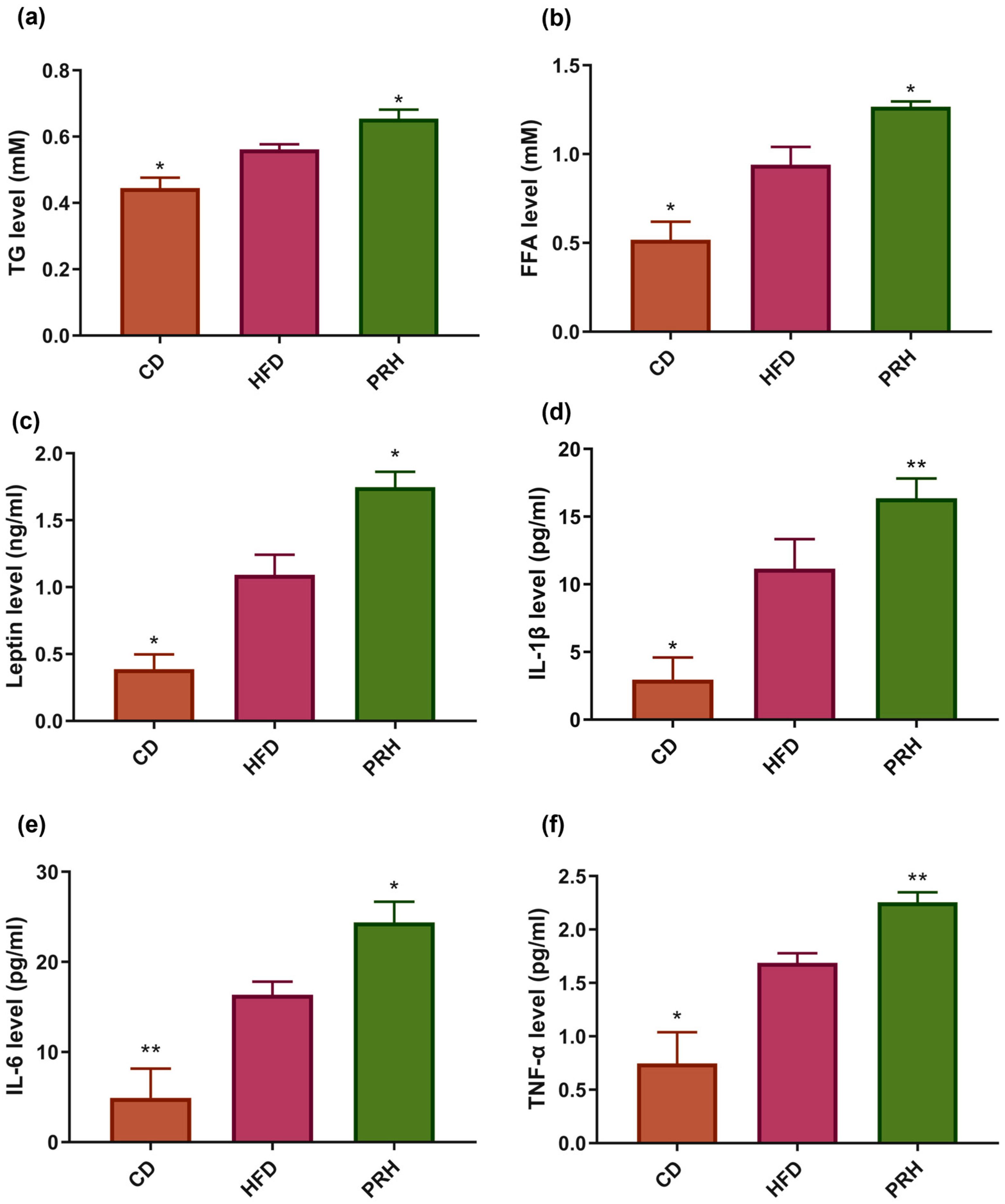
2.3. Effect of PRS1 on Lipid Profile and Serum Inflammatory Factors
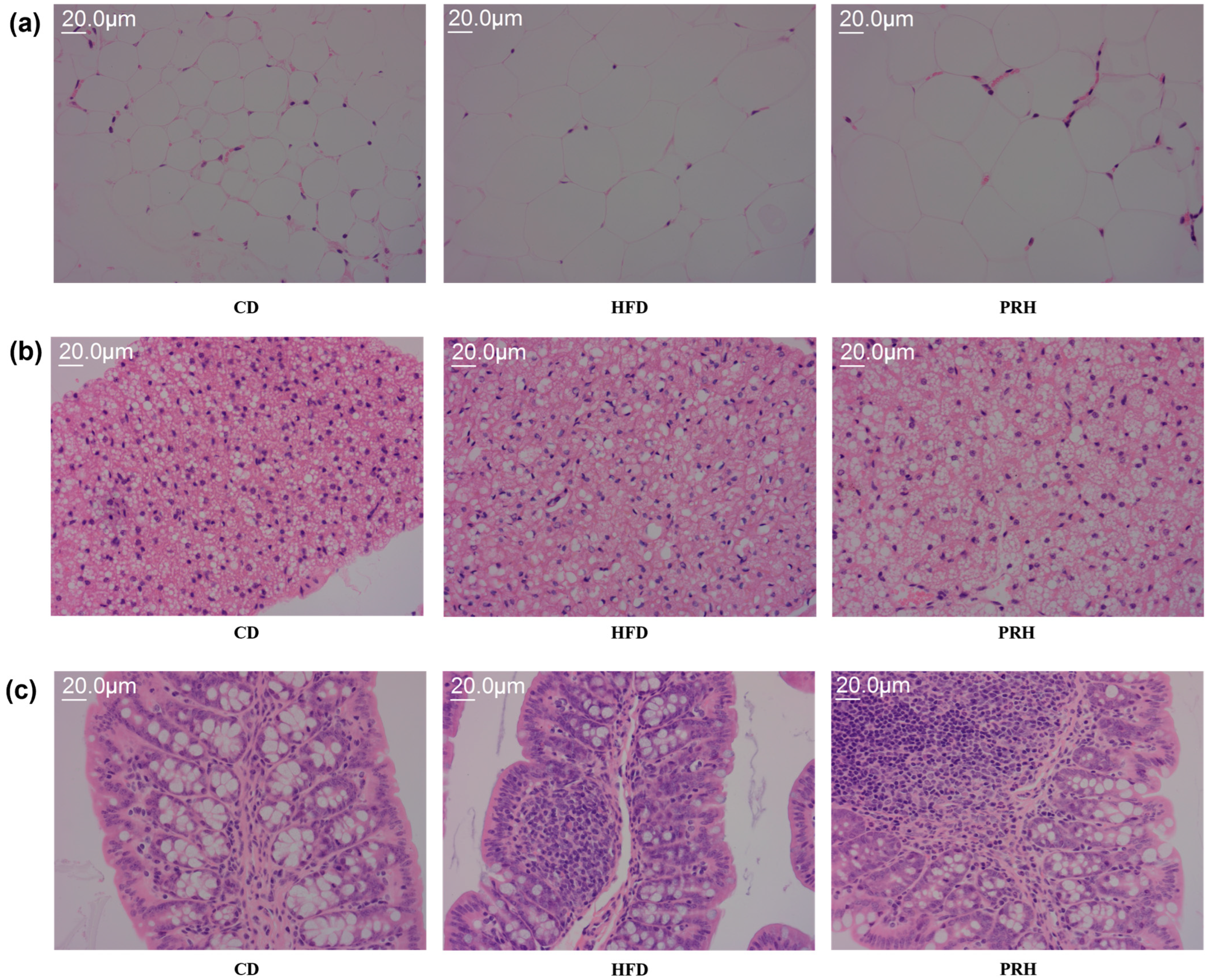
2.4. Effect of PRS1 on the Micromorphology of White Adipose, Brown Adipose, and Liver Tissues
2.5. PRS1 Increases Inflammatory Factor Expression and Intestinal Permeability in Colon Tissue
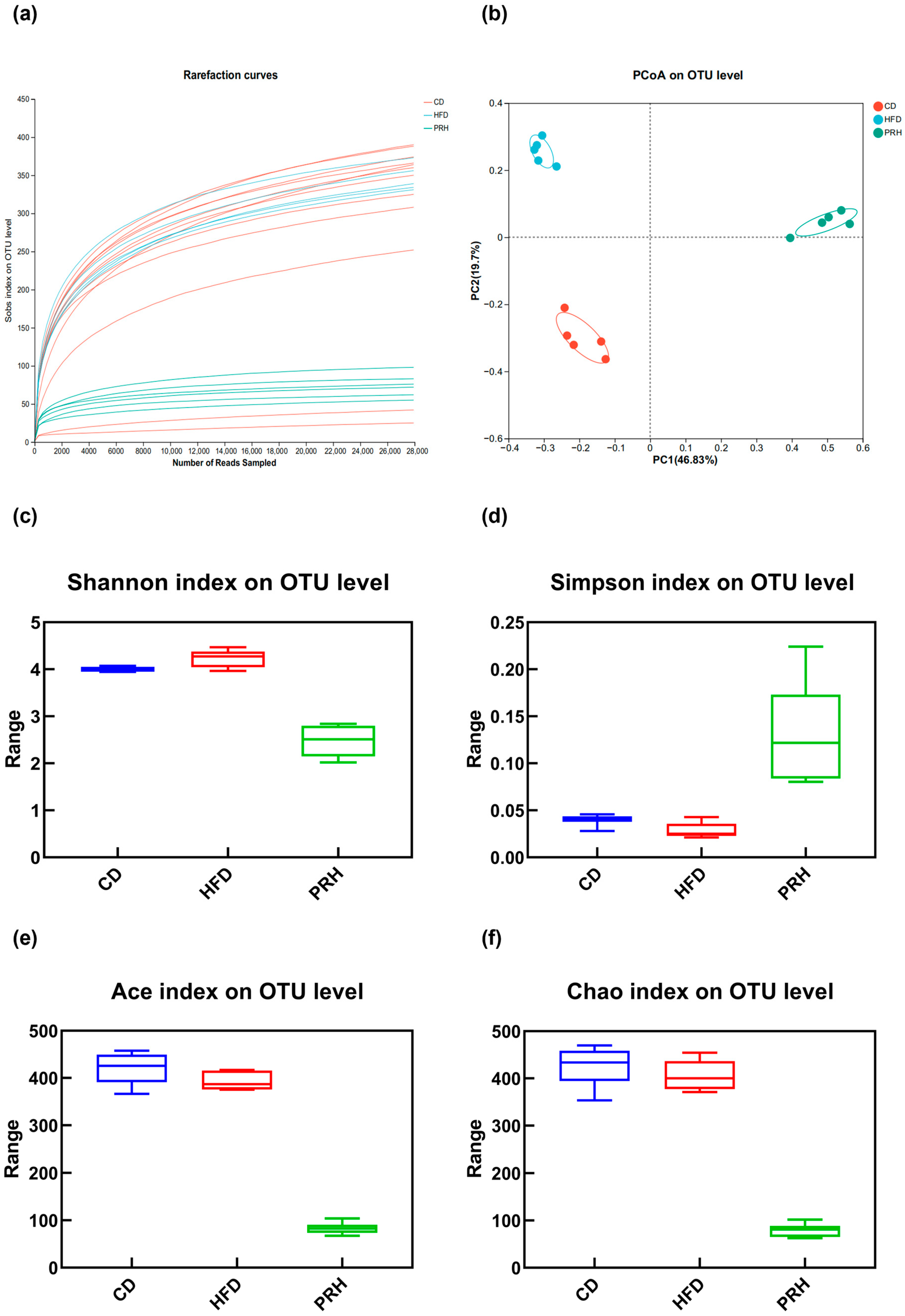
2.6. PRS1 Changes the Abundance and Diversity of the Intestinal Flora in Mice
2.6.1. Alpha Diversity Analysis
2.6.2. Beta Diversity Analysis
2.6.3. Gut Microbiota Composition
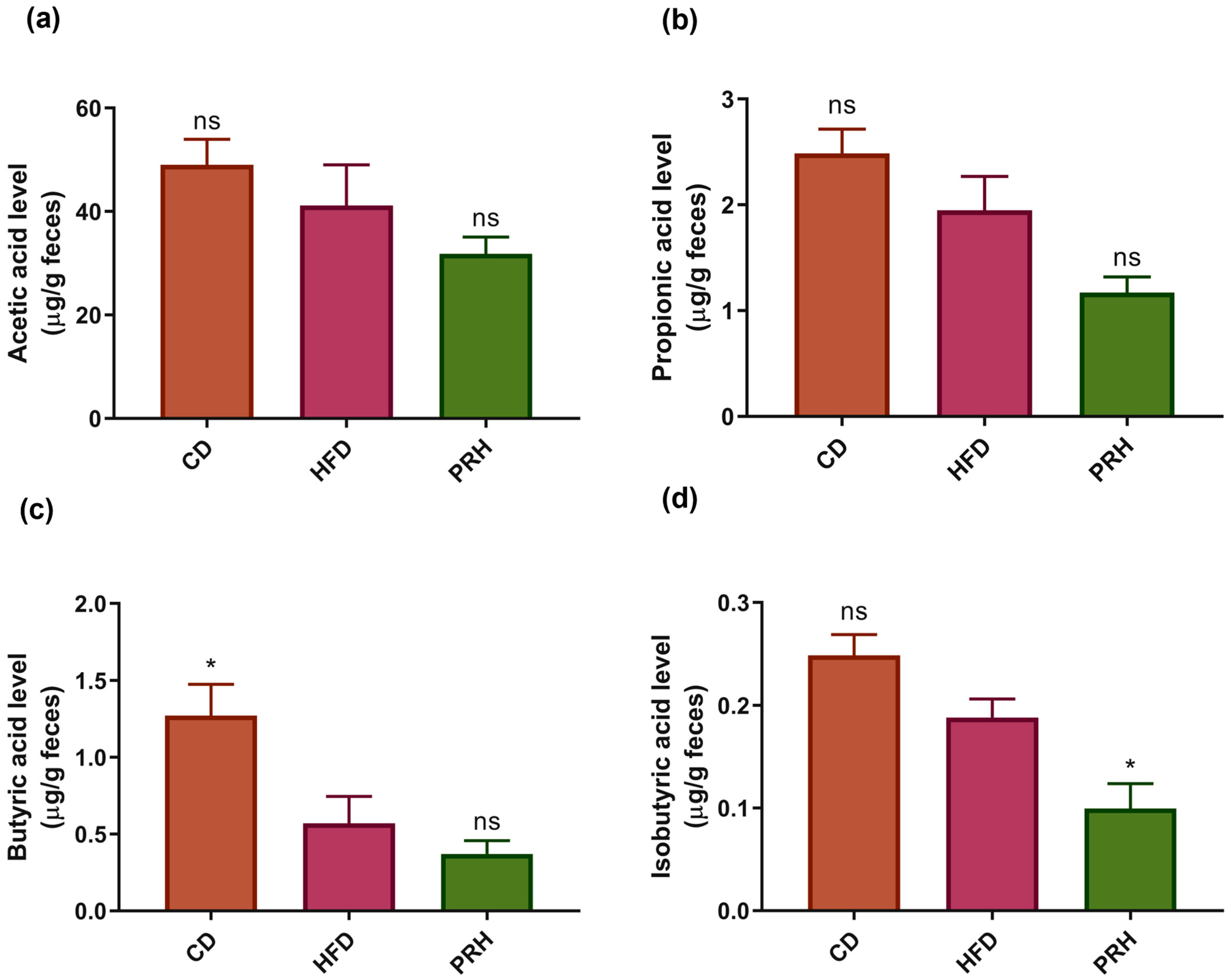
2.7. PRS1 Reduces SCFA Secretion
3. Discussion
3.1. Effect of PRS1 on HFD-Induced Obesity and Inflammatory Responses
3.2. Effect of PRS1 on Blood Lipid Levels, Inflammatory Response, and Intestinal Barrier in HFD Mice
3.3. Effect of PRS1 on the Gut Microbiota Composition of HFD-Fed Mice
3.4. Effect of PRS1 Intervention on SCFA Content
4. Materials and Methods
4.1. Mouse Experimental Environment and Groups
4.2. Mouse Adipose, Colon, and Liver Tissue Samples
4.3. Blood Biochemical Indexes
4.4. OGTT and ITT
4.5. Hematoxylin and Eosin Staining (H&E)
4.6. 16S rRNA Gene Sequence Analysis
4.7. Real Time-qPCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity among Adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC) Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19.2 Million Participants. Lancet 2016, 387, 1377–1396. [CrossRef]
- Kolarić, V.; Svirčević, V.; Bijuk, R.; Zupančič, V. Chronic Complications of Diabetes and Quality of Life. Acta Clin. Croat. 2022, 61, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heshka, S.; Kelley, D.E.; Thornton, J.; Boxt, L.; Pi-Sunyer, F.X.; Patricio, J.; Mancino, J.; Clark, J.M.; MRI Ancillary Study Group of Look AHEAD Research Group. Changes in Adipose Tissue Depots and Metabolic Markers Following a 1-Year Diet and Exercise Intervention in Overweight and Obese Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wu, X.; Bin, S.; Li, T.