Abstract
This paper deals with the synergy between Nuclear Magnetic Resonance (NMR) spectroscopic investigations and DFT calculations, mainly of NMR parameters. Both the liquid and the solid states are discussed here. This text is a mix of published results supplemented with new findings. This paper deals with examples in which useful results could not have been obtained without combining NMR measurements and DFT calculations. Examples of such cases are tautomeric systems in which NMR data are calculated for the tautomers; hydrogen-bonded systems in which better XH bond lengths can be determined; cage compounds for which assignment cannot be made based on NMR data alone; revison of already published structures; ionic compounds for which reference data are not available; assignment of solid-state spectra and crystal forms; and the creation of libraries for biological molecules. In addition to these literature cases, a revision of a cage structure and substituent effects on pyrroles is also discussed.
1. Introduction
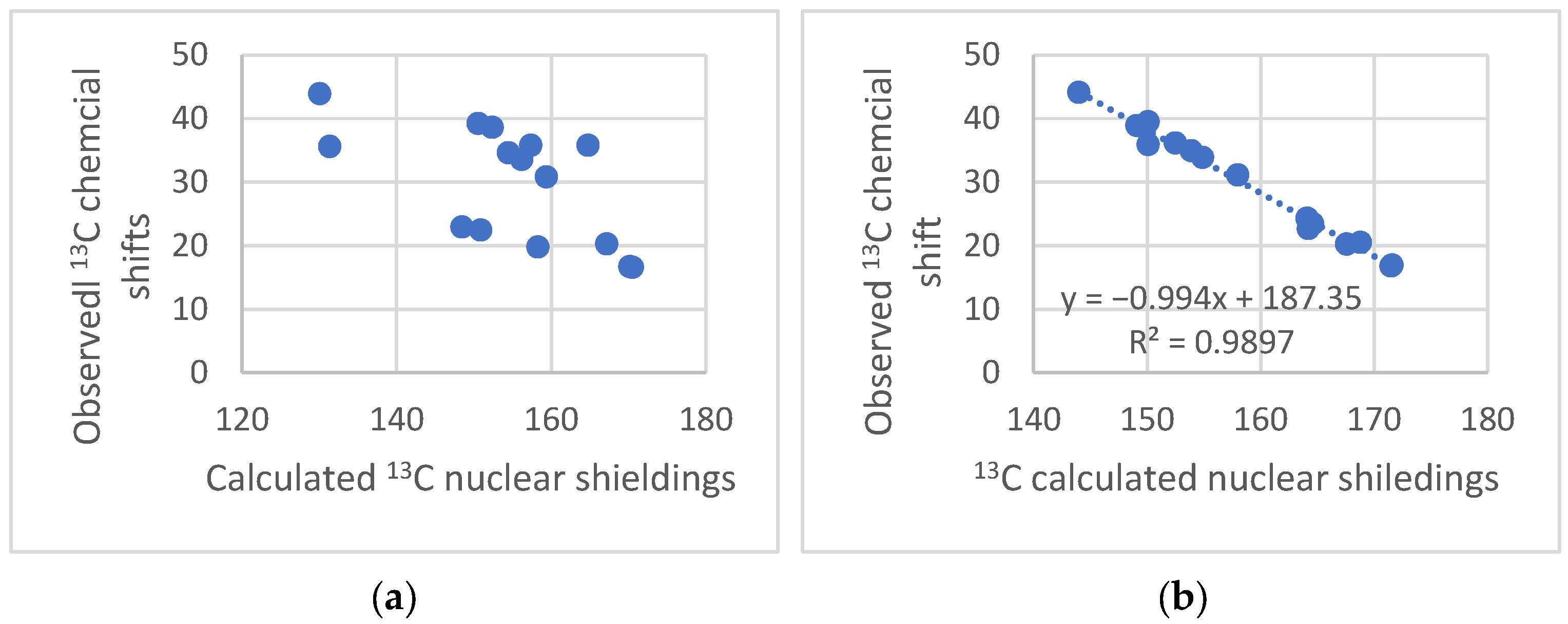
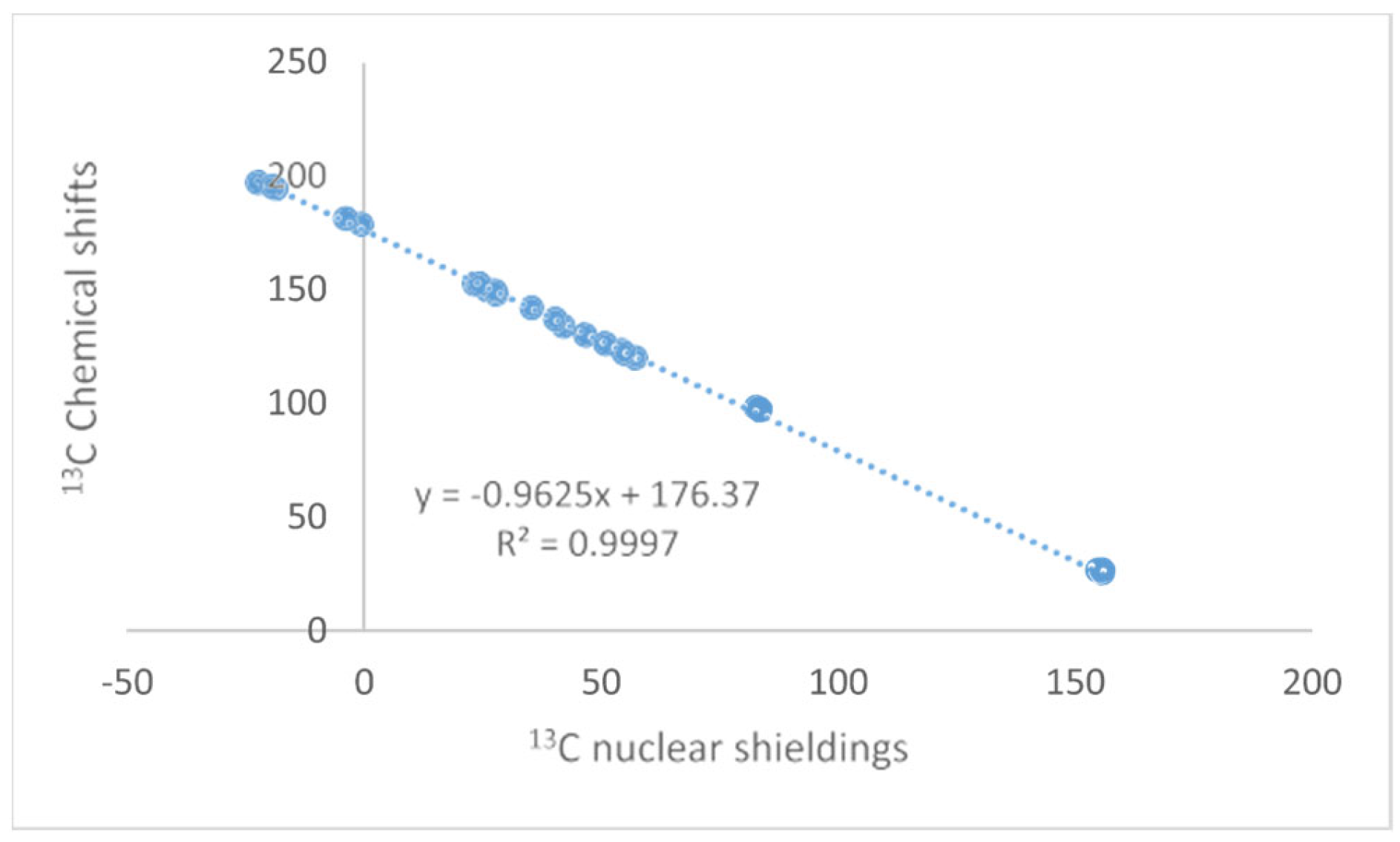
The NMR technique has reached maturity in many ways, and so have Density Functional Theory (DFT) calculations. Unfortunately, no golden rule about a functional or basis set can be given, so inspiration about the choice of functional and basis set can be found in the papers discussed here. Papers finding the optimal DFT functional and basis set can also be found elsewhere [1,2,3]. Some authors use calculated values for TMS as a reference; this should be avoided, as the correlation between experimental and clouted values is seldom one (see Figure 1). This paper will focus on the synergy obtained by combining these two methods. The examples will be NMR parameters calculated for non-existing structures to be used in tautomeric equilibria, assignment of stereoisomers, assignment of cage compounds, structural studies of charged species, accurate structures of hydrogen bonded systems, a revision of already published structures of natural products [4], creating libraries for biological molecules, and the assignment of solid-state NMR spectra. The present paper covers primarily recent work. Numerous reviews dealing with DFT calculations and NMR at some point are available [5,6].
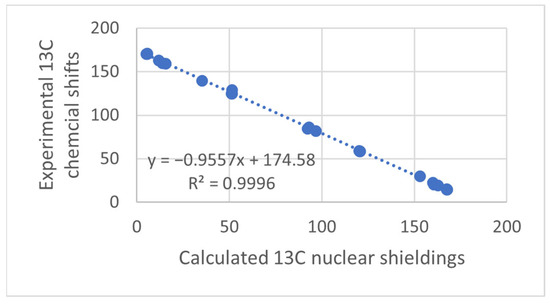
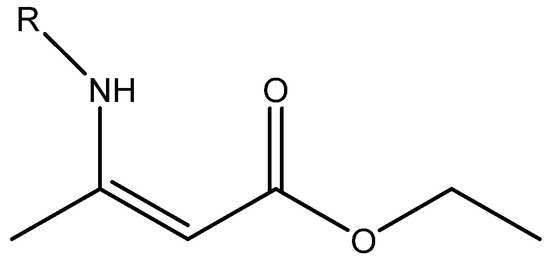
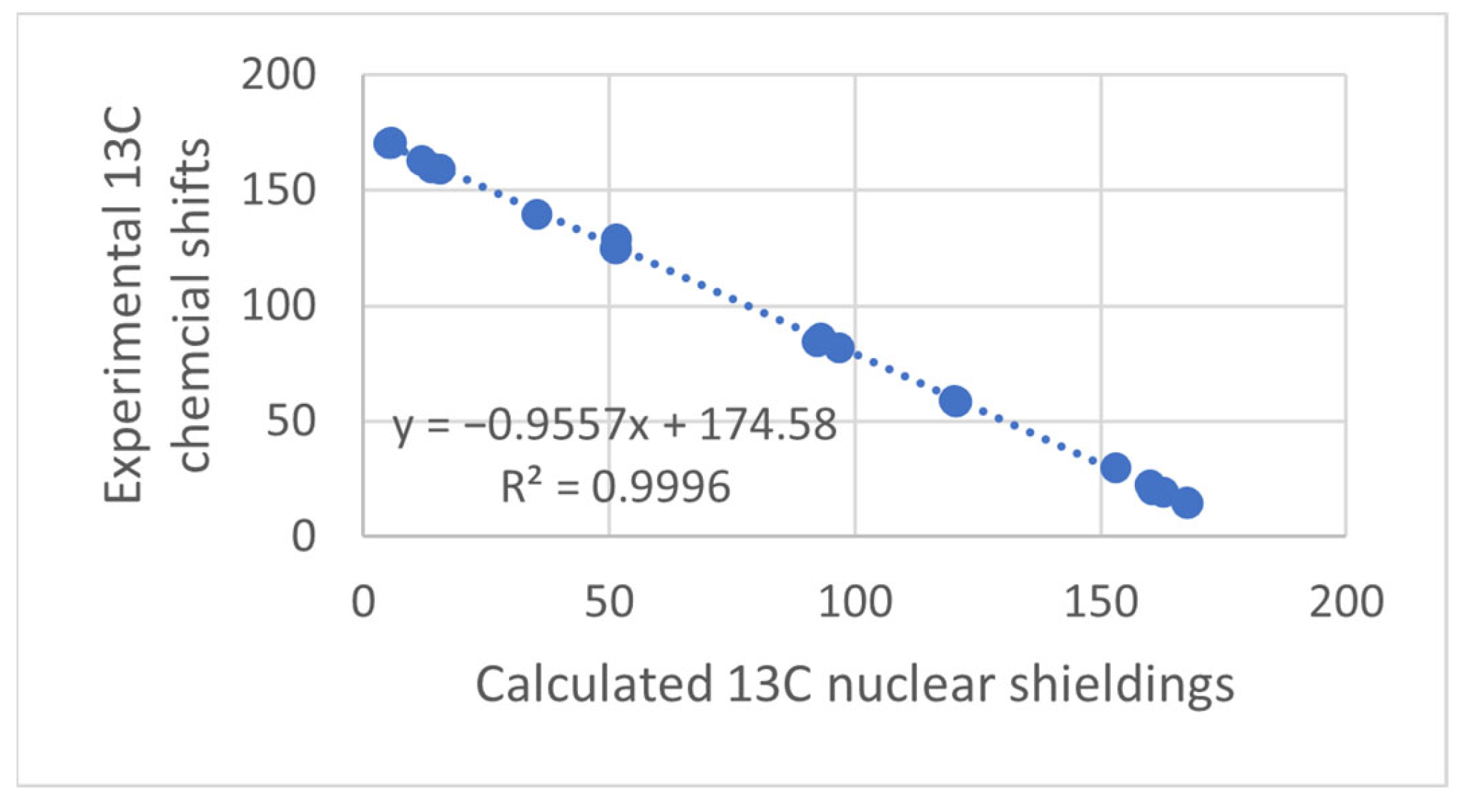
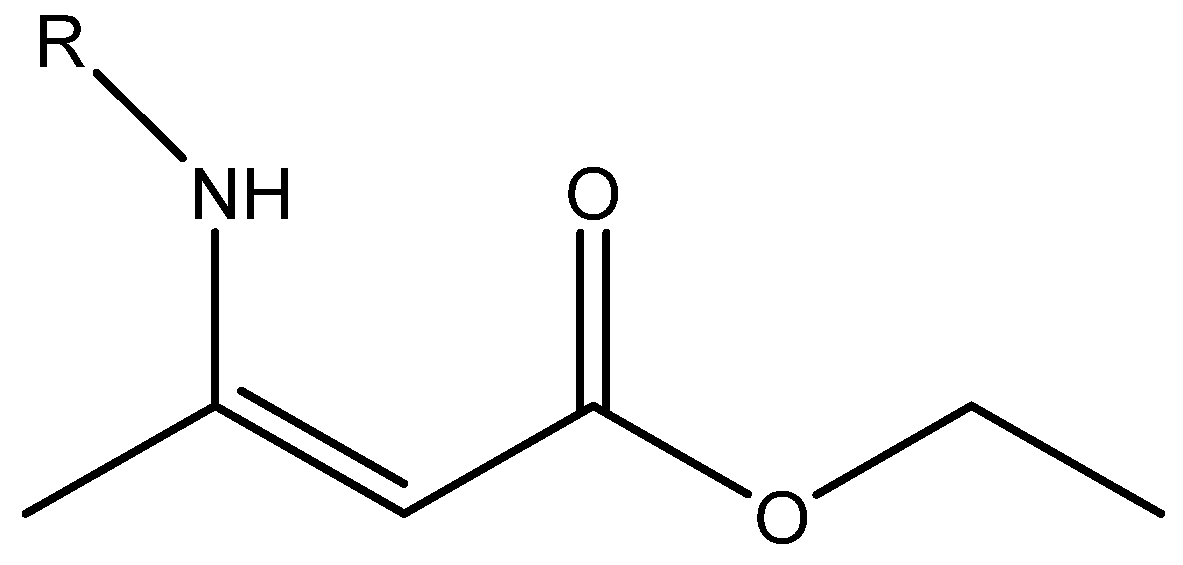
Figure 1.
Plot of calculated vs. experimental 13C chemical shifts for ethyl (Z)-R-3-(amino)but-2-enoate (see Figure 2). B3LYP is the functional and 6-311 ++ G(d,p) is the basis set. A similar plot is found in Ref. [7].
2. Results and Discussion
2.1. DFT Calculations
When the text refers to DFT calculations, this normally involves both structure optimization and calculation of nuclear shielding using the Gage Independent Atomic Orbital (GIAO) method [8,9]. For an example, see Figure 1. The same applies to solvent effects if mentioned, e.g., through a Polarizable Continuum Model (PCM) approximation [10].
