A Rare Benzothiazole Glucoside as a Derivative of ‘Albedo Bluing’ Substance in Citrus Fruit and Its Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
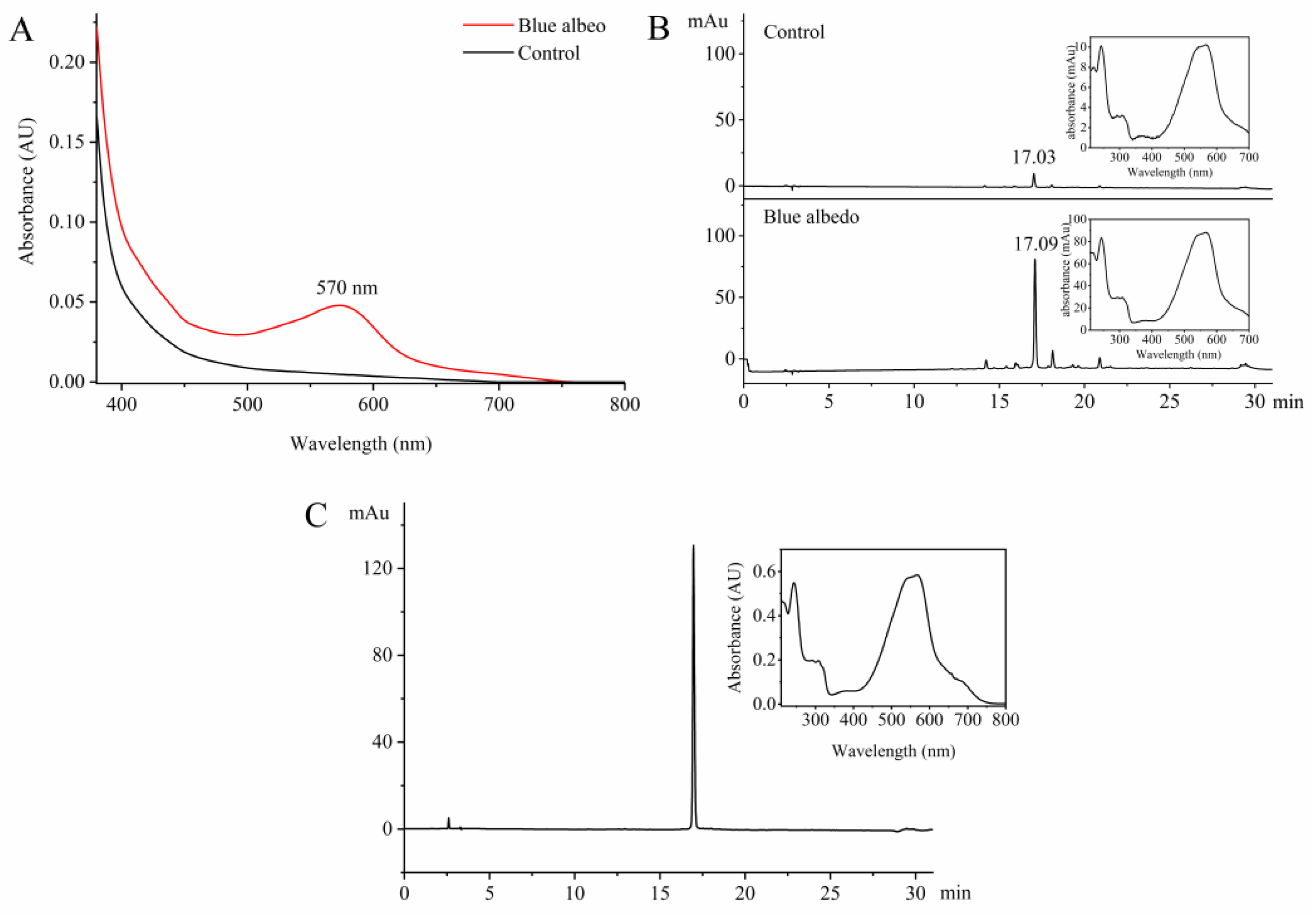
2.1. Extraction and Purification of ABS from ‘Gonggan’
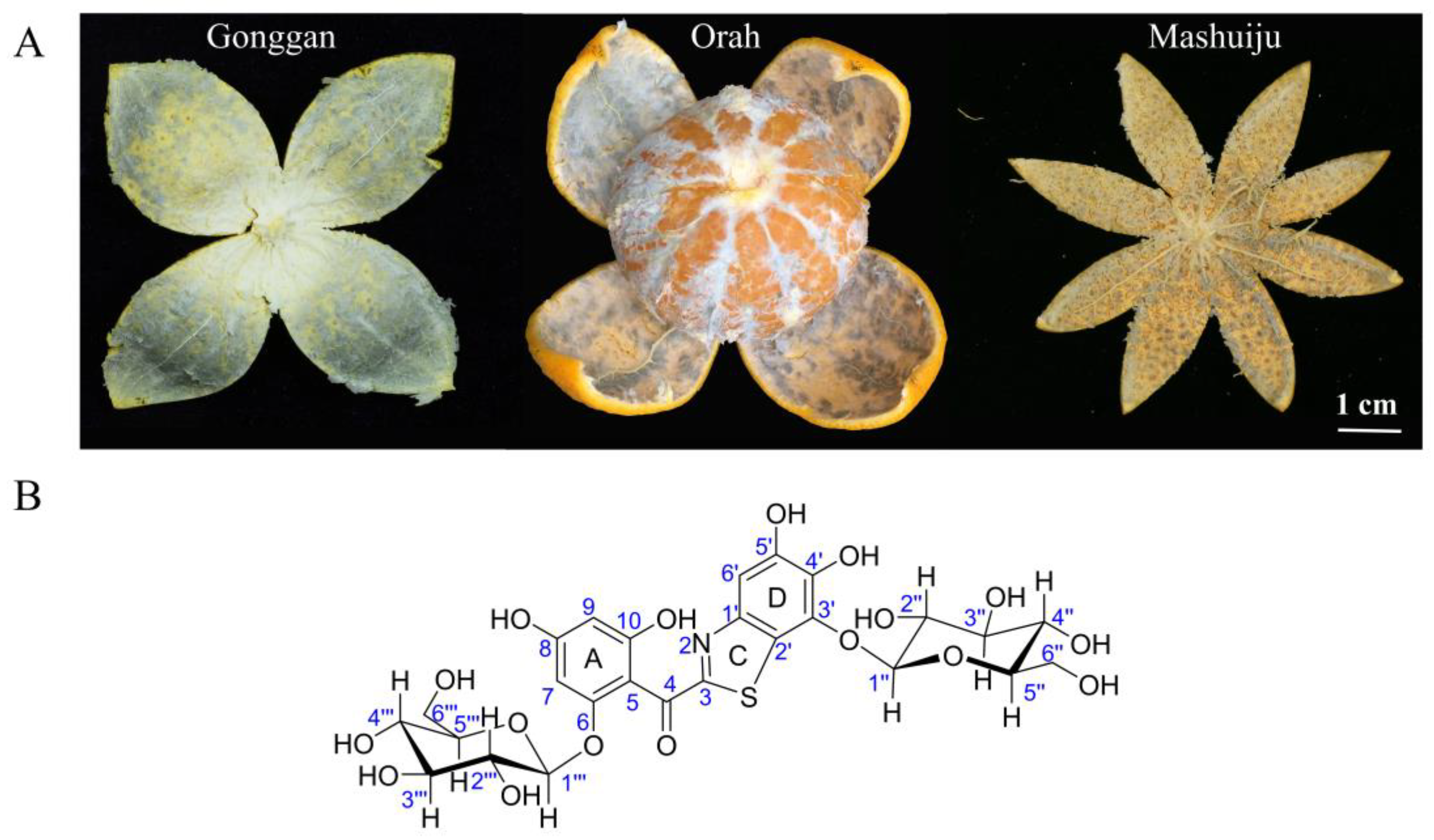
2.2. Comparative Analysis of ABS among Different Citrus Varieties
2.3. Preparation of ABS-D