-J.; Huang, R.; Liu, Z.; Liu, Y.; Ruan, Z.; Deng, Z.; Hou, Y.; et al. Dietary Amylose and Amylopectin Ratio and Resistant Starch Content Affects Plasma Glucose, Lactic Acid, Hormone Levels and Protein Synthesis in Splanchnic Tissues. J. Anim. Physiol. Anim. Nutr. 2010, 94, 220–226. [Google Scholar] [CrossRef]
- Venkataraman, A.; Sieber, J.R.; Schmidt, A.W.; Waldron, C.; Theis, K.R.; Schmidt, T.M. Variable Responses of Human Microbiomes to Dietary Supplementation with Resistant Starch. Microbiome 2016, 4, 33. [Google Scholar] [CrossRef]
- Effects of Dietary Beef and Chicken with and without High Amylose Maize Starch on Blood Malondialdehyde, Interleukins, IGF-I, Insulin, Leptin, MMP-2, and TIMP-2 Concentrations in Rats—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20432166/ (accessed on 7 December 2023).
- Nilsson, A.C.; Johansson-Boll, E.V.; Björck, I.M.E. Increased Gut Hormones and Insulin Sensitivity Index Following a 3-d Intervention with a Barley Kernel-Based Product: A Randomised Cross-over Study in Healthy Middle-Aged Subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef]
- Barouei, J.; Bendiks, Z.; Martinic, A.; Mishchuk, D.; Heeney, D.; Hsieh, Y.-H.; Kieffer, D.; Zaragoza, J.; Martin, R.; Slupsky, C.; et al. Microbiota, Metabolome, and Immune Alterations in Obese Mice Fed a High-Fat Diet Containing Type 2 Resistant Starch. Mol. Nutr. Food Res. 2017, 61, 1700184. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.; Bergeron, N.; Krauss, R.M.; Jansson, J.K.; Tiedje, J.M. Metagenomic Insights into the Degradation of Resistant Starch by Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01562-18. [Google Scholar] [CrossRef]