Reviews on the calculation of nuclear shielding are available [11,12]. An overview of considerations related to DFT calculations is given in Ref. [13]. Their use in natural products has also been demonstrated [14,15,16].
2.2. XH Positions
Determining the position of OH or NH protons in hydrogen-bonded systems based on X-ray measurements is often difficult. This is improved by optimizing X-ray structures using 1H chemical shifts as constraints in DFT calculations and by taking advantage of the great sensitivity of 1H-NMR shielding to hydrogen-bonding properties [17]. The positon of OH protons has also been addressed as a function of solvent. [18]
X-ray structures of proteins are not accurate with respect to the position of NH protons. This is a problem when calculating deuterium isotope effects on 15N chemical shifts. This can be improved by performing DFT calculations. In the case of ubiquitin, this was performed by introducing formamide as a hydrogen bond acceptor, with the position of amide oxygen being the same as position of the acceptor in the protein, and then by optimizing the structure using BPW91/6-31G(d,p) calculations [19].
2.3. Assignments
Despite the power of 2D NMR, NMR spectra can be so complex that an assignment can be difficult. This has been demonstrated in a large number of natural products [4,20].
An example is given below. An attempt to determine the absolute structure of a molecule isolated from H. angustifolia by 13C NMR shows that a comparison of experimental and calculated nuclear shielding by Density Functional Theory (DFT) calculations (GIAO approach) gives a very poor fit (Figure 3a), indicating that the assigned structure probably needs revision. This also suggests a reassignment of the 13C data. However, the experimentally reassigned 13C chemical shifts are very close to those of (−)-ishwarane (Figure 4a). Plotting those new experimental data vs. calculated nuclear shielding gives a very good correlation, which leads to the conclusion that the compound isolated from H. angustifolia is (−)-ishwarane (Figure 4a).
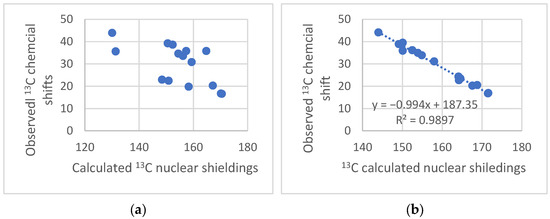
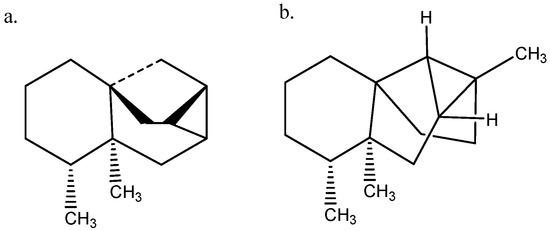
Figure 4.
(a) (−)-Ishwarane. (b) (−)-Hortoishwarane.
2.4. Stereochemistry
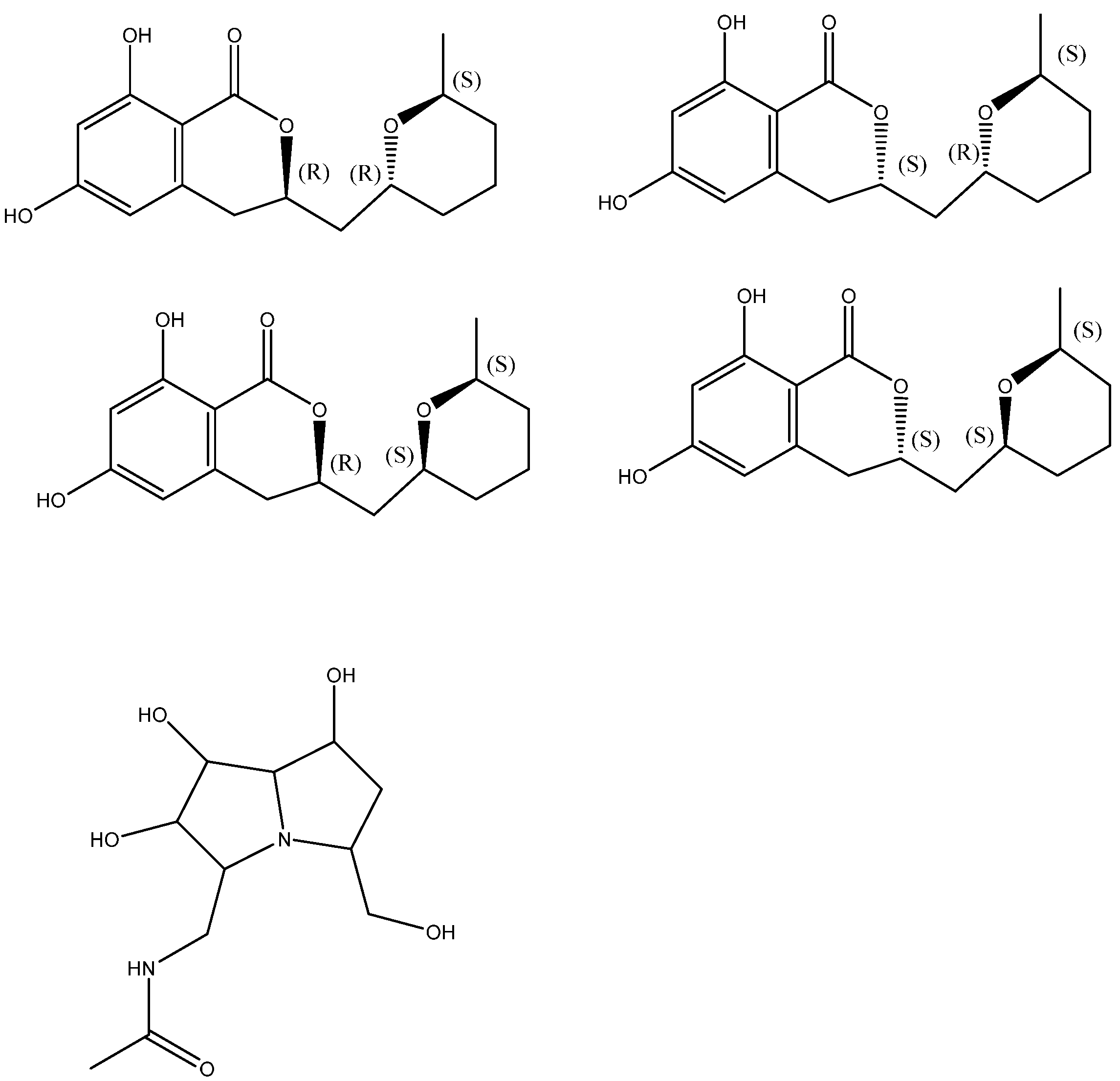
The stereochemical assignment of cladosporin was demonstrated. It has three stereocenters featuring 3R, 10R, and 14S absolute configuration (see Figure 5). The four cladologs shown in Figure 5 were calculated and 13C chemical shifts were measured. It was fond that chemical shift differences between pairs of carbons gave better results and the parameter Mean Average Error, MAEΔΔδ, was used for the evaluation of the six sets of data comparing the calculated and experimental data. MAEΔΔδ = Σ(ΔΔδ)/nΔΔδ. Σ is the summation of n computed Δδ absolute error values (ΔΔδ) normalized to the number of ΔΔδ errors considered (nΔΔδ) [22]. The method was also tested successfully on pochonicine (Figure 5 below) with four chiral centers.
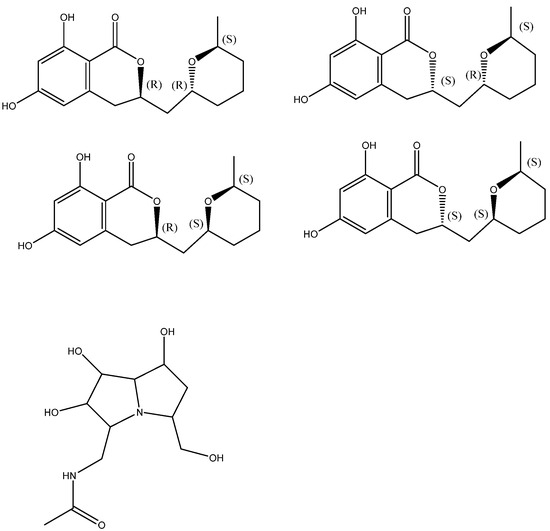
Figure 5.
Top four structures: stereoisomers of cladosporin. Bottom: pochonicine. Taken from Ref. [22].
All 13C NMR chemical shift data were assigned to each specific isomer [23].
2.5. Isotope Effects
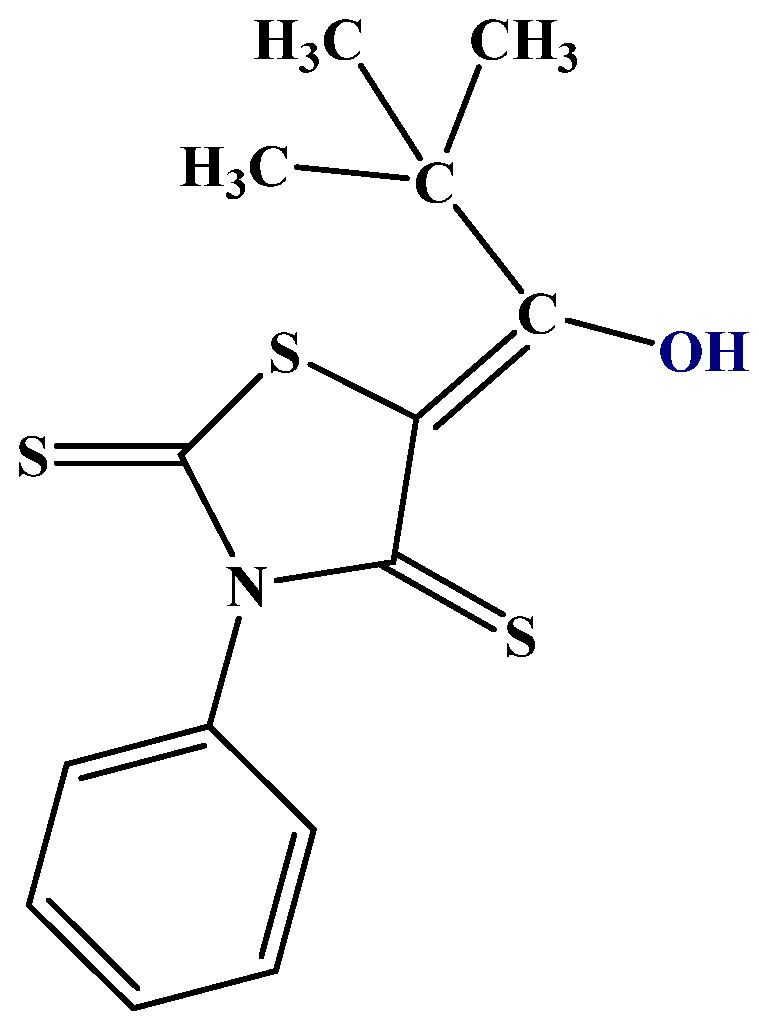
In the study of hydrogen bonding and tautomerism, a useful NMR parameter are isotope effects on chemical shifts. Especially, deuterium isotope effects on 13C, 15N, and 19F chemical shifts have been studied [24]. These can be calculated using the Jameson approach [25]. In its most simple form, deuterium isotope effects can be calculated by assuming that the XH bond is shortened upon deuteration. Assuming a reasonable value and that all the isotope effects of the molecule depend on the same shortening, a plot vs. experimental values can be used to scale the shortening. Isotope effects have been demonstrated in 5-acylrhodanines and the corresponding thiorhodanines. Structure and isotope effects have been calculated using the B3LYP-GD3BJ functional and the 6-311++G(d,p) basis set. Based on these calculations, the compounds were shown to exist in the enol form (Figure 6) rather than the thiol form or the keto-thione form [26]. This is rather unusual as β-thioxoketones have been reported to be tautomeric [24].
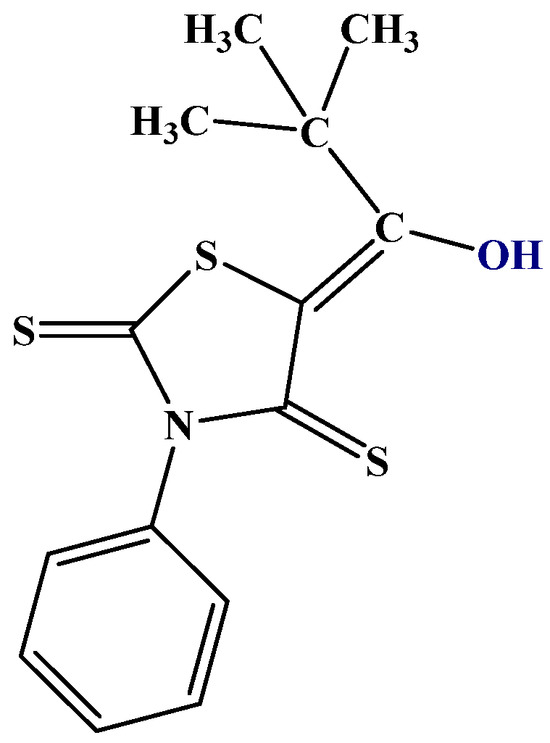
Figure 6.
5-tert-butyl-3-phenyl-4-thiorhodanine. Other investigated thiorhodanines with bulky substituents are 5-adamantoyl-3-methyl-4-thiorhodanine and 5-adamantoyl-3-phenyl-4-thiorhodanine [27].
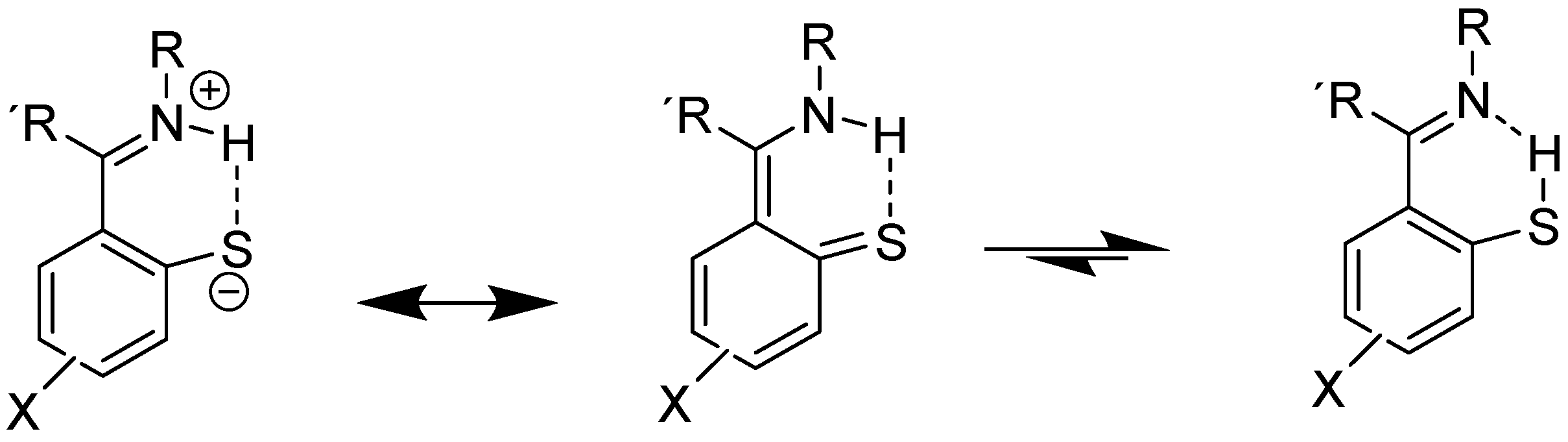
An example involving NH and C=S bonds is thiophenoxyketimines (Figure 7). In this case, the best DFT functional is B3LYP/6-311+G(2d,p), including solvent effects. The chemical shift for the C=S carbon is very low, 166 ppm, showing a large contribution of the zwitter ionic form (Figure 7). A small percentage of the SH form is possible based on the calculated 13C chemical shifts [27].
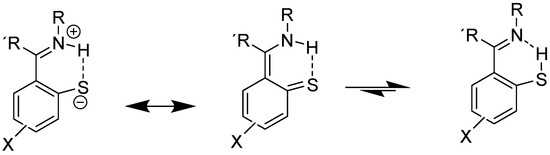
Figure 7.
Resonance and tautomeric forms of thiophenoxyketimines. R alkyl or aryl, R methyl.