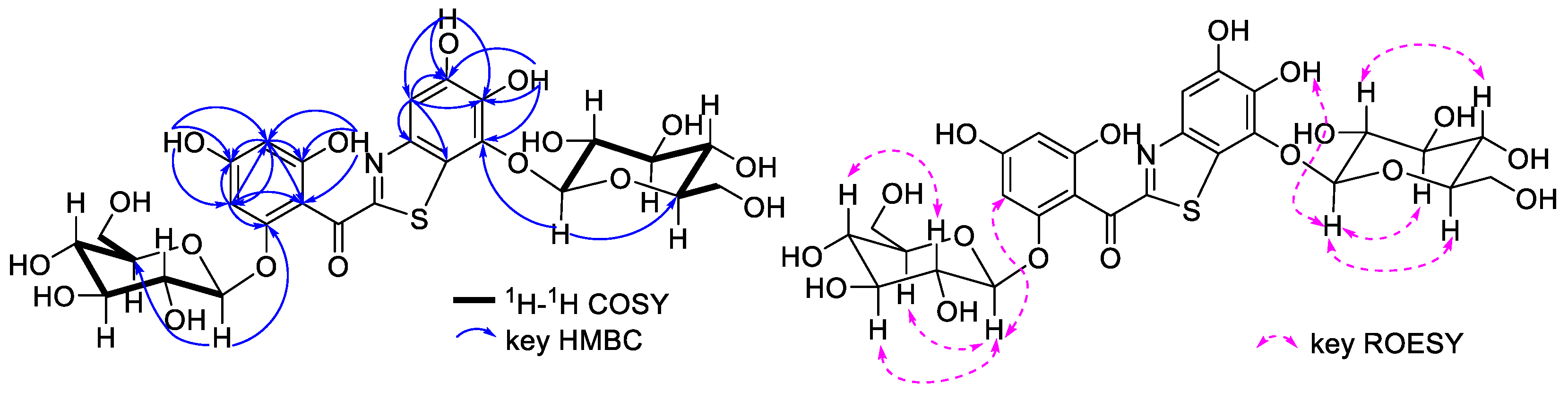
2.4. Structural Identification of ABS-D
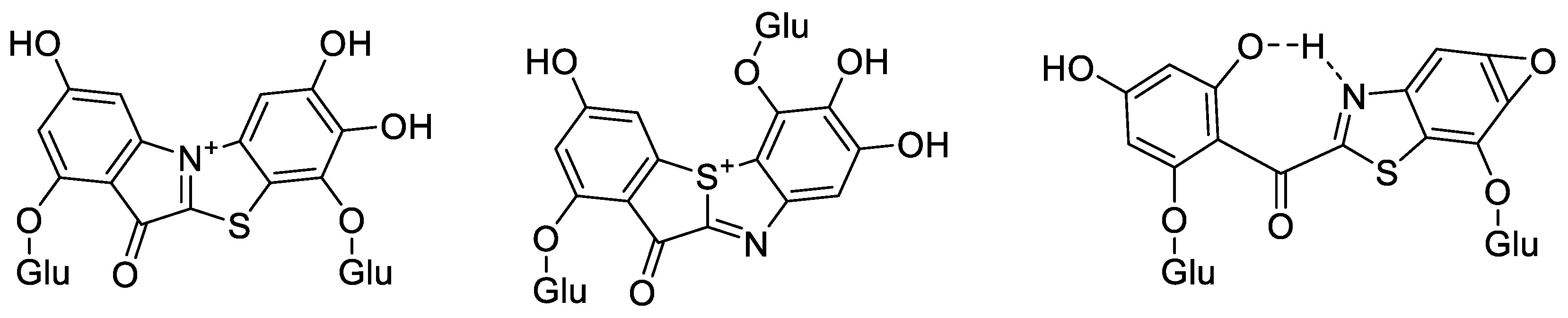
2.5. Structure Prediction of ABS
2.6. Antioxidant Activities of ABS and ABS-D
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction and Purification of ABS
3.3. HPLC Analysis of ABS
3.4. UPLC-QTOF-MS/MS Analysis of ABS
3.5. Comparative Analysis of ABS among Different Citrus Varieties
3.6. Preparation of ‘Albedo Bluing’ Substance Derivative (ABS-D)
3.7. UV-Vis and NMR Analysis of ABS-D
3.8. Calculation of the Chemical Shifts of ABS-D
3.9. Antioxidant Activity Assays
3.9.1. DPPH Radical-Scavenging Activity
3.9.2. ABTS Radical-Scavenging Activity
3.9.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 March 2023).