- Hughes, R.L.; Horn, W.H.; Finnegan, P.; Newman, J.W.; Marco, M.L.; Keim, N.L.; Kable, M.E. Resistant Starch Type 2 from Wheat Reduces Postprandial Glycemic Response with Concurrent Alterations in Gut Microbiota Composition. Nutrients 2021, 13, 645. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Wang, D.; Kan, J. Effects of Resistant starch III on the Serum Lipids Levels and Gut Microbiota of Kunming Mice under High-Fat Diet. Food Sci. Hum. Health 2023, 12, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, D.; Liu, N.; Qiao, O.; Gao, W.; Li, X. Preparation and Characterization of Resistant Starch Type 3 from Yam and Its Effect on the Gut Microbiota. Tradit. Med. Res. 2022, 7, 11–19. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, L.; Chen, H.; Zhang, H.; Hu, H.; Dai, X. Potato Resistant Starch Inhibits Diet-Induced Obesity by Modifying the Composition of Intestinal Microbiota and Their Metabolites in Obese Mice. Int. J. Biol. Macromol. 2021, 180, 458–469. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, M.; Kim, J.J.; Lin, R.; Xu, J.; Fan, L.; Qi, Y.; Wang, L.; Liu, W.; et al. Dietary Type 2 Resistant Starch Improves Systemic Inflammation and Intestinal Permeability by Modulating Microbiota and Metabolites in Aged Mice on High-Fat Diet. Aging 2020, 12, 9173–9187. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of Dietary Resistant Starch Type 4 on Human Gut Microbiota and Immunometabolic Functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef]
- Lau, E.; Zhou, W.; Henry, C.J. Effect of Fat Type in Baked Bread on Amylose-Lipid Complex Formation and Glycaemic Response. Br. J. Nutr. 2016, 115, 2122–2129. [Google Scholar] [CrossRef]