2.6. Tautomerism
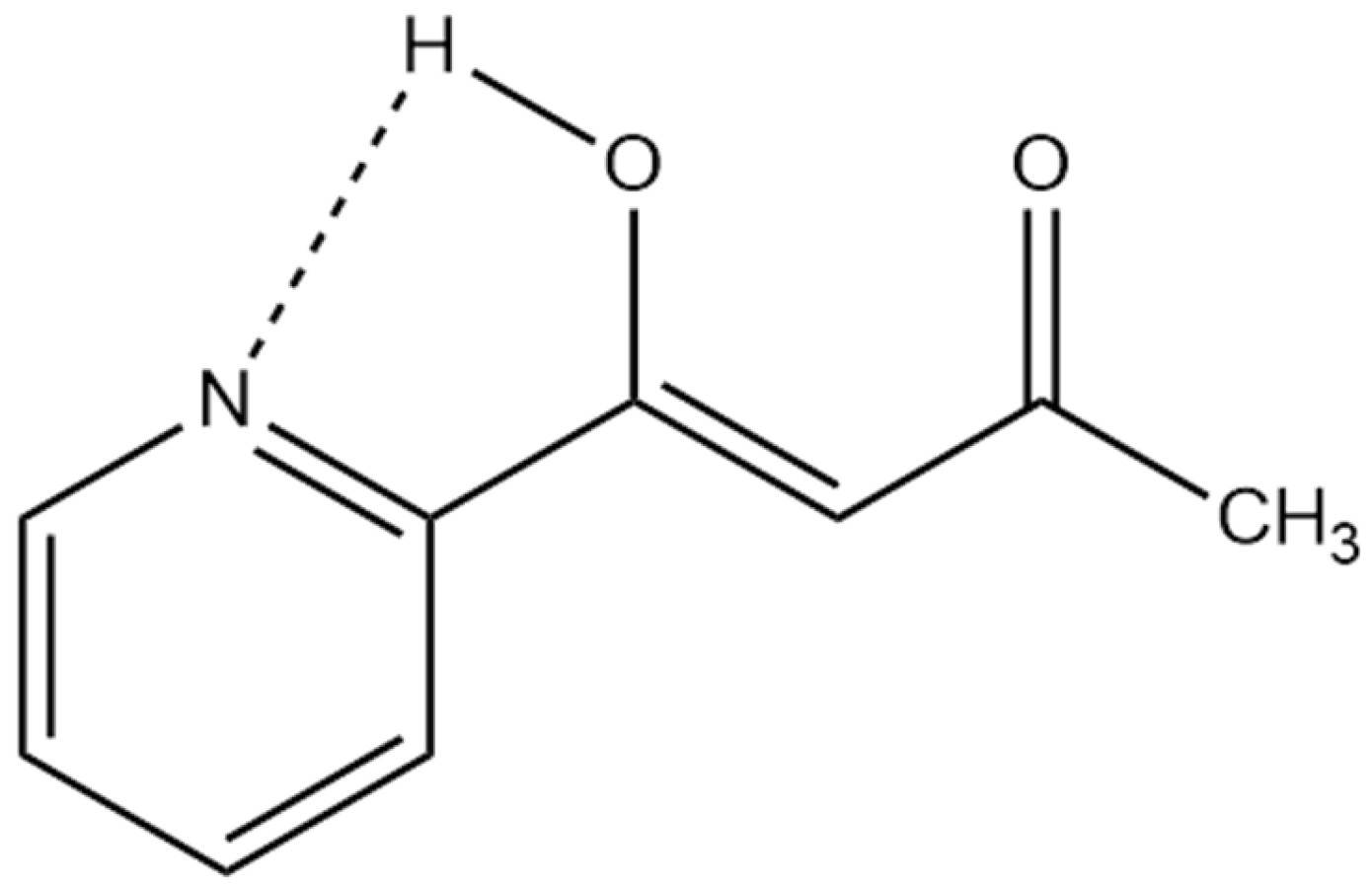
DFT calculations can provide structural information on and energies of the two tautomers, and also, in this context, nuclear shieldings of the nuclei. Knowing the latter, equilibrium constants can be determined. Examples are many, including β-diketones, β-thioxoketones, o-hydroxySchiff bases, or o-hydroxyazo compounds [24]. One case is shown in Figure 8 for 1-(n-pyridinyl)butane-1,3-diones. The calculated nuclear shieldings (B3LYP/6-311++G(d,p)) of both structure and nuclear shielding calculations for the two tautomers are weighted according to their calculated energies [28].
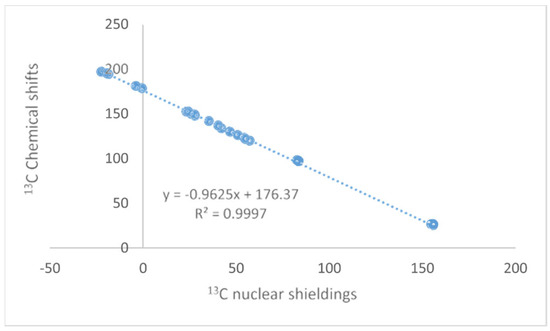
Figure 8.
Plot of experimental 13C chemical shifts vs. calculated 13C nuclear shieldings keto-enol forms of 1-(n-pyridinyl)butane-1,3-diones. Taken from Ref. [28]. Copyright Wiley 2023.
In cases in which other intramolecular effects, such as hydrogen bonding to nitrogen, is present, DFT calculations of the structure are invaluable (see Figure 9).
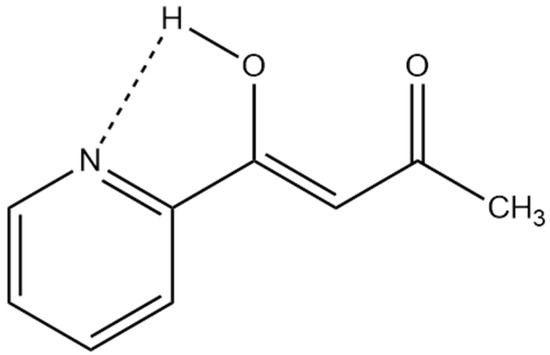
Figure 9.
Possible hydrogen bond in 1-(n-pyridinyl)butane-1,3-dione other than the strong one to oxygen [28].
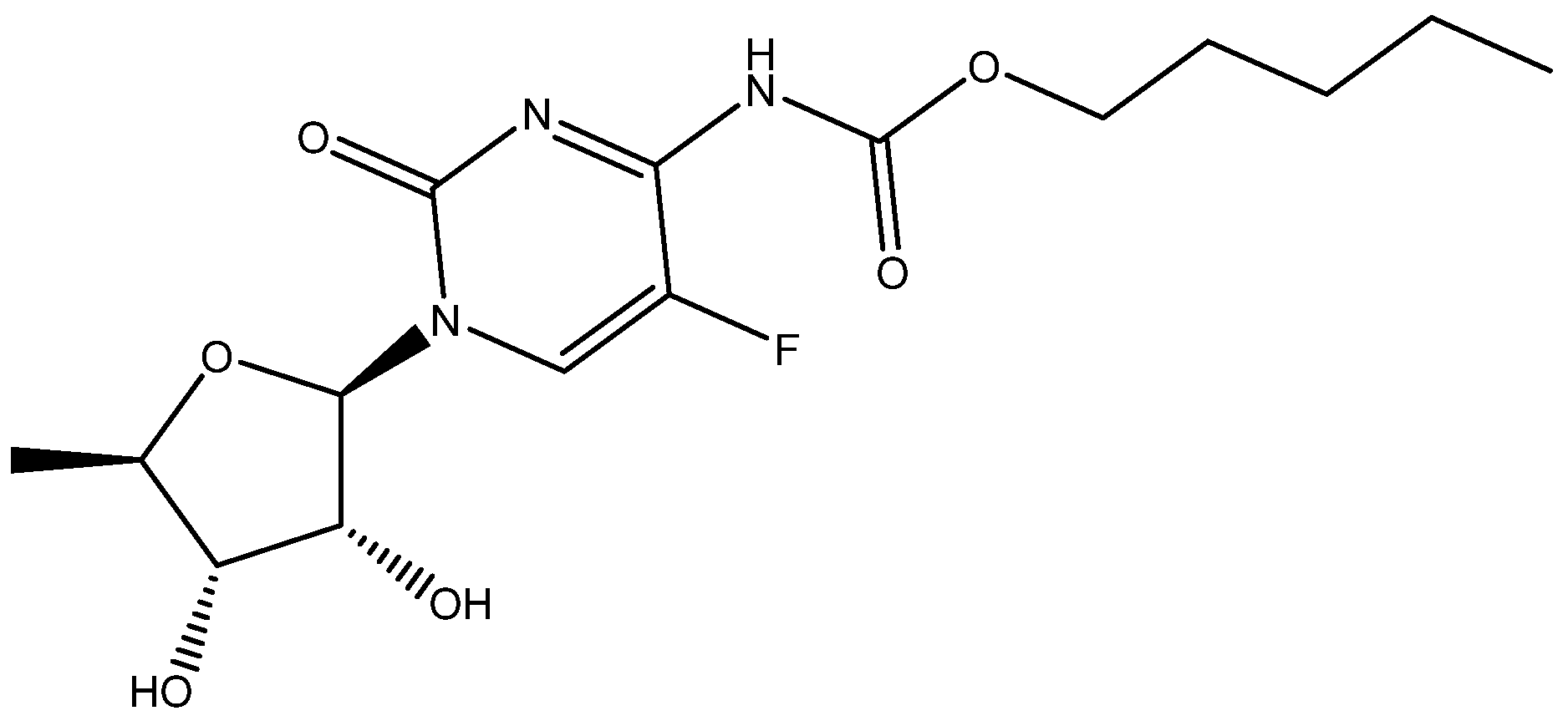
Cmoch et al. [29] investigated the structure of capecitane (see Figure 10) in various solvents and used DFT calculations to produce chemical shifts which could be observed not unambiguously.
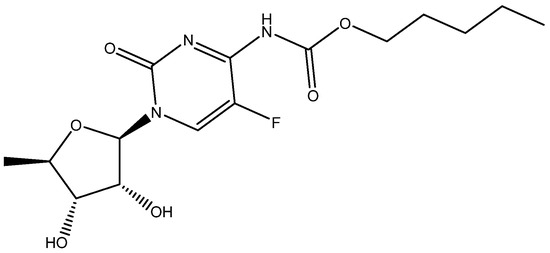
Figure 10.
One of the tautomeric structures of capecitabene.
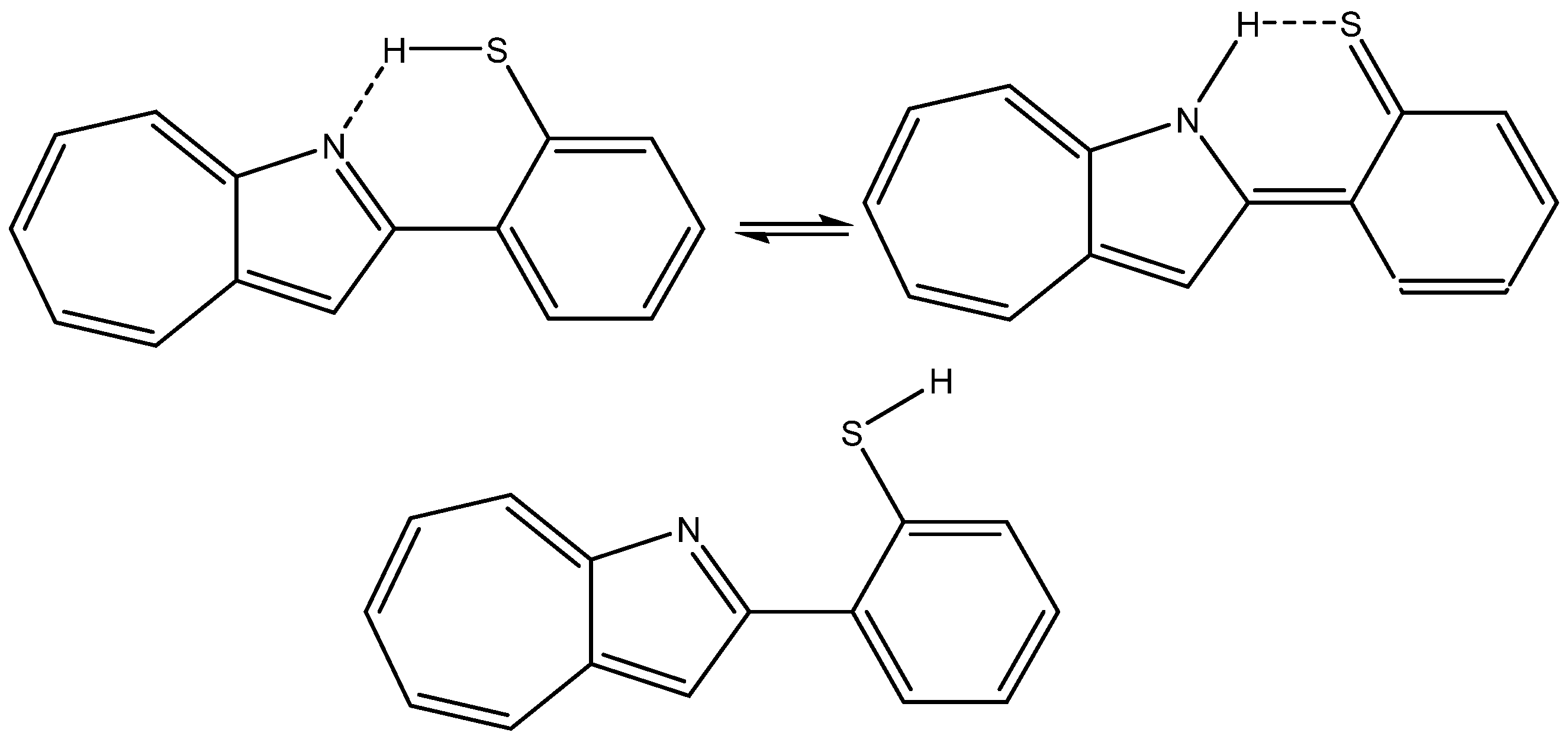
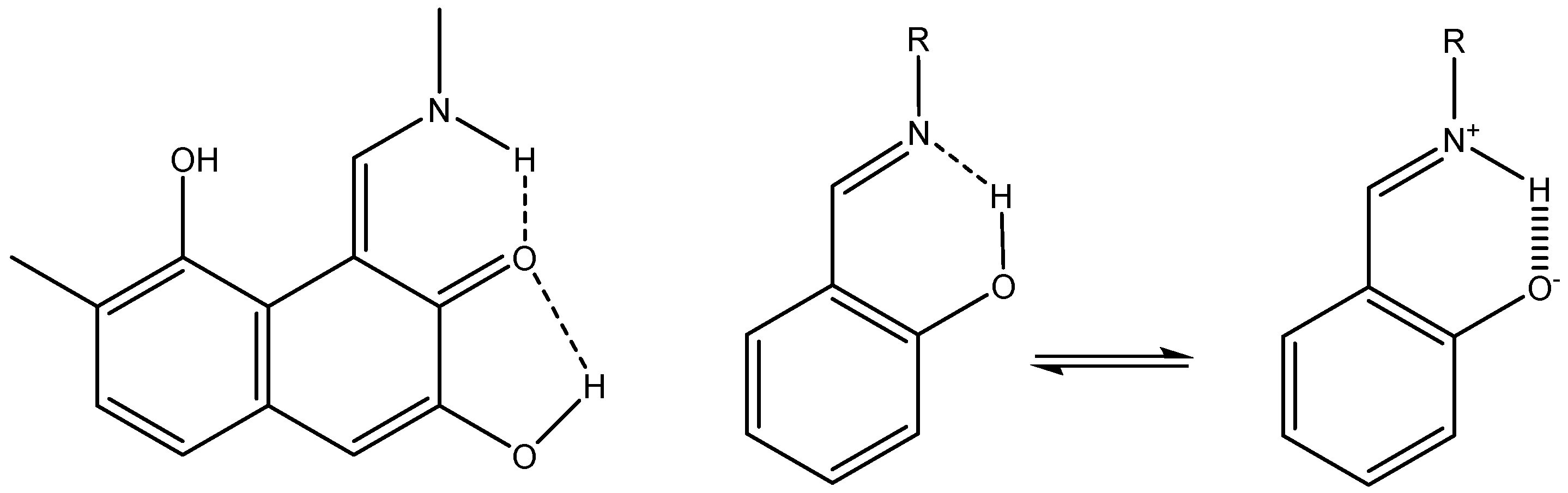
A case in which the calculated nuclear shielding of both hydrogen bonds and non-hydrogen bonds (XH, X=N or S) have been calculated but not used is 2-(2-Mercaptophenyl)-1-azaazulene (see Figure 11), despite the fact that the large spread in 1H chemical shifts (from ~18 to ~3 ppm) offers a platform for analysis [30].