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Available online: https://data.stats.gov.cn/english/easyquery.htm?cn=C01 (accessed on 20 March 2023).
- Browing, H.W.; Mcgovern, R.J.; Jackson, L.K.; Calvert, V.; Wardowski, W.F. Florida Citrus Diagnostic Guide; Florida Science Source: Florida, FL, USA, 1995; pp. 225–235. ISBN 978-0944961018. [Google Scholar]
- Yu, Q.M.; Li, G.G.; Xu, R.W.; Peng, Z.X.; Yuan, Z.Y.; Li, W.Y.; Tian, J.; Zeng, J.W.; Peng, S.A.; Xu, J. Physiological mechanisms for the phenomenon of ‘blue albedo’ fruits of Citrus reticulate in Guangxi. Acta Hortic. Sin. 2020, 47, 1172–1182. [Google Scholar] [CrossRef]
- Pina, F. Chemical applications of anthocyanins and related compounds. A Source of Bioinspiration. J. Agric. Food Chem. 2014, 62, 6885–6897. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Ohata, J.; Otsugu, M.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Nakano, K. Inhibitory effects of flavedo, albedo, fruits, and leaves of Citrus unshiu extracts on Streptococcus mutans. Arch. Oral Biol. 2021, 124, 105056. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xue, J.; Xu, S.X.; Zhang, R.Q. Chemical constituents of the leaves of Diospyros Kaki and their cytotoxic effects. J. Asian Nat. Prod. Res. 2007, 9, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.X.; Wang, S.; Zeng, K.W.; Tu, P.F.; Jiang, Y. Inhibitory constituents from the aerial parts of Polygala tenuifolia on LPS-induced NO production in BV2 microglia cells. Bioorg. Med. Chem. Lett. 2013, 23, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xie, H.H.; Jiang, Y.M.; Wei, X.Y. Phenolics and sesquiterpenes from litchi pericarp. J. Funct. Foods. 2014, 9, 156–161. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Phenolics from Garcinia mangostana alleviate exaggerated vasoconstriction in metabolic syndrome through direct vasodilatation and nitric oxide generation. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Beelders, T.; Brand, D.J.; de Beer, D.; Malherbe, C.J.; Mazibuko, S.E.; Muller, C.J.F.; Joubert, E. Benzophenone C- and O-glucosides from Cyclopia genistoides (Honeybush) inhibit mammalian alpha-glucosidase. J. Nat. Prod. 2014, 77, 2694–2699. [Google Scholar] [CrossRef]
- Pandey, R.P.; Li, T.F.; Kim, E.H.; Yamaguchi, T.; Park, Y.I.; Kim, J.S.; Sohng, J.K. Enzymatic synthesis of novel phloretin glucosides. Appl. Environ. Microbiol. 2013, 79, 3516–3521. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Xia, F.; Wang, K.Y.; Chen, J.M.; Tu, P.F. Five new benzophenone glycosides from the leaves of Aquilaria sinensis (Lour.) Gilg. Chin. Chem. Lett. 2014, 25, 1573–1576. [Google Scholar] [CrossRef]