- Guo, J.; Ellis, A.; Zhang, Y.; Kong, L.; Tan, L. Starch-Ascorbyl Palmitate Inclusion Complex, a Type 5 Resistant Starch, Reduced in Vitro Digestibility and Improved in Vivo Glycemic Response in Mice. Carbohydr. Polym. 2023, 321, 121289. [Google Scholar] [CrossRef]
- Härle, P.; Straub, R.H. Leptin Is a Link between Adipose Tissue and Inflammation. Ann. N. Y. Acad. Sci. 2006, 1069, 454–462. [Google Scholar] [CrossRef]
- Blum, W.F. Leptin: The Voice of the Adipose Tissue. Horm. Res. 1997, 48 (Suppl. S4), 2–8. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Yu, S.; Zhou, X.; Liu, Z.; Zhang, X.; Cai, H.; Hu, Z. Analysis of Bacterial Community Structure of Fuzhuan Tea with Different Processing Techniques. Open Life Sci. 2023, 18, 20220573. [Google Scholar] [CrossRef]
- Independence of Alpha and Beta Diversities—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20715617/ (accessed on 31 May 2023).
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A Dietary Intervention with Functional Foods Reduces Metabolic Endotoxaemia and Attenuates Biochemical Abnormalities by Modifying Faecal Microbiota in People with Type 2 Diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- You, H.; Tan, Y.; Yu, D.; Qiu, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Therapeutic Effect of SCFA-Mediated Regulation of the Intestinal Environment on Obesity. Front. Nutr. 2022, 9, 886902. [Google Scholar] [CrossRef] [PubMed]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R.D.C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; Oliveira, L.C.S.D.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef]