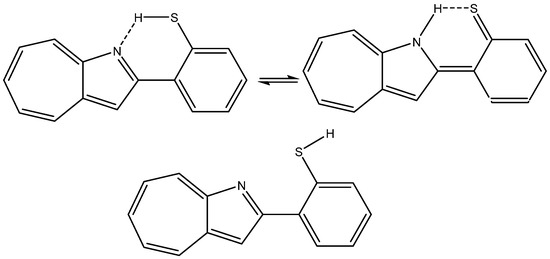
Figure 11.
Tautomers and rotamers of 2-(2-mercaptophenyl)-1-azaazulene from Ref. [30].
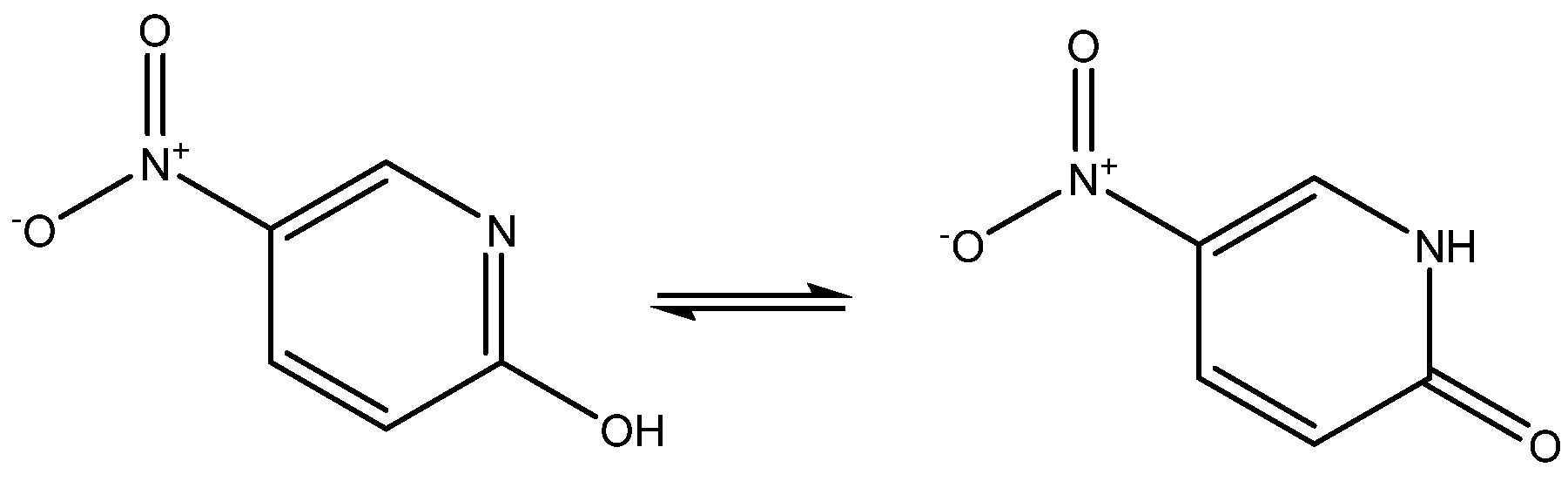
2-Hydroxy-5-nitropyridine may exist as two keto-eno tautomers (see Figure 12). Its NMR parameters were calculated and it was found that they fitted best with the keto-form [31], although the NMR parameter possibly should have been averaged, as the energy difference between the two tautomers was as low as 1.35 Kcal/mol.
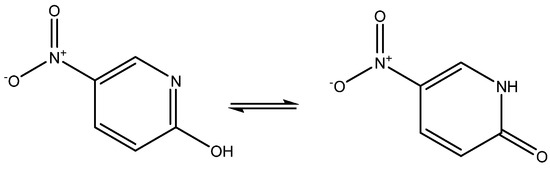
Figure 12.
Tautomers of 2-hydroxy-5-nitropyridine.
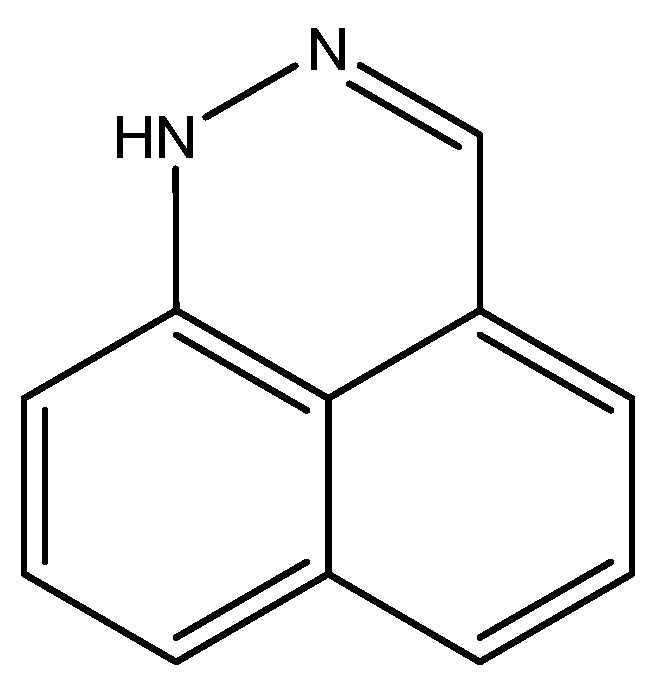
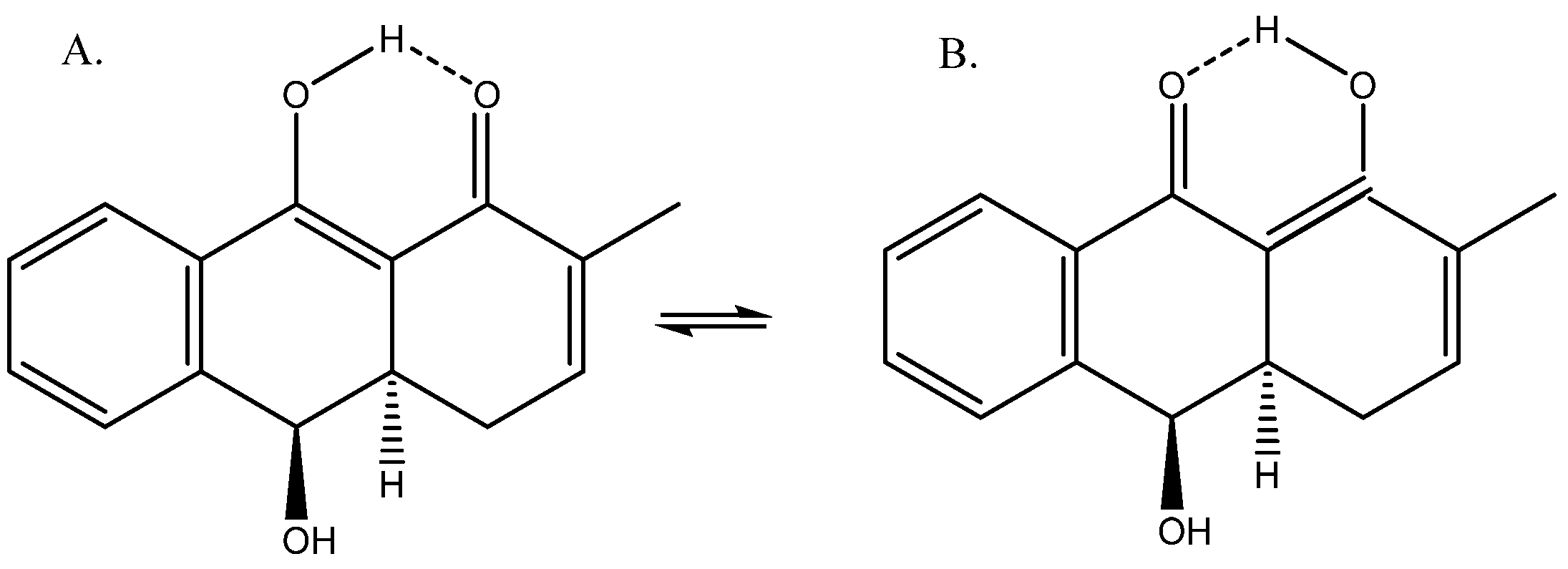
For benzo[d,e]cinnoline, a good fit was found between 1H calculated chemical shifts (B3LYP/6-311++G(d,p) for both structure and for GIAO calculations) and experimental values for the tautomer, which are shown in Figure 13. Notice that many other tautomers are possible. The calculated 13C chemical shifts form a good base for further studies [32].
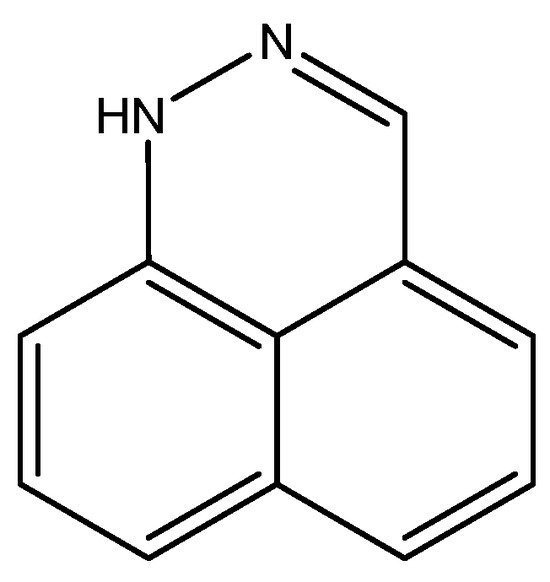
Figure 13.
Structure of benzo[d,e]cinnoline.
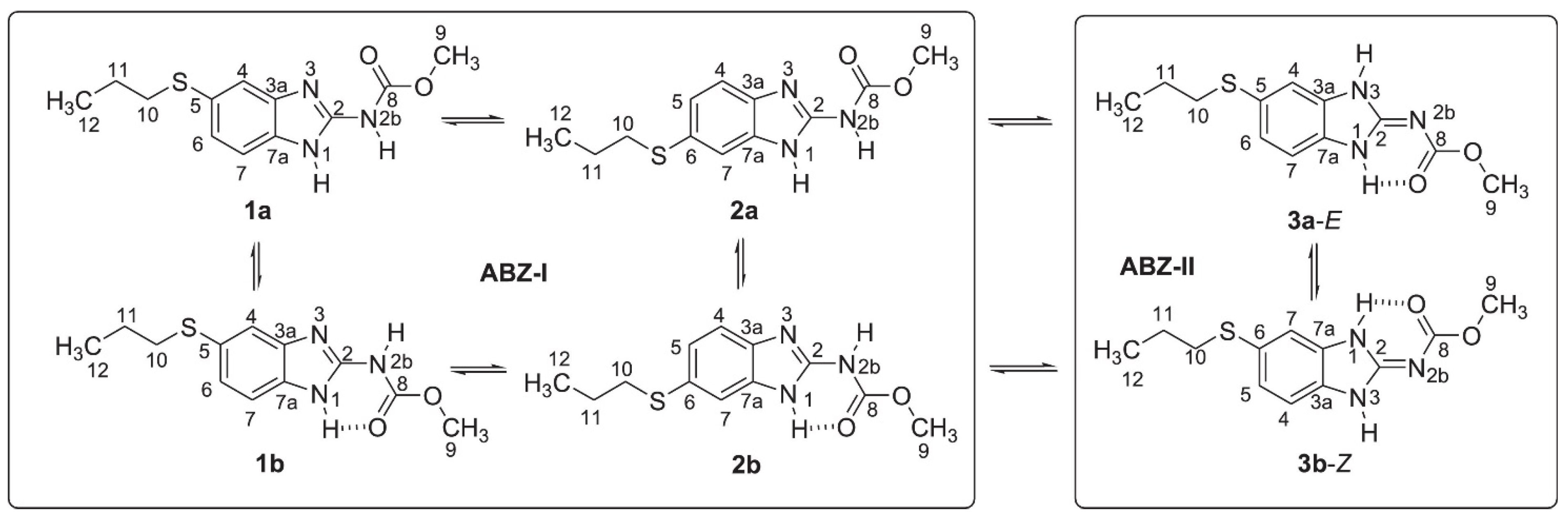
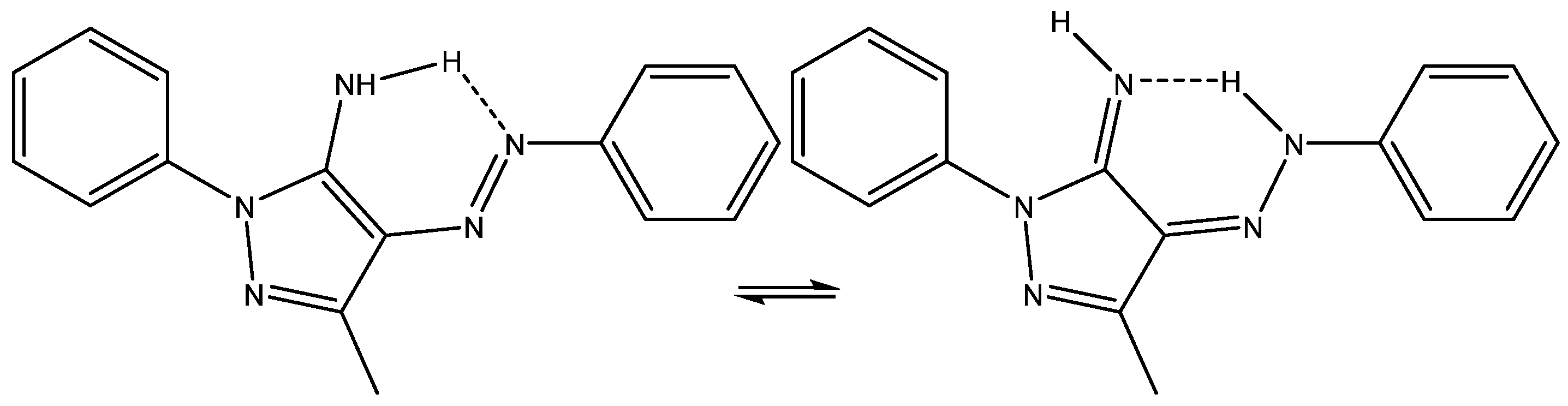
Albendazole may exist both as tautomers and rotamers, as shown in Figure 14 [33]. As seen, the interplay of a very complex set of structures may occur. DFT calculations turn out to be absolutely essential.
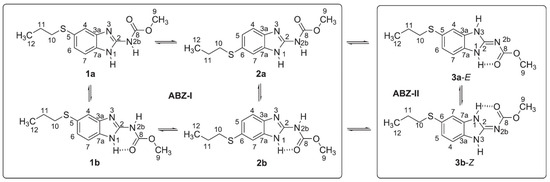
Figure 14.
Tautomers and rotamers of albendazole. Taken from Ref. [33]. Copyright Elsevier 2022.
Mebendazole is very similar to albendazole. The former is missing the SCH2CH2CH3 side chain. Mebendazole may exist as three different phases related to tautomers [34] and can be defined as desmotropes, i.e., crystal phases in which different tautomers are isolated [35].