- Spadaro, A.; Frotscher, M.; Hartmann, R.W. Optimization of hydroxybenzothiazoles as novel potent and selective inhibitors of 17 beta-HSD1. J. Med. Chem. 2012, 55, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Q.; Danger, D.P.; Dock, S.T.; Hawley, L.; Roller, S.G.; Smith, C.D.; Handlon, A.L. Synthesis and SAR of benzisothiazole and indolizine-beta-D-glucopyranoside inhibitors of SGLT2. ACS Med. Chem. Lett. 2010, 1, 19–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marcarino, M.O.; Cicetti, S.; Zanardi, M.M.; Sarotti, A.M. A critical review on the use of DP4+ in the structural elucidation of natural products: The good, the bad and the ugly. A practical guide. Nat. Prod. Rep. 2022, 39, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.L.P.; De Albuquerque, A.C.F.; Fiorot, R.G.; Lião, L.M.; Martorano, L.H.; Mota, G.V.S.; Valverde, A.L.; Carneiro, J.W.M.; Dos Santos Junior, F.M. Structural characterisation of natural products by means of quantum chemical calculations of NMR parameters: New insights. Org. Chem. Front. 2021, 8, 2019–2058. [Google Scholar] [CrossRef]
- Le Bozec, L.; Moody, C.J. Naturally occurring nitrogen-sulfur compounds. The benzothiazole alkaloids. Aust. J. Chem. 2009, 62, 639–647. [Google Scholar] [CrossRef]
- Muramoto, H.; Fukuda, K.; Hasegawa, T.; Okamoto, K.; Kotani, T. Preparation of Hypolipemic Aroylbenzothiazoles. European Patent Organization. Patent EP735029, 28 March 1996. [Google Scholar]
- Tonks, N.; Gauss, C.M.; Venkataramani, P.; Joshua-Tor, L.; Elkayam, E.; Abed, Y.A.; Cheng, K.F.; Altiti, A.; Orfi, L.; Szabadkai, I.; et al. Preparation of Substituted Dioxolobenzothiazoles and Benzothiazoles as Inhibitors of DYRK and PIM. World Intellectual Property Organization. Patent WO2022159436, 28 July 2022. [Google Scholar]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B.S. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Li, W.; Dai, X.; Pu, E.; Bian, H.; Chen, Z.; Zhang, X.; Guo, Z.; Li, P.; Li, H.; Yong, Y.; et al. HLB-MCX-based solid-phase extraction combined with liquid chromatography-tandem mass spectrometry for the simultaneous determination of four agricultural antibiotics (Kasugamycin, Validamycin A, Ningnanmycin, and Polyoxin B) residues in plant-origin foods. J. Agric. Food Chem. 2020, 68, 14025–14037. [Google Scholar] [CrossRef]
- Becke, A.D.; Johnson, E.R. A density-functional model of the dispersion interaction. J. Chem. Phys. 2005, 123, 154101. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Ma, R.; Yang, L.; Bai, X.; Li, J.; Yuan, M.; Wang, Y.; Xie, Y.; Hu, J.; Zhou, J. Phenolic constituents with antioxidative, tyrosinase inhibitory and anti-aging activities from Dendrobium loddigesii rolfe. Nat. Prod. Bioprospect. 2019, 9, 329–336. [Google Scholar] [CrossRef]
- Yang, M.L.; Ma, Y.L.; Wang, Z.Y.; Khan, A.; Zhou, W.B.; Zhao, T.R.; Cao, J.X.; Cheng, G.G.; Cai, S.B. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crops Prod. 2020, 143, 111433. [Google Scholar] [CrossRef]