- Wang, A.; Guo, T.; An, R.; Zhuang, M.; Wang, X.; Ke, S.; Zhou, Z. Long-Term Consumption of Resistant Starch Induced Changes in Gut Microbiota, Metabolites, and Energy Homeostasis in a High-Fat Diet. J. Agric. Food Chem. 2023, 71, 8448–8457. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Ma, Q.; Tian, B.; Nie, C.; Chen, Z.; Li, J. Health Beneficial Effects of Resistant Starch on Diabetes and Obesity via Regulation of Gut Microbiota: A Review. Food Funct. 2020, 11, 5749–5767. [Google Scholar] [CrossRef] [PubMed]
- Yamei, Y.; Yujia, P.; Jilong, T.; Jia, M.; Lu, L.; Xiaoying, L.; Linwu, R.; Xiaoxiong, Z.; Youlong, C. Effects of Anthocyanins from the Fruit of Lycium Ruthenicum Murray on Intestinal Microbiota. J. Funct. Foods 2018, 48, 533–541. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between Body Mass Index and Firmicutes/Bacteroidetes Ratio in an Adult Ukrainian Population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Ignacio, A.; Fernandes, M.R.; Rodrigues, V.a.A.; Groppo, F.C.; Cardoso, A.L.; Avila-Campos, M.J.; Nakano, V. Correlation between Body Mass Index and Faecal Microbiota from Children. Clin. Microbiol. Infect. 2016, 22, 258.e1-8. [Google Scholar] [CrossRef] [PubMed]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, Composition, Diversity and Novelty of Soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.-H.; Yu, T.; Chen, Q.-K. Effects of Berberine and Metformin on Intestinal Inflammation and Gut Microbiome Composition in Db/Db Mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ma, K.; Li, J.; Ren, Z.; Zhang, J.; Shan, A. Lactobacillus Rhamnosus GG Ameliorates DON-Induced Intestinal Damage Depending on the Enrichment of Beneficial Bacteria in Weaned Piglets. J. Anim. Sci. Biotechnol. 2022, 13, 90. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Jiang, Y.; Wu, J.; Chen, L.; Ding, Y.; Zhou, Y.; Deng, L.; Chen, X. Poria Cocos Polysaccharide Ameliorated Antibiotic-Associated Diarrhea in Mice via Regulating the Homeostasis of the Gut Microbiota and Intestinal Mucosal Barrier. Int. J. Mol. Sci. 2023, 24, 1423. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Perdigones, C.M.; Muñoz-Garach, A.; Álvarez-Bermúdez, M.D.; Moreno-Indias, I.; Tinahones, F.J. Gut Microbiota of Patients with Type 2 Diabetes and Gastrointestinal Intolerance to Metformin Differs in Composition and Functionality from Tolerant Patients. Biomed. Pharmacother. 2022, 145, 112448. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.-E.; Li, W.-B.; Wang, H.-Y.; Ma, Y.-M.; Zhao, X.-H.; Yang, H.; Qian, J.-M.; Li, J.-N. VSL#3 Can Prevent Ulcerative Colitis-Associated Carcinogenesis in Mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef]
- Du, H.; Zhao, A.; Wang, Q.; Yang, X.; Ren, D. Supplementation of Inulin with Various Degree of Polymerization Ameliorates Liver Injury and Gut Microbiota Dysbiosis in High Fat-Fed Obese Mice. J. Agric. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, C.; Lin, S.; Zheng, M.; Chen, C.; Zheng, B.; Zhang, Y. Lotus Seed Resistant Starch Regulates Gut Microbiota and Increases Short-Chain Fatty Acids Production and Mineral Absorption in Mice. J. Agric. Food Chem. 2017, 65, 9217–9225. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-S.; Song, E.-J.; Nam, Y.-D.; Nam, T.G.; Kim, H.-J.; Lee, B.-H.; Seo, M.-J.; Seo, D.-H. Effects of Enzymatically Modified Chestnut Starch on the Gut Microbiome, Microbial Metabolome, and Transcriptome of Diet-Induced Obese Mice. Int. J. Biol. Macromol. 2020, 145, 235–243. [Google Scholar] [CrossRef]
- Duan, M.; Sun, X.; Ma, N.; Liu, Y.; Luo, T.; Song, S.; Ai, C. Polysaccharides from Laminaria Japonica Alleviated Metabolic Syndrome in BALB/c Mice by Normalizing the Gut Microbiota. Int. J. Biol. Macromol. 2019, 121, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Manzano, M.J.; Andrés, V.; Dorado, B. Oil Red O and Hematoxylin and Eosin Staining for Quantification of Atherosclerosis Burden in Mouse Aorta and Aortic Root. Methods Mol. Biol. 2015, 1339, 85–99. [Google Scholar] [CrossRef]