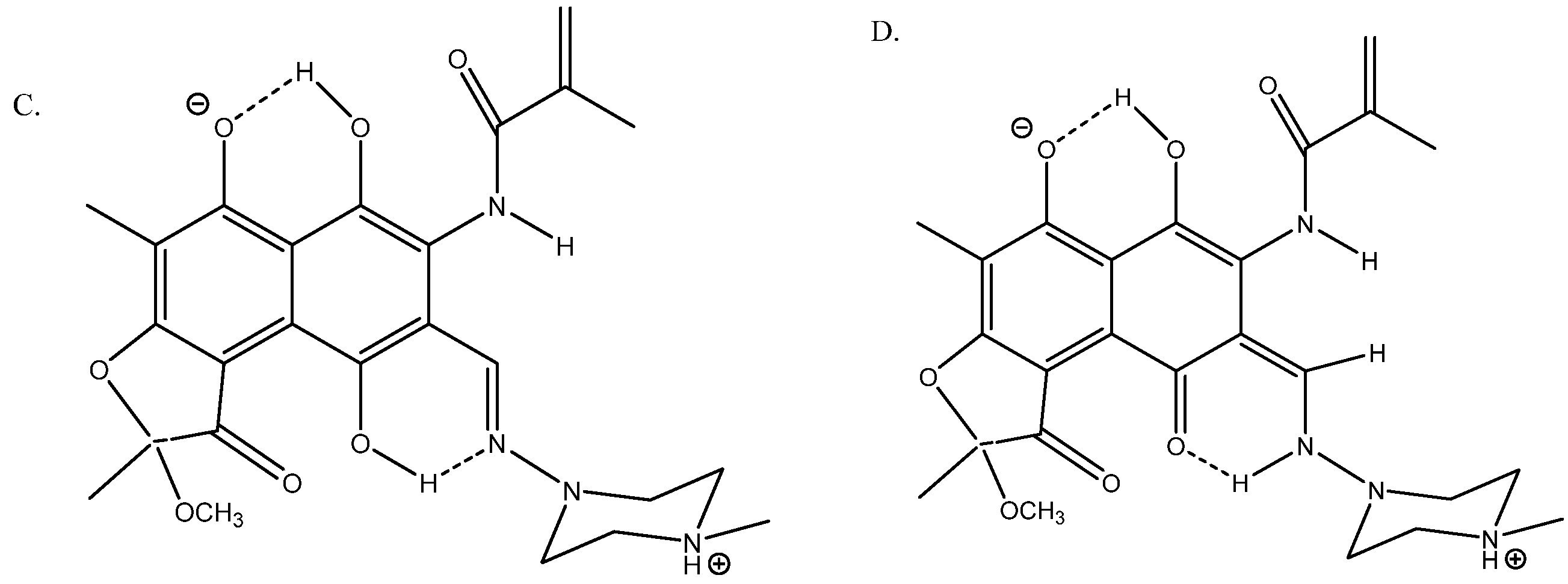
Rifamycin is a complex multi-hydrogen-bonded system [36] (see Figure 15). Its geometries were optimized using the B3LYP functional with the Pople basis set 6-31G(d) [37] and the solvent (DMSO) was taken into account in the PCM approach in the calculations of 13C chemical shifts. A very good fit was obtained between calculated and experimental 13C chemical shifts; R2 = 0.9949 for structure A.
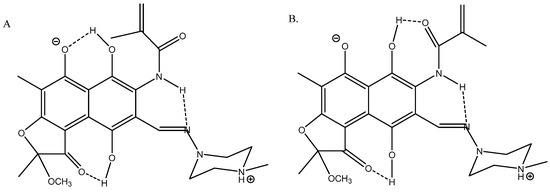
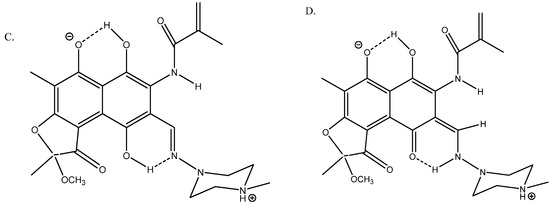
Figure 15.
Truncated structures of rifamycin. (A) Hydrogen bonds to O- and C=O. (B) Hydrogen bonds to C=O(NH) and to C=O. (C) Hydrogen bonds to O- and to C=N. (D) Is a tautomer of C. Taken from Ref. [36]. Copyright MDPI 2023.
A compound isolated from Rubia phillipinensis [38] showed a β-diketone-like structure (Figure 16). The author only suggested measurements for structure A based on low temperature, but as the barrier was low, this was not conclusive evidence. DFT calculations of the 13C chemical shifts suggested a tautomeric equilibrium between the two forms, with A dominating (9:1) [39].
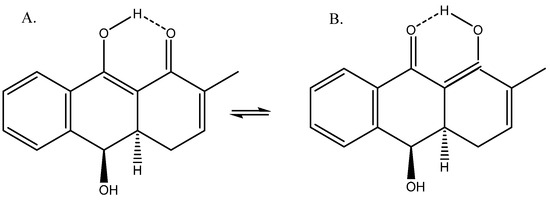
Figure 16.
(A) Enolic forms. (B) Tautomeric structures of dihydroanthracene-1(4H)one.
The tautomerism of 3-methyl-1-phenyl-4-(phenyldiazenyl)-1H-pyrazol-5-amine (see Figure 17) was studied in detail by calculating 1H and 13C chemical shifts and comparing them to the experimental results in order to find the best functional and basis set. However, it is strange to see the chemical shift of the methyl group being taken into account, as this is very, very far from the remaining data points [40].
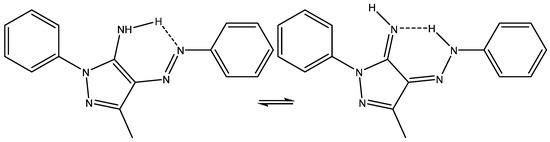
Figure 17.
Tautomerism of 3-methyl-1-phenyl-4-(phenyldiazenyl)-1H-pyrazol-5-amine.
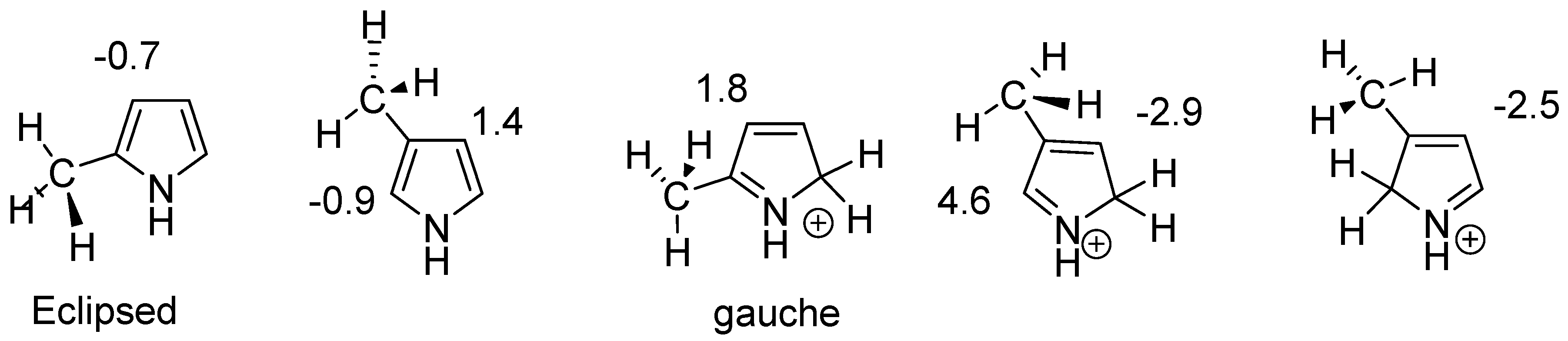
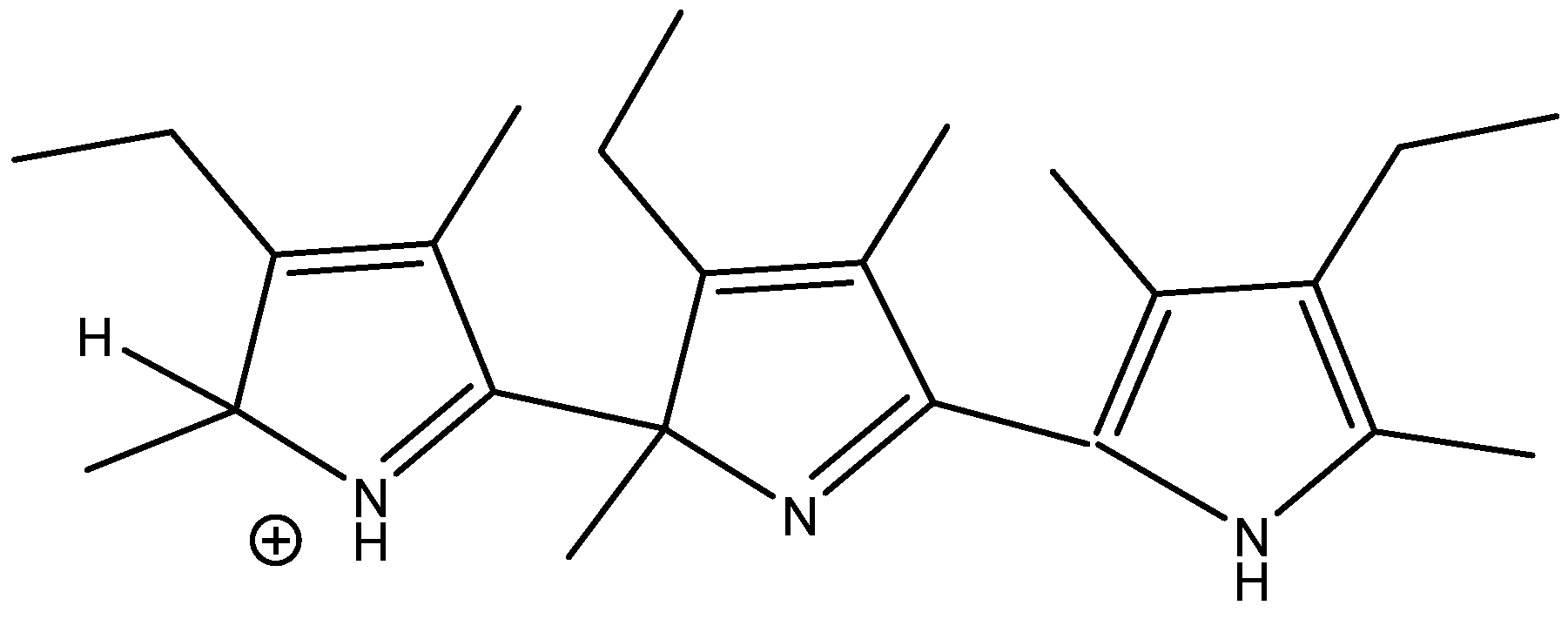
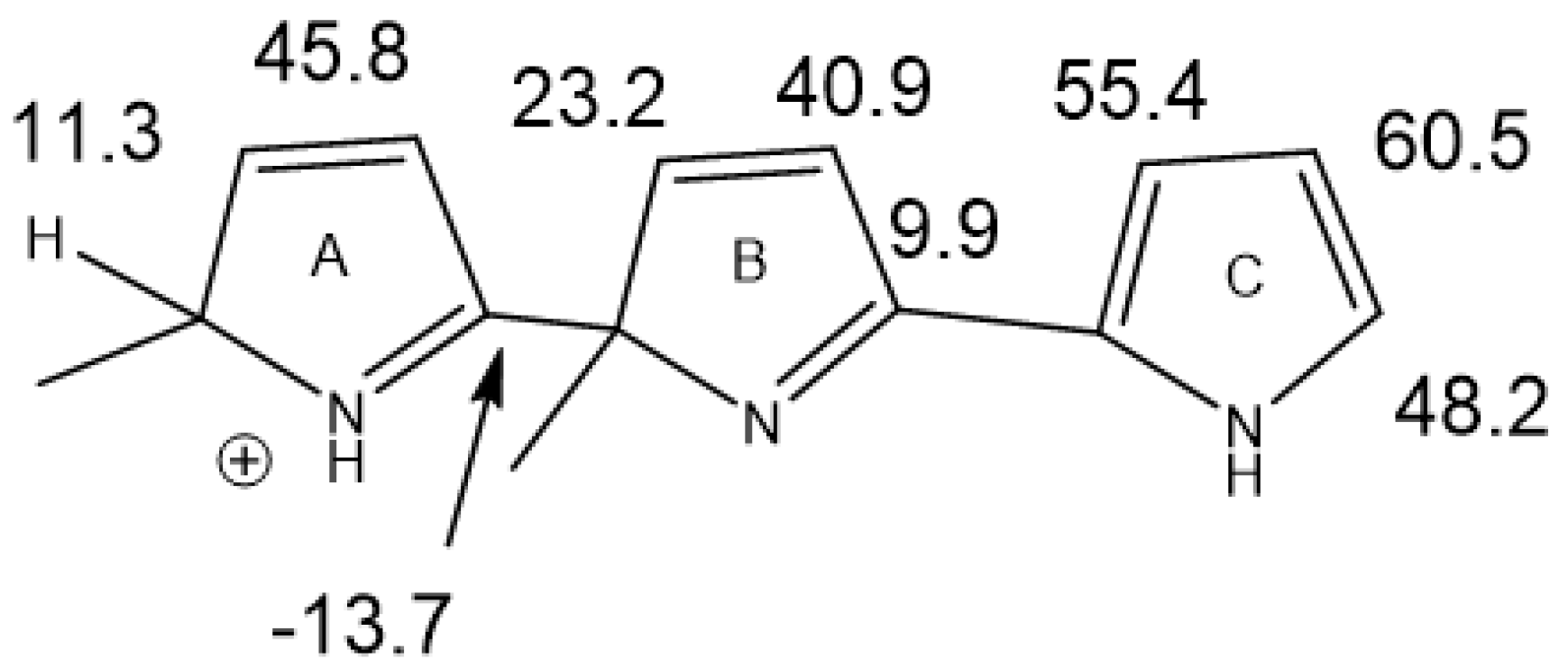
Conformers clearly influence chemical shifts but often are not available directly if the barrier to interconversion is low. Conformers are discussed in Ref. [30]. An example of conformers of protonated alkylpyrroles is given in Figure 18. Different substituent effects are found over the same bond. These substituent effects are later used to estimate the effects of protonation of dimers and trimers (see Section 2.10).
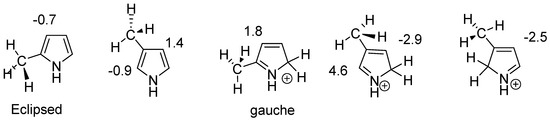
Figure 18.
Calculated substituent effects on 13C chemical shifts for protonated alkyl pyrroles.
2.7. Geometric Isomers
Chemical shifts in the geometric isomers of 18:3 conjugated linolenic acids (CLnAs), hexadecatrienyl pheromones, and model triene-containing compounds were calculated using functionals such as B3LYP and PBE0 as well as functionals including corrections for dispersion interactions (B3LYP-D3, APFD, M06-2X and ωB97XD). 1H NMR chemical shifts were computed at the GIAO/B3LYP/6-311+G(2d,p) level, including PCM, or with even less demanding functionals and basis sets. The very good linear correlation found between experimental NMR chemical shifts, δexp, and calculated shifts, δcalc, provides a strong indication that the assignment procedure is correct. The procedure led to a correction of previous assignments [41].