| Position | δH (J in Hz) a | δC | 1H-1H COSY b | HMBC | ROESY |
|---|---|---|---|---|---|
| 2 | |||||
| 3 | 165.1 | ||||
| 4 | 187.6 | ||||
| 5 | 108.0 | ||||
| 6 | 157.1 | ||||
| 7 | 6.12, br s | 94.0 | 5, 6, 8, 9 | 1‴, 8-OH | |
| 8 | 160.5 | ||||
| 8-OH | 9.79, s | 7, 8, 9 | 7, 9 | ||
| 9 | 6.05, br s | 96.5 | 5, 7, 8, 10 | 8-OH, 10-OH | |
| 10 | 157.3 | ||||
| 10-OH | 9.71, br s | 5, 9, 10 | 9 | ||
| 1′ | 146.1 | ||||
| 2′ | 122.7 | ||||
| 3′ | 137.7 | ||||
| 4′ | 139.7 | ||||
| 4′-OH | 9.38, s | 3′, 4′, 5′ | 5′-OH, 1″ | ||
| 5′ | 147.9 | ||||
| 5′-OH | 9.77, s | 4′, 5′, 6′ | 4′-OH, 6′ | ||
| 6′ | 7.25, s | 106.1 | 1′, 2′, 4′, 5′ | 5′-OH | |
| 1″ | 4.80, d (7.8) | 105.4 | 2″ | 3′, 3″, 5″ | 3″, 5″, 4′-OH |
| 2″ | 3.35, t (7.9) | 73.6 | 1″, 3″ | 1″, 3″ | 4″ |
| 3″ | 3.27, t (7.6) | 75.9 | 2″, 4″ | 2″, 4″ | 1″ |
| 4″ | 3.26 | 69.3 | 3″, 5″ | 3″, 6″ | 2″, 6″ |
| 5″ | 3.24 | 77.3 | 4″, 6″ | 1″ | 1″ |
| 6″ | 3.68, br d (10.7) | 60.5 | 5″ | 4″, 5″ | 4″ |
| 3.55, dd (11.6, 4.3) | 4″, 5″ | 4″ | |||
| 1‴ | 4.75, d (7.8) | 100.5 | 2‴ | 6, 3‴, 5‴ | 7, 3‴, 5‴ |
| 2‴ | 2.93, t (8.2) | 73.1 | 1‴, 3‴ | 1‴, 3‴ | 4‴ |
| 3‴ | 3.17, t (8.9) | 76.5 | 2‴, 4‴ | 2‴, 4‴ | 1‴ |
| 4‴ | 3.06, t (9.2) | 69.4 | 3‴, 5‴ | 3‴, 6‴ | 2‴, 6‴ |
| 5‴ | 3.22 | 77.0 | 4‴, 6‴ | 1‴ | 1‴ |
| 6‴ | 3.64, br d (10.4) 3.43, dd (11.7, 5.3) | 60.6 | 5‴ | 4‴, 5‴ 4‴, 5‴ | 4‴ 4‴ |
| Compounds | DPPH (IC50 μM) a | ABTS+ (IC50 μM) a | FRAP a,b |
|---|---|---|---|
| ABS | 10.28 ± 0.15 | 38.88 ± 0.39 | 314.61 ± 4.88 |
| ABS-D | 28.30 ± 0.34 | 19.63 ± 0.09 | 218.36 ± 1.96 |
| Ascorbic acid c | 34.64 ± 0.21 | 46.26 ± 0.20 | 91.18 ± 2.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Yu, C.; Li, Q.; Peng, L.; Chun, C.; Tang, X.; Liu, S.; Hu, C.; Ling, L. A Rare Benzothiazole Glucoside as a Derivative of ‘Albedo Bluing’ Substance in Citrus Fruit and Its Antioxidant Activity. Molecules 2024, 29, 302. https://doi.org/10.3390/molecules29020302
Yang C, Yu C, Li Q, Peng L, Chun C, Tang X, Liu S, Hu C, Ling L. A Rare Benzothiazole Glucoside as a Derivative of ‘Albedo Bluing’ Substance in Citrus Fruit and Its Antioxidant Activity. Molecules. 2024; 29(2):302. https://doi.org/10.3390/molecules29020302
Chicago/Turabian StyleYang, Chao, Chuanxiu Yu, Qiang Li, Liangzhi Peng, Changpin Chun, Xiaolong Tang, Song Liu, Chengbo Hu, and Lili Ling. 2024. "A Rare Benzothiazole Glucoside as a Derivative of ‘Albedo Bluing’ Substance in Citrus Fruit and Its Antioxidant Activity" Molecules 29, no. 2: 302. https://doi.org/10.3390/molecules29020302
APA StyleYang, C., Yu, C., Li, Q., Peng, L., Chun, C., Tang, X., Liu, S., Hu, C., & Ling, L. (2024). A Rare Benzothiazole Glucoside as a Derivative of ‘Albedo Bluing’ Substance in Citrus Fruit and Its Antioxidant Activity. Molecules, 29(2), 302. https://doi.org/10.3390/molecules29020302