- Clarridge, J.E. Impact of 16S rRNA Gene Sequence Analysis for Identification of Bacteria on Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Rev. 2004, 17, 840–862. [Google Scholar] [CrossRef] [PubMed]
- Watts, G.S.; Youens-Clark, K.; Slepian, M.J.; Wolk, D.M.; Oshiro, M.M.; Metzger, G.S.; Dhingra, D.; Cranmer, L.D.; Hurwitz, B.L. 16S rRNA Gene Sequencing on a Benchtop Sequencer: Accuracy for Identification of Clinically Important Bacteria. J. Appl. Microbiol. 2017, 123, 1584–1596. [Google Scholar] [CrossRef]
- Heid, C.A.; Stevens, J.; Livak, K.J.; Williams, P.M. Real Time Quantitative PCR. Genome Res. 1996, 6, 986–994. [Google Scholar] [CrossRef]
- McKinzie, P.B.; Myers, M.B. A Brief Practical Guide to PCR. Methods Mol. Biol. 2023, 2621, 3–13. [Google Scholar] [CrossRef]
- Maren, N.A.; Duduit, J.R.; Huang, D.; Zhao, F.; Ranney, T.G.; Liu, W. Stepwise Optimization of Real-Time RT-PCR Analysis. Methods Mol. Biol. 2023, 2653, 317–332. [Google Scholar] [CrossRef]



| CD | HFD | PRL | PRM | PRH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ingredient | gm | kcal | gm | kcal | gm | kcal | gm | kcal | gm | kcal |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 |
| Maltodextrin 10 | 150 | 600 | 100 | 400 | 100 | 400 | 100 | 400 | 100 | 400 |
| Sucrose | 0 | 0 | 172.8 | 691.2 | 172.8 | 691.2 | 172.8 | 691.2 | 172.8 | 691.2 |
| PRS1 | 0 | 0 | 0 | 0 | 42.5 | 0 | 90 | 0 | 269 | 0 |
| Soybean Oil | 25 | 225 | 25 | 225 | 25 | 225 | 25 | 225 | 25 | 225 |
| Lard | 20 | 180 | 177.5 | 1597.5 | 177.5 | 1597.5 | 177.5 | 1597.5 | 177.5 | 1597.5 |