2.8. Coupling Constants
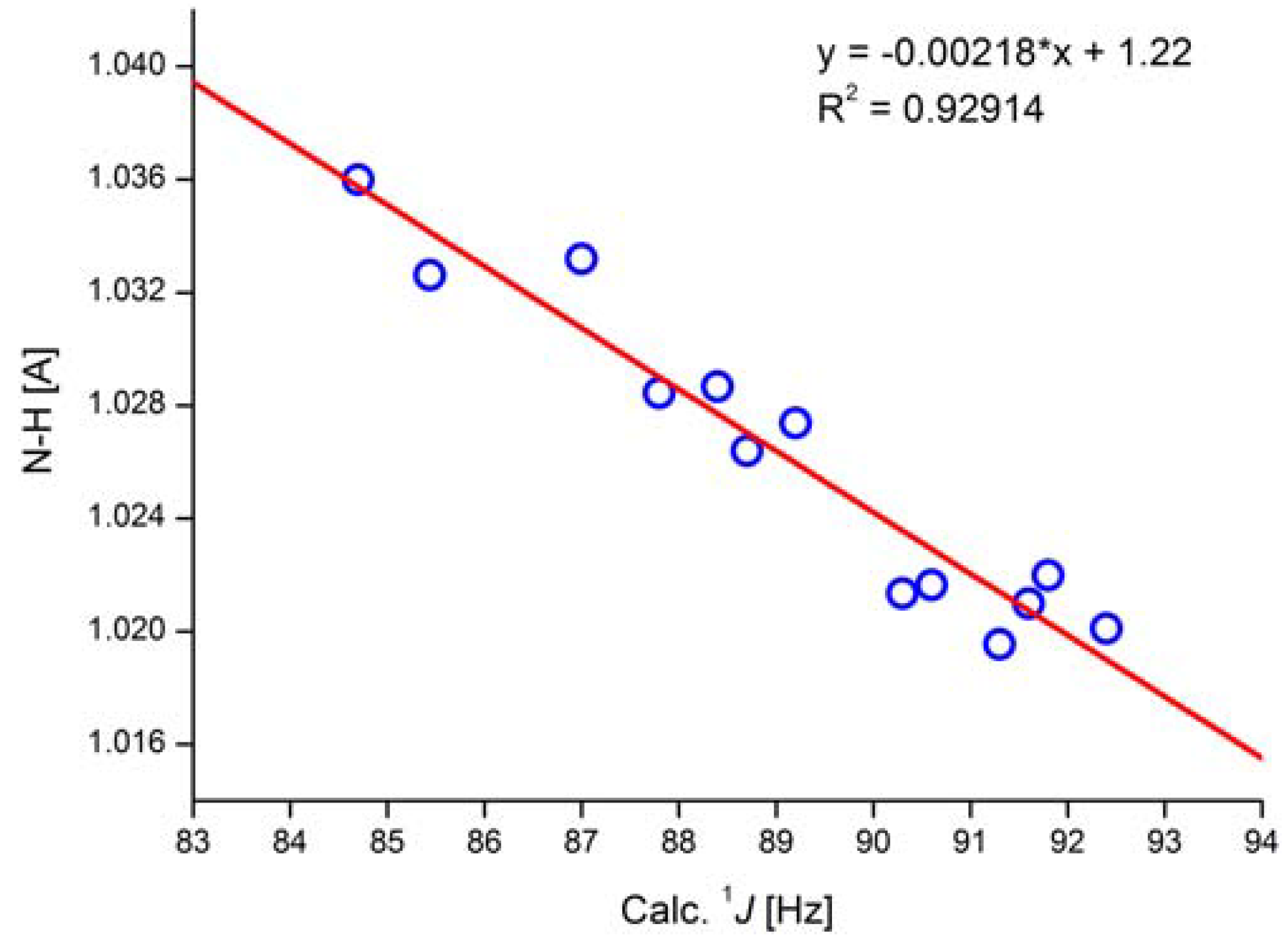
A parameter often used in systems in which NH groups are central, e.g., tautomerism, is 1J(N,H). The study of 1J(N,H) has been pioneered by Lycka and his group [42]. 1J(N,H) couplings are typically of the order of -90 Hz. However, the magnitude may vary due to substituent effects. A truncated gossypol molecule was chosen as the test system for calculations (Figure 19). It was demonstrated that structure optimization using B3LYP/6-311++G** in PCM approximation (CDCl3 as solvent) and APFD/6-311++G** (mixed) for the calculation of coupling constants gave the best result. Studying a number of compounds, a correlation between 1J(N,H) and NH bond length was found (see Figure 20) [43].
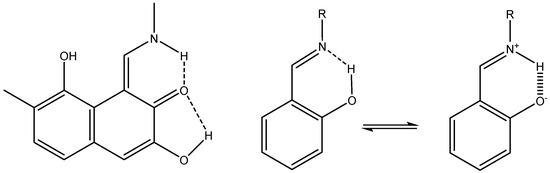
Figure 19.
Left: truncated version of gossypol. Right: tautomers of o-hydroxyazo compounds.
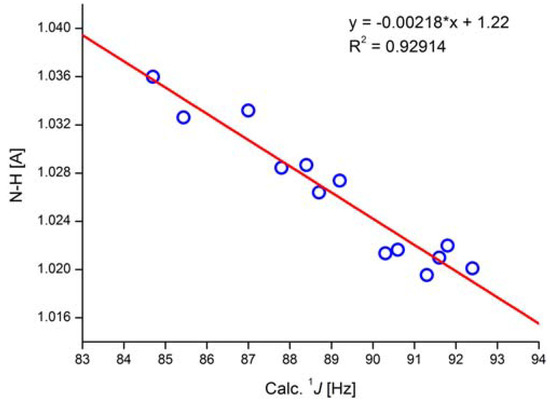
Figure 20.
Correlation of calculated NH bond length and calculated one-bond NH coupling constants. In the equation * is a multiplication sign. APFD/6- 311++G** (mixed) functional/basis set. Taken from Ref. [43]. Copyright Wiley 2020.
Taking the equation in Figure 20, coupling constants can be predicted for the NH tautomer of o-hydroxyazo compounds (Figure 19). More importantly, it could also be determined that the experimental coupling constant of 45 Hz must come from a tautomeric equilibrium and not from a stretch of the NH bond [43].
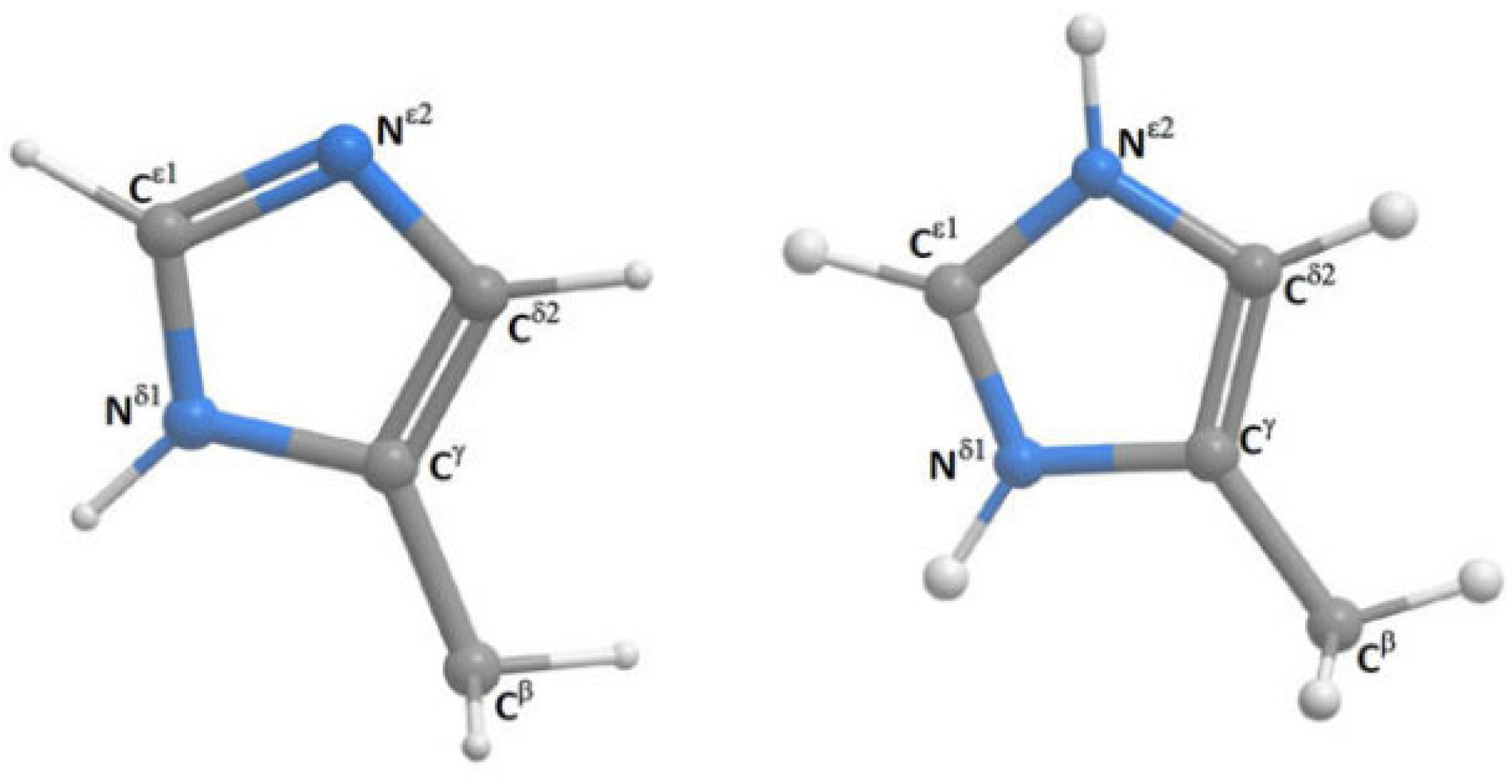
Histidine may at high pH exist as two different tautomers (see Figure 21). DFT calculations are used to determine the 1J(C,H) couplings. 1J(CεH) is not very sensitive to the position of hydrogen, whereas 1J(Cδ2H) couplings show a difference of ~15Hz between the two tautomers and are a good probe to identify the tautomeric composition of a non-protonated histine [44].
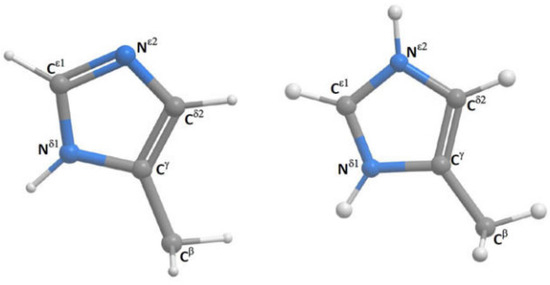
Figure 21.
Histidine and protonated histidine. Taken from Ref. [44]. Copyright Elsevier 2017.
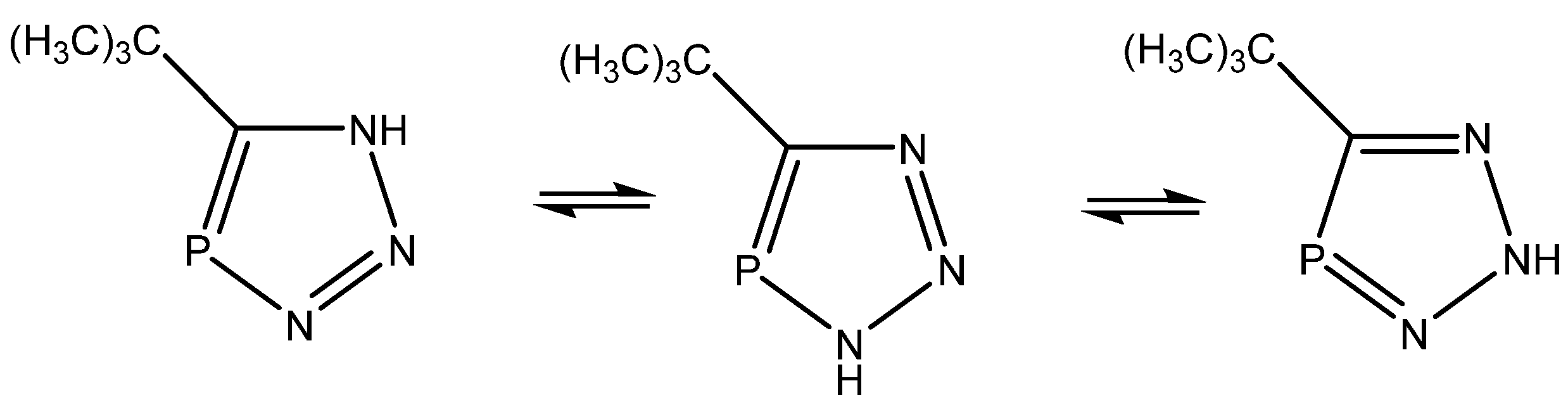
Alkorta and Elguero [45] studied triazaphospholes (Figure 22) and found that the calculated coupling constants (B3LYP/6-311++G(d,p)) in general fitted the experimental ones, except for 1J(C,P), and pointed towards the tautomer to the right, whereas the calculated chemical shfits pointed towards the middle one. The authors leave the presence of both tautomers as a possibility. Synergy was not very obvious in the present case.
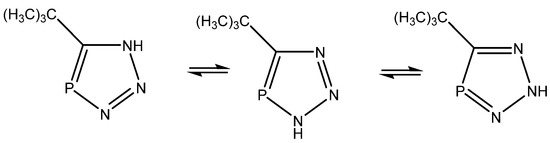
Figure 22.
Tautomeric equilibrium of triazaphospholes.
Bifulco et al. [46] calculated 13C-13C one-bond coupling constants of the 12 possible stereoisomers of strychnine and assigned the correct stereoisomer by comparison with the experimental values. The functional/basis set was B3LYP/6-311+G(d,p).
2.9. Ring Current and Anisotropy Effects
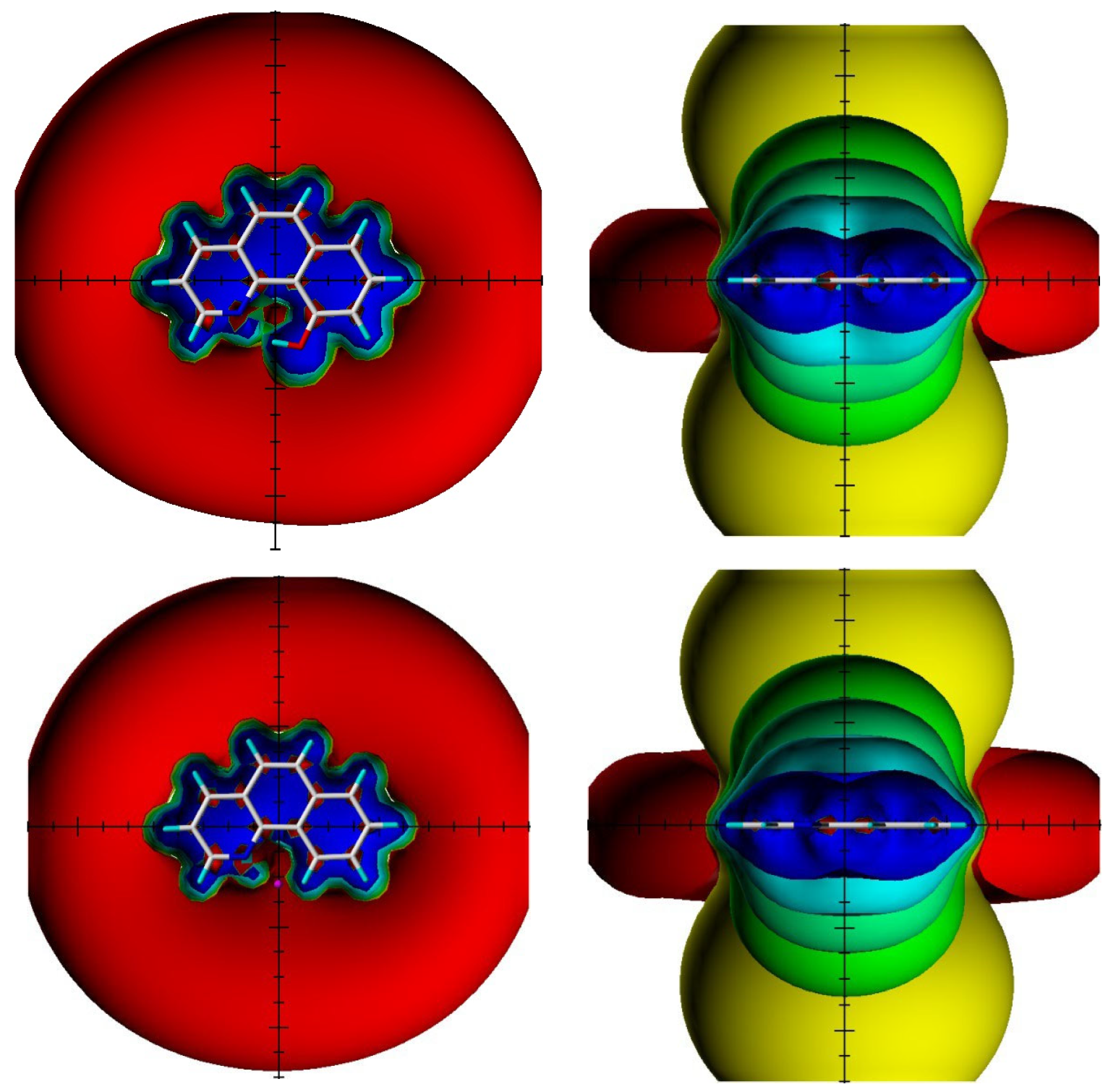
XH (X=O,N,S) chemical shifts are often used as an indicator of hydrogen bonding and as a measure of hydrogen bond strength. However, in aromatic systems, ring currents may influence chemical shifts. A modern way of estimating those is through space NMR shieldings (TSNMRS) [47].