| Mineral Mix S10026 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 |
| Dicalcium Phosphate | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 | 13 | 0 |
| Calcium Carbonate | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 |
| Potassium Citrate, 1 H2O | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 |
| Vitamin Mix V10001 | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| Total | 1055.1 | 4057 | 858.15 | 4057 | 850.65 | 4057 | 898.15 | 4057 | 1077.1 | 4057 |
| Gene | Forward and Reverse Primers |
|---|---|
| ZO-1 | F: 5′-TTTGAGACGACTCGGGGGAT-3′ |
| R: 5′-TCTCGTTTTCTGGTTGGCAGT-3′ | |
| Occludin | F: 5′-CGCGTGCACACACACAATAA-3′ |
| R: 5′-TAGTAACGGAAAGGACCCCC-3′ | |
| Muc2 | F: 5′-GTTTGGACACGCACAAGGAC-3′ |
| R: 5′-CTCGGGTAGCTTCCACTGTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, N.; Guo, X.; Fan, B.; Cheng, S.; Wang, F. Potato Resistant Starch Type 1 Promotes Obesity Linked with Modified Gut Microbiota in High-Fat Diet-Fed Mice. Molecules 2024, 29, 370. https://doi.org/10.3390/molecules29020370
Zhang W, Zhang N, Guo X, Fan B, Cheng S, Wang F. Potato Resistant Starch Type 1 Promotes Obesity Linked with Modified Gut Microbiota in High-Fat Diet-Fed Mice. Molecules. 2024; 29(2):370. https://doi.org/10.3390/molecules29020370
Chicago/Turabian StyleZhang, Weiyue, Nana Zhang, Xinxin Guo, Bei Fan, Shumei Cheng, and Fengzhong Wang. 2024. "Potato Resistant Starch Type 1 Promotes Obesity Linked with Modified Gut Microbiota in High-Fat Diet-Fed Mice" Molecules 29, no. 2: 370. https://doi.org/10.3390/molecules29020370
APA StyleZhang, W., Zhang, N., Guo, X., Fan, B., Cheng, S., & Wang, F. (2024). Potato Resistant Starch Type 1 Promotes Obesity Linked with Modified Gut Microbiota in High-Fat Diet-Fed Mice. Molecules, 29(2), 370. https://doi.org/10.3390/molecules29020370