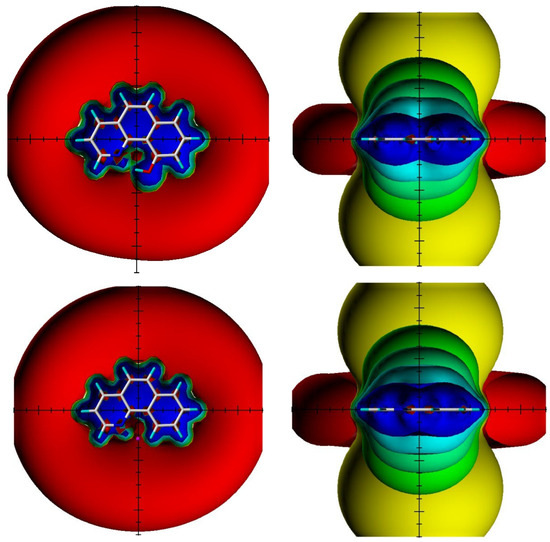
Figure 23.
Visualization of the spatial magnetic properties (TSNMRS) of 10-hydroxybenzo[h]quinoline (above) and benzo[h]quinoline (below) as ICSS of different direction and size (blue represents 5 ppm shielding, cyan 2 ppm shielding, green-blue 1 ppm shielding, green 0.5 ppm shielding, yellow 0.1 ppm shielding, and red −0.1 ppm deshielding). The pink dot in the top view of benzo[h]quinoline shows the position of the OH atom in 10-hydroxybenzo[h]quinolone. Taken from Ref. [48]. Copyright Elsevier 2018.
TSNMRS can also be used to estimate anisotropy effects [49]. TSNMRS values are a combination of ring current effects and anisotropy effects. The TSNMRS value for 10-hydroxybenzo[h]quinoline is as high as −1.91 ppm. For 1,3,5-triacetyl-2,4,6-trihydroxybenzene, this value is 0.17 ppm; for salicylaldehyde, it is −0.67 ppm; for thiosalicylaldehyde, −0.03 ppm; for the enol form of acetylacetone, 0.40 ppm; for o-hydroxyacetophenone, −0.65 ppm; and for o-hydroxythioacetophenone, 0.08 ppm [48]. It is interesting to notice the very large difference between salicylaldehyde and thiosalicylaldehyde as well as between o-hydroxyacetophenone and o-hydroxythioacetophenone. This illustrates the effects of anisotropy. Kleinpeter et al. [50] used TSNMRS to estimate the anisotropy effects of three-membered rings.
2.10. Charged Species
NMR of charged species has recently been reviewed [51,52]. Standard chemical shifts of charged molecules are few. DFT calculations of chemical shifts can be extremely useful in structure elucidation. This is demonstrated in the product of acid dimerization of 2,4-dimethyl-3-ethylpyrrole (kryptopyrrole). The best functional and basis set turned out to be B3LYP/6-311++G(2d,p) including PCM. Obviously, the effect of counter ions should be considered [53].
Working on a set of monoprotonated alkylpyrroles, a large set of functionals and basis sets were tested [54].
The treatment of alkylpyrroles, e.g., kryptopyrrole, may also lead to trimers. Leaving the reaction mixture for a while leads only to one species, as shown in Figure 24. Chemical shifts were calculated based on the values of a scaffold (Figure 25) and substituent effects were calculated based on monomers. A very decent fit between the experimental and calculated values (R2 = 0.984) was obtained. It is important to realize that monomers corresponding to rings A or B cannot be synthesized. The calculated results also revealed that only ring A carried a positive charge (notice the large difference in chemical shifts between the charged and the neutral ring). This method can successfully be used for polymers.
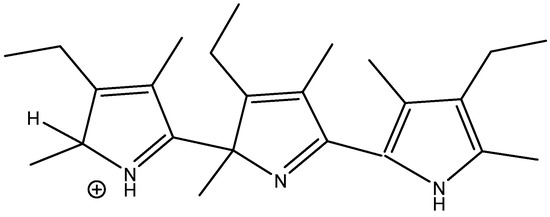
Figure 24.
Trimer from acid treatment of krytopyrrole after long standing.
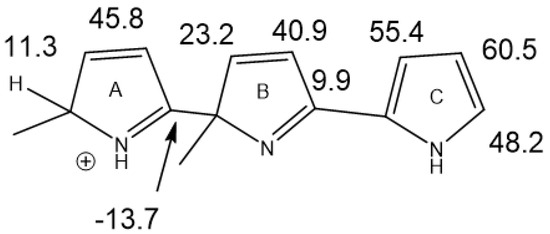
Figure 25.
Calculated 13C nuclear shielding for a trimer scaffold. The structure was optimized with the functional/basis set B3LYP/cc-pVDZ and the nuclear shielding calculated as B3LYP/6-311++G(2d,p).
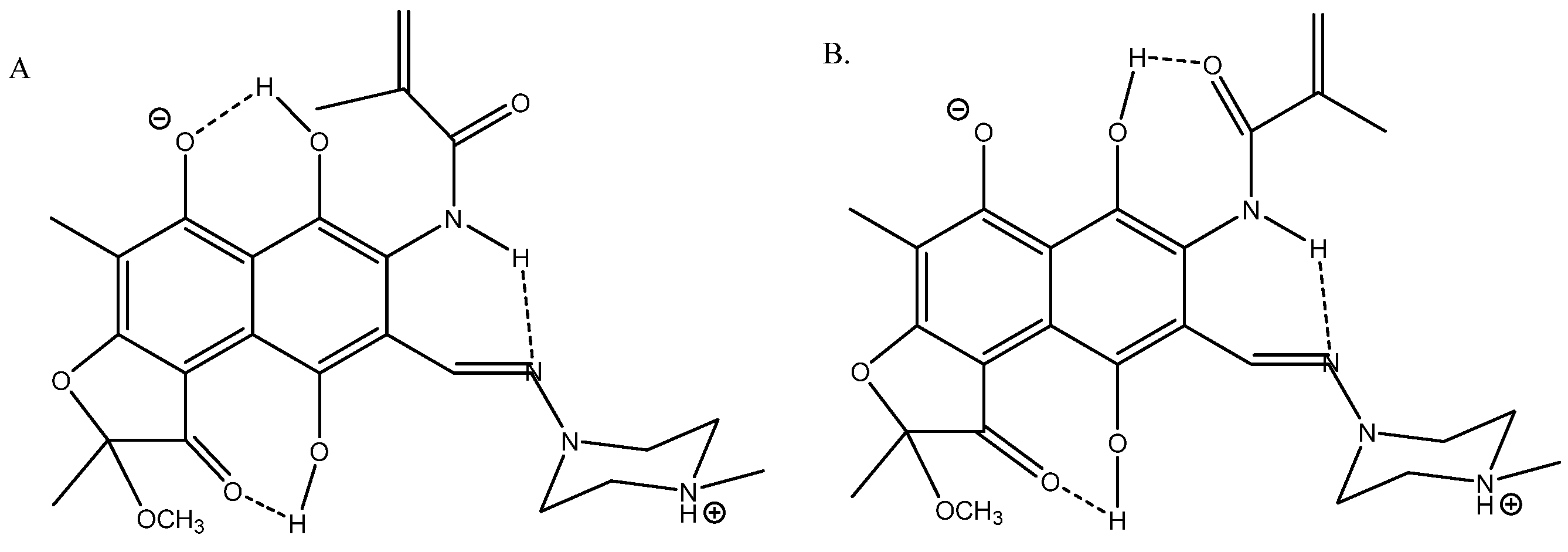
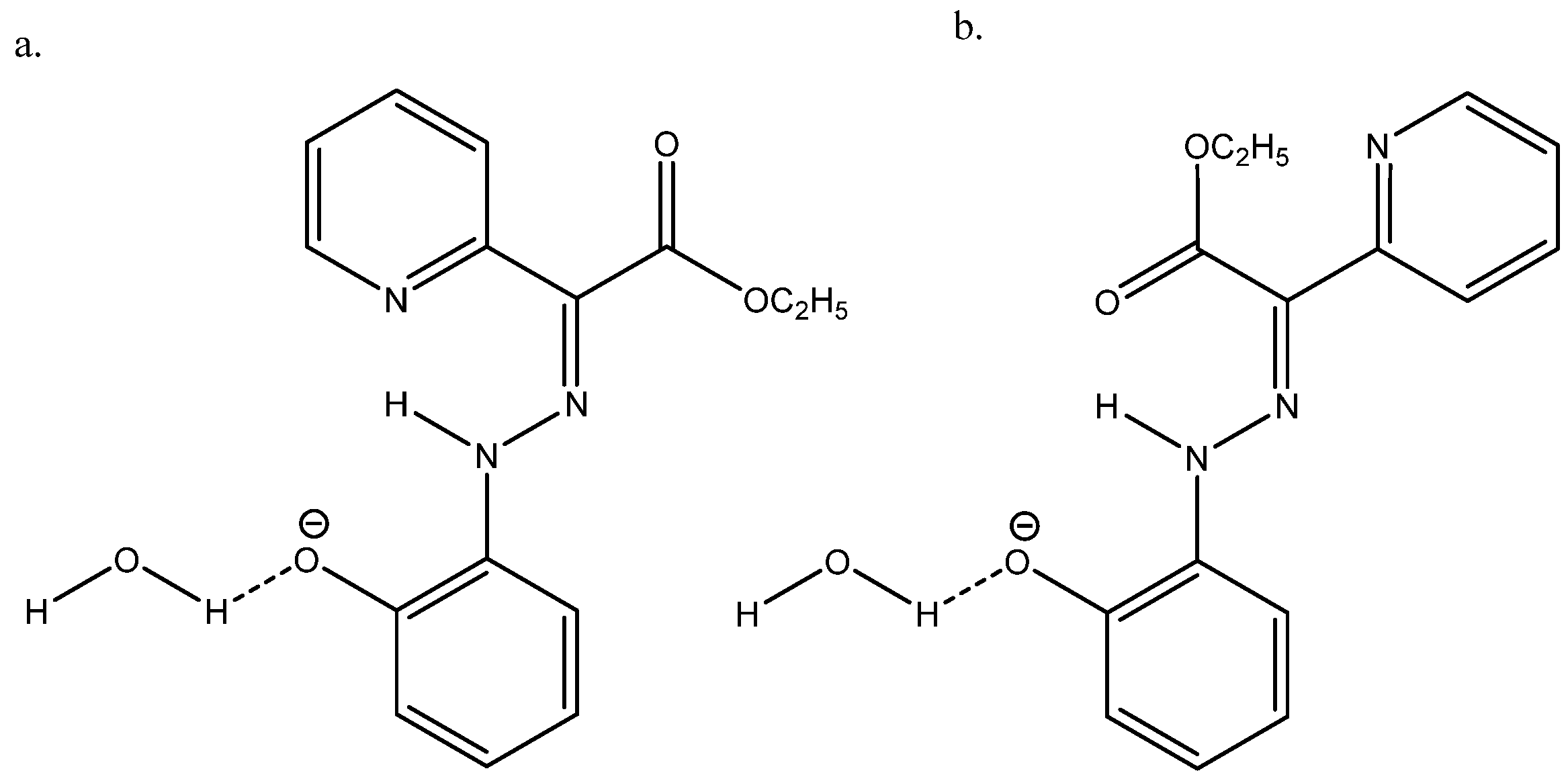
Nuclear shieldings can be difficult to calculate in negatively charged species due to large self-interaction errors [55]. In the case of the azo compound ethyl 2-(2-(2-hydroxyphenyl)hydrazineylidine)-2-(pyridin-2-yl)acetate (see Figure 26), the best agreement between the experimental 13C chemical shifts and calculated ones was obtained with B3LYP/6-311++G(2d,p) + PCM and adding a water molecule, as seen in Figure 26 [56].
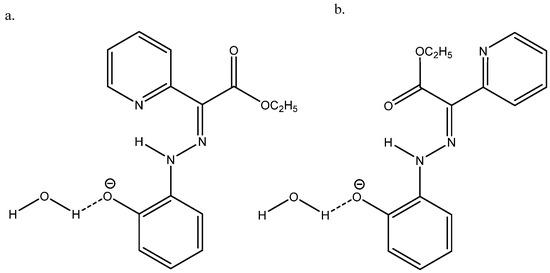
Figure 26.
(a) is the major form and (b) is the minor form. Taken from Ref. [56]. Copyright Wiley 2021.
2.11. Chemical Libraries
Investigating complex biological samples requires access to NMR spectra of a very large number of compounds. For some compounds, reference data are not available. This has been overcome by creating a database of DFT calculated spectra using a method called ISCLE (In Silico Chemical Library Engine) [57].
2.12. Solid State
The assignment of solid-state (SS) spectra is often difficult due to overlap. This can be helped by periodic DFT calculations. This is demonstrated in perovskites [58]. Blanc et al. [59] used DFT calculations for a series of silicon catalysts [(≡SiO)M(ER)(=CHtBu)(R′)] (M = Re, Ta, Mo or W; ER = CtBu, NAr or CH2tBu; R′ = CH2tBu, NPh2, NC4H4) on an oxide support to integrate static values for Chemical Shift Anisotropy (CSA) values. A comparison with experimental data showed motional averaging on the NMR time scale. The data also showed that the motion is non-isotropic.
A series of functionalized (NH or OH) azo-compounds (for an example, see Figure 27) has been studied in the solid state by indirect detection of 14N. CASTEP calculations [60] can be corrected by using a hydride PBE0 functional to give better nuclear shieldings:
s(crystal, PBE0) = s(crystal, PBE) + s(gas PBE0)
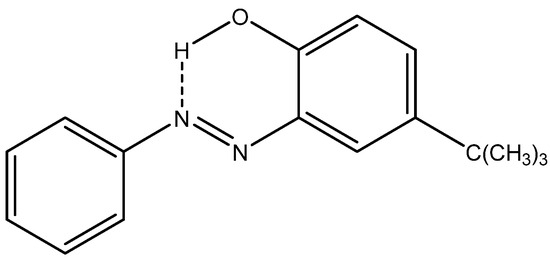
Figure 27.
o-Hydroxyazo compound on the azo form.
The tautomeric form can thus be determined. The authors find that “even when the crystal structure is not known, a comparison of gas-phase calculated 13C chemical shifts of both possible tautomeric structures with those obtained experimentally by SS-NMR can be used for the determination of the tautomeric form” [61].
The examples above (Figure 27) determine one of the tautomeric forms. It becomes more complex when a tautomeric equilibrium is possible between two species.
1H chemical shifts in six solid amino acids are determined and calculated at the DFT and coupled-cluster levels, including spin-orbit coupling, molecular dynamics, and nuclear quantum effects. Including these effects leads to a more accurate prediction of 1H chemical shifts [62].
3. Conclusions
NMR is a very strong tool in structural studies and a very large amount of data are now available. Where this is not the case, DFT calculations of both structures and NMR parameters can be of great importance. This paper illustrates this in cases like tautomerism, in which the necessary structures and NMR parameters of non-isolable tautomers can be calculated. The same is true for ionic species or for compounds found in biological samples. A revision of these structures is also demonstrated. A new case is that of hortoishware. It is also demonstrated that substituent effects on chemical shifts can be calculated for use in polymers. Structural studies of monounsaturated and ω-3 polyunsaturated free fatty acids is discussed in a paper in this Special Issue [63]. The assignment of solid-state spectra and crystal forms also profit greatly from DFT calculations.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author would like to thank Ole Hammerich and Evanildo G. Lacerda for their help with alkylpyrrole calculations and Shashikumar K. Paknikar for the involvement in work on ishwarane.
Conflicts of Interest
The author declares no conflict of interest.
References
- Rzepiela, K.; Buczek, A.; Kupka, T.; Broda, M.A. Factors Governing the Chemical Stability and NMR Parameters of Uracil Tautomers and Its 5-Halogen Derivatives. Molecules 2020, 25, 3931. [Google Scholar] [CrossRef] [PubMed]
- Holtomo, O.; Nsangou, M.; Motapon, O. Basis set dependence of 1H–X spin–spin coupling constants in non-empirical pure DFT framework, X = 1H, 13C, 19F, 35Cl: Case of CHCl=CH–CF3 stereoisomers. AIP Adv. 2021, 11, 035113. [Google Scholar] [CrossRef]
- Antonov, L. (Ed.) Tautomerism: Concepts and Applications in Science and Technology; Wiley-VCH Verlag: Weinheim, Germany, 2016. [Google Scholar]
- Chhetri, B.K.; Lavoie, S.; Sweeney-Jones, A.M.; Kubanek, J. Recent trends in the structural revision of natural products. Nat. Prod. Rep. 2018, 35, 514–531. [Google Scholar] [CrossRef] [PubMed]
- Antonov, L. (Ed.) Tautomerism: Methods and Theories; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2014. [Google Scholar]
- Bazrafshan, M.; Vakili, M.; Tayyari, S.F.; Kamounah, F.S.; Hansen, P.E.; Shiri, A. Synthesis, molecular structure, conformational, and intramolecular hydrogen bond strength of ethyl 3-amino-2-butenoate and its N-Me, N-Ph, and N-Benzyl derivatives; an experimental and theoretical study. J. Mol. Struct. 2023, 1274, 34479. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. 1. A Gauge-invariant LCAO method for N.M.R. chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, F.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Kutateladze, A.G.; Reddy, D.S. High-Throughput In Silico Structure Validation and Revision of Halogenated Natural Products Is Enabled by Parametric Corrections to DFT-Computed 13C NMR Chemical Shifts and Spin–Spin Coupling Constants. J. Org. Chem. 2017, 82, 3368–3381. [Google Scholar] [CrossRef]
- Kupka, T. Theory and Computation of Nuclear Shielding. In Nuclear Magnetic Resonance; Hodgkinson, P., Sauri, J., Eds.; Royal Society of Chemistry: London, UK, 2022; Volume 48. [Google Scholar]
- Grimblat, N.; Sarotti, A.M. Computational Chemistry to the Rescue: Modern Toolboxes for the Assignment of Complex Molecules by GIAO NMR Calculations. Chem. A Eur. J. 2016, 22, 12246–12261. [Google Scholar] [CrossRef]
- Beran, G.J.O. Calculating Nuclear Magnetic Resonance Chemical Shifts from Density Functional Theory: A Primer. eMagRes 2019, 8, 215–226. [Google Scholar]
- Bagno, A.; Rastrelli, F.; Saielli, G. Toward the Complete Prediction of the 1H and 13C NMR Spectra of Complex Organic Molecules by DFT Methods: Application to Natural Substances. Chem. Eur. J. 2006, 12, 5514–5525. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, A.C.F.; Ribeiro, D.J.; de Amorim, M.B. Structural determination of complex natural products by quantum mechanical calculations of 13C NMR chemical shifts: Development of a parameterized protocol for terpenes. J. Mol. Model. 2016, 22, 183. [Google Scholar] [CrossRef]
- Elyashberg, M.; Tyagarajan, S.; Mandal, M.; Buevich, A.V. Enhancing Efficiency of Natural Product Structure Revision: Leveraging CASE and DFT over Total Synthesis. Molecules 2023, 28, 3796. [Google Scholar] [CrossRef] [PubMed]
- Siskos, M.G.; Choudhary, M.I.; Gerothanassis, I.P. Hydrogen Atomic Positions of O–H···O Hydrogen Bonds in Solution and in the Solid State: The Synergy of Quantum Chemical Calculations with 1H-NMR Chemical Shifts and X-ray Diffraction Methods. Molecules 2017, 22, 415. [Google Scholar] [CrossRef] [PubMed]
- Mari, S.H.; Varras, P.C.; Atia-tul-Wahab; Choudhary, I.M.; Siskos, M.G.; Gerothanassis., I.P. Solvent-Dependent Structures of Natural Products Based on the Combined Use of DFT Calculations and 1H-NMR Chemical Shifts. Molecules 2019, 24, 2290. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, J.; LiWang, A.; Manalo, M.N.; Hansen, P.E. Peptide Deuterium Isotope Effects on Backbone 15N Chemical Shifts in Proteins. J. Biol. NMR 2009, 44, 119–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, H.; Wang, Y.; Nafie, L.A. Computational methods and points for attention in absolute configuration determination. Front. Nat. Prod. 2023, 1, 1086897. [Google Scholar] [CrossRef]
- Ratnayake, R.; Wikramratne, N. A novel Tetracyclic Sesquiterpene from the Genus Hortonia. Ceylon J. Sci. 2017, 46, 115–118. [Google Scholar] [CrossRef][Green Version]
- Lauro, G.; Das, P.; Riccio, R.; Reddy, D.S.; Bifulco, G. DFT/NMR Approach for the Configuration Assignment of Groups of Stereoisomers by the Combination and Comparison of Experimental and Predicted Sets of Data. J. Org. Chem. 2020, 85, 3297–3306. [Google Scholar] [CrossRef]
- Das, P.; Babbar, P.; Malhotra, N.; Sharma, M.; Jachak, G.R.; Gonnade, R.G.; Shanmugam, D.; Harlos, K.; Yogavel, M.; Sharma, A.; et al. Specific Stereoisomeric Conformations Determine the Drug Potency of Cladosporin Scaffold against Malarial Parasite. J. Med. Chem. 2018, 61, 5664–5678. [Google Scholar] [CrossRef]
- Hansen, P.E. Isotope Effects on Chemical shifts of small molecules. Molecules 2022, 27, 2405. [Google Scholar] [CrossRef] [PubMed]
- Jameson, C.J.; Osten, H.-J. The NMR isotope shift in polyatomic molecules. Estimation of the dynamic factors. J. Chem. Phys. 1984, 81, 4300–4305. [Google Scholar] [CrossRef]
- Elias, R.S.; Saeed, B.A.; Kamounah, F.S.; Duus, F.; Hansen, P.E. Strong Intramolecular Hydrogen Bonds and steric Effects. A NMR and Computational Study. Magn. Reson. Chem. 2020, 58, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.A.; Elias, R.S.; Kamounah, F.S.; Hansen, P.E. A NMR, MP2 and DFT Study of Thiophenoxyketenimines (o-ThioSchiff bases). Magn. Reson. Chem. 2018, 56, 172–182. [Google Scholar] [CrossRef]
- Hansen, P.E.; Darugar, V.; Vakili, M.; Kamounah, F.S. Tautomerism of pyridinylbutane-1,3-diones: An NMR and DFT study. Magn. Reson. Chem. 2023, 61, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Cmoch, P.; Krzeczyński, P.; Leś, A. Multinuclear NMR Measurements and DFT Calculations for Capecitabine Tautomeric form Assignment in a Solution. Molecules 2018, 23, 161. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, S.H.; Halim, S.A.; El-Nahas, A.M.; El-Meligy, A.B. A density functional theory study of the molecular structure, reactivity, and spectroscopic properties of 2-(2-mercaptophenyl)-1-azaazulene tautomers and rotamers. Sci. Rep. 2023, 13, 15626. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Shaaban, I.A.; Soliman, U.A.; Zoghaib, W.M. 2-Hydroxy-5-nitropyridine and 5-nitro-2-pyridone: Tautomerism, infrared, Raman, and NMR spectral interpretations, normal coordinate analysis, and DFT calculations. J. Chin. Chem. Soc. 2021, 68, 1863–1879. [Google Scholar] [CrossRef]
- Elguero, J.; Alkorta, I. A DFT study of the tautomerism of 1H-benzo[de]cinnolines and their protonated forms. Theor. Chem. Accounts 2022, 141, 26. [Google Scholar] [CrossRef]
- Claramunt, R.M.; López, C.; Sanz, D.; Elguero, J.; Alkorta, I. Determination of the tautomerism of albendazole desmotropes using solution and solid state NMR together with DFT theoretical calculations, both energies and chemical shifts. J. Mol. Struct. 2022, 1261, 132883. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Glass, B.D.; Mangan, M.; Smithson, J. Analysing the crystal purity of mebendazole raw material and its stability in a suspension formulation. Int. J. Pharm. 2008, 361, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Bravetti, F.; Bordignon, S.; Alig, E.; Eisenbeil, D.; Fink, L.; Nervi, C.; Gobetto, R.; Schmidt, M.U.; Chierotti, M.R. Solid-State NMR-Driven Crystal Structure Prediction of Molecular Crystals: The Case of Mebendazole. Chem. Eur. J. 2022, 28, e202103589. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E.; Kamounah, F.S. Multiple Intramolecular Hydrogen Bonding in large Biomolecules. DFT Calculations and Deuterium Isotope Effects on 13C chemical shifts as a tool in structural studies. Chemistry 2023, 5, 1317–1328. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Oh, J.; Quan, K.T.; Lee, J.S.; Park, I.; Kim, C.S.; Ferreira, D.; Thuong, P.T.; Kim, Y.H.; Na, M. NMR-Based Investigation of Hydrogen Bonding in a Dihydroanthracen-1(4H)one from Rubia philippinensis and Its Soluble Epoxide Hydrolase Inhibitory Potential. J. Nat. Prod. 2018, 81, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E. NMR of natural products as potential drugs. Molecules 2021, 26, 3763. [Google Scholar] [CrossRef] [PubMed]
- Safi, Z.S.; Wazzan, N. DFT calculations of 1H- and 13C-NMR chemical shifts of 3-methyl-1-phenyl-4-(phenyldiazenyl)-1H-pyrazol-5-amine in solution. Sci. Rep. 2022, 12, 17798. [Google Scholar] [CrossRef]
- Venianakis, T.; Oikonomaki, C.; Siskos, M.G.; Primikyri, A.; Gerothanassis, I.P. DFT Calculations of 1H NMR Chemical Shifts of Geometric Isomers of Conjugated Linolenic Acids, Hexadecatrienyl Pheromones, and Model Triene-Containing Compounds: Structures in Solution and Revision of NMR Assignments. Molecules 2021, 26, 3477. [Google Scholar] [CrossRef]
- Lyčka, A.; Šnobl, D. Coupling constants nitrogen-15-nitrogen-15 and nitrogen-15-hydrogen in phenylhydrazones forming hydrogen bond. Collect. Czech. Chem. Commun. 1981, 46, 892–897. [Google Scholar] [CrossRef]
- Hansen, P.E.; Saeed, B.A.; Rutu, R.S.; Kupka, T. One-bond 1J(15N,H) coupling constants at sp2 hybridized nitrogen of Schiff bases, enaminones and similar compounds. A theoretical study. Magn. Reson. Chem. 2020, 58, 750–762. [Google Scholar] [CrossRef]
- Vila, J.A.; Scheraga, H.A. Limiting Values of the one-bond C–H Spin-Spin Coupling Constants of the Imidazole Ring of Histidine at High-pH. J. Mol. Struct. 2017, 1134, 576–581. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J. Theoretical studies of the tautomerism of two azaphospholes: 5-tert-butyl-1,2,3,4-triazaphosphole and N,N-dimethyl-1,2,4,3-triazaphosphole-5-amine. Moroc. J. Heterocycl. Chem. 2016, 15, 92–98. [Google Scholar]
- Bifulco, G.; Riccio, R.; Martin, G.E.; Buevich, A.V.; Williamson, R.T. Quantum Chemical Calculations of 1JCC Coupling Constants for the Stereochemical Determination of Organic Compounds. Org. Lett. 2013, 15, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Klod, S.; Koch, A.; Kleinpeter, E. Ab-initio quantum-mechanical GIAO calculation of the anisotropic effect of C–C and X–C single bonds—Application to the 1H NMR spectrum of cyclohexane. J. Chem. Soc., Perkin Trans. 2002, 2, 1506–1509. [Google Scholar] [CrossRef]
- Hansen, P.E.; Koch, A.; Kleinpeter, E. Ring Current and Anisotropy Effects on OH Chemical Shifts. Tetrahedron Lett. 2018, 59, 22188–22192. [Google Scholar] [CrossRef]
- Kleinpeter, E. Quantification and Visualization of the Anisotropy Effect in 1H NMR Spectroscopy by Through-Space-NMR-Shieldings. Ann. Rep. NMR Spectr. 2014, 82, 115. [Google Scholar]
- Kleinpeter, E.; Krüger, S.; Koch, A. Anisotropy Effect of Three-Membered Rings in 1H NMR Spectra: Quantification by TSNMRS and Assignment of the Stereochemistry. J. Phys. Chem. A. 2015, 119, 4268–4276. [Google Scholar] [CrossRef]
- Krivdin, L.B. Computational NMR of charged systems. Magn. Reson. Chem. 2022, 60, 8–79. [Google Scholar] [CrossRef]
- Kupka, T. Theory and computation of nuclear shielding. In Nuclear Magnetic Resonance; Hodgkinson, P., Ed.; Royal Society of Chemistry: London, UK, 2021; Volume 46, pp. 1–33. [Google Scholar]
- Lacerda, E.G., Jr.; Kamounah, F.S.; Coutinho, K.; Sauer, S.P.A.; Hansen, P.E.; Hammerich, O. Computational prediction of the 1H and 13C NMR chemical shifts for protonated alkylpyrroles–electron correlation and not solvation is the salvation. Chem. Phys. Chem. 2019, 20, 78–91. [Google Scholar] [CrossRef]
- Zahn, S.L.V.; Hammerich, O.; Hansen, P.E.; Sauer, S.P.A. The Best DFT Functional for the Prediction of 1H and 13C Chemical Shifts of Protonated Alkylpyrroles. J. Comput. Chem. 2021, 42, 1248–1262. [Google Scholar] [CrossRef]
- Nattino, F.; Dupont, C.; Marzari, N.; Andreussi, O.; Nattino, F.; Dupont, C.; Marzari, N.; Andreussi, O.J. Functional extrapolations to tame unbound anions in density-functional theory calculations. Chem. Theory Comput. 2019, 15, 6313–6322. [Google Scholar] [CrossRef] [PubMed]
- Kurutos, A.; Sauer, S.P.A.; Kamounah, F.S.; Hansen, P.E. Azo-hydrazone derived molecular switches: Synthesis and conformational investigation. Magn. Reson. Chem. 2021, 59, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Yesiltepe, Y.; Nuñez, J.R.; Colby, S.M.; Thomas, D.G.; Borkum, M.I.; Reardon, P.N.; Washton, N.M.; Metz, T.O.; Teeguarden, J.G.; Govind, N.; et al. An automated framework for NMR chemical shift calculations of small organic molecules. J. Cheminform. 2018, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Quarti, C.; Furet, E.; Katan, C. DFT Simulations as Valuable Tool to Support NMR Characterization of Halide Perovskites: The Case of Pure and Mixed Halide Perovskites. Helv. Chim. Acta 2021, 104, e2000231. [Google Scholar] [CrossRef]
- Blanc, F.; Basset, J.-M.; Copéret, C.; Sinha, A.; Tonzetich, Z.J.; Schrock, R.R.; Solans-Monfort, X.; Clot, E.; Eisenstein, O.; Lesage, A.; et al. Dynamics of Silica-Supported Catalysts Determined by Combining Solid-State NMR Spectroscopy and DFT Calculations. J. Am. Chem. Soc. 2008, 130, 5886–5900. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, P.C.J.; Hasnip, J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Bartov, K.; Císarova, I.; Lycka, A.; Dracínský, M. Tautomerism of azo dyes in the solid state studied by 15N, 14N, 13C and 1H NMR spectroscopy, X-ray diffraction and quantum-chemical calculations. Dyes Pigments 2020, 178, 108342. [Google Scholar] [CrossRef]
- Dračínský, M.; Vícha, J.; Bártová, K.; Hodgkinson, P. Towards Accurate Predictions of Proton NMR Spectroscopic Parametersin Molecular Solids. ChemPhysChem 2020, 21, 2075–2083. [Google Scholar] [CrossRef]
- Venianakis, T.; Siskos, M.G.; Papamokos, G.; Gerothanassis, I.P. Structural Studies of Monounsaturated and ω-3 Polyunsaturated Free Fatty Acids in Solution with the Combined Use οf NMR and DFT Calculations—Comparison with the Liquid State. Molecules 2023, 28, 6144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).