Fractionation and Lability of Phosphorus Species in Cottonseed Meal-Derived Biochars as Influenced by Pyrolysis Temperature
Abstract
1. Introduction
2. Results and Discussion
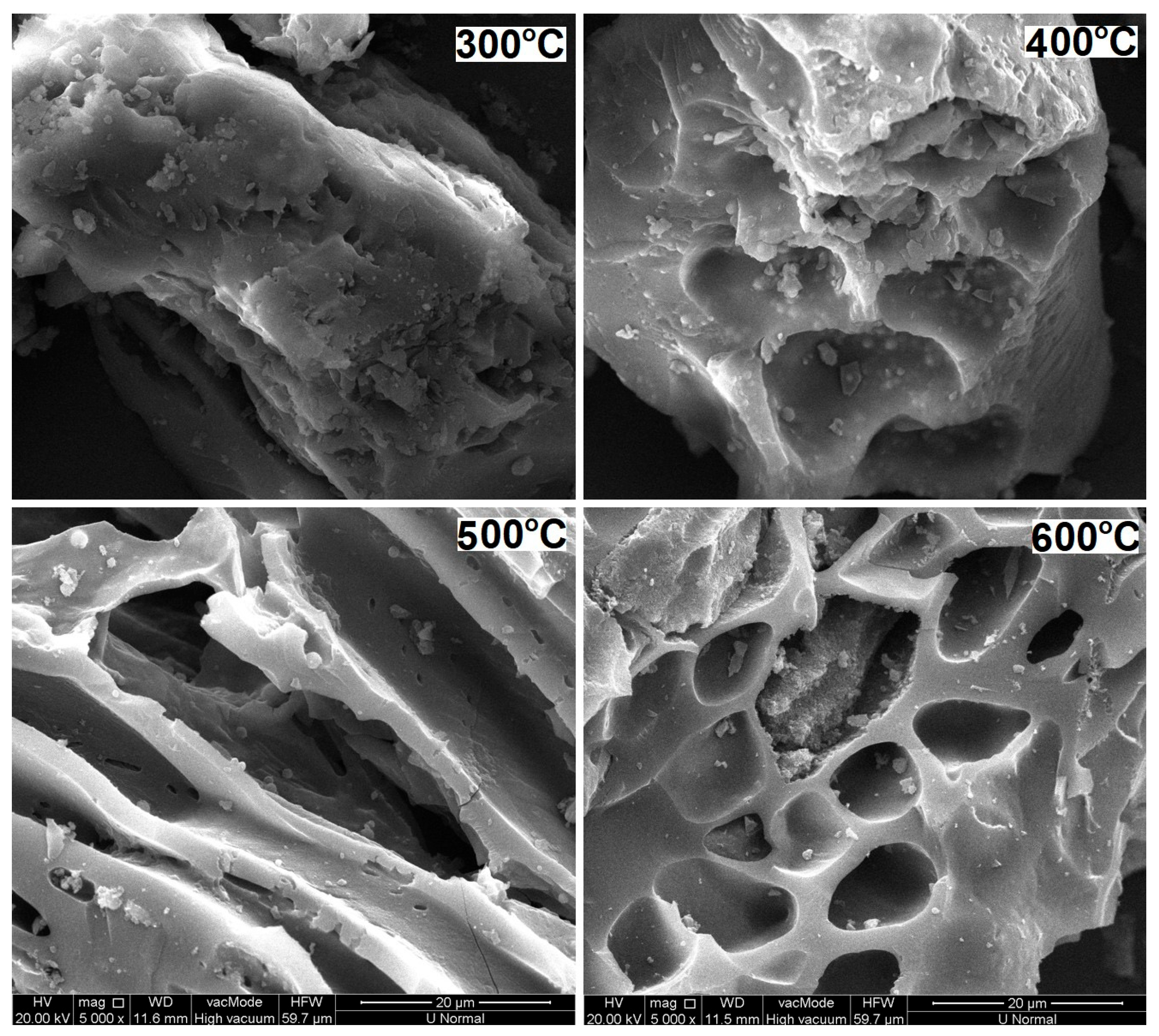
2.1. General Characteristics of CSM-Derived Biochars
2.2. Thermochemical Transformation of P in CSM during Pyrolysis
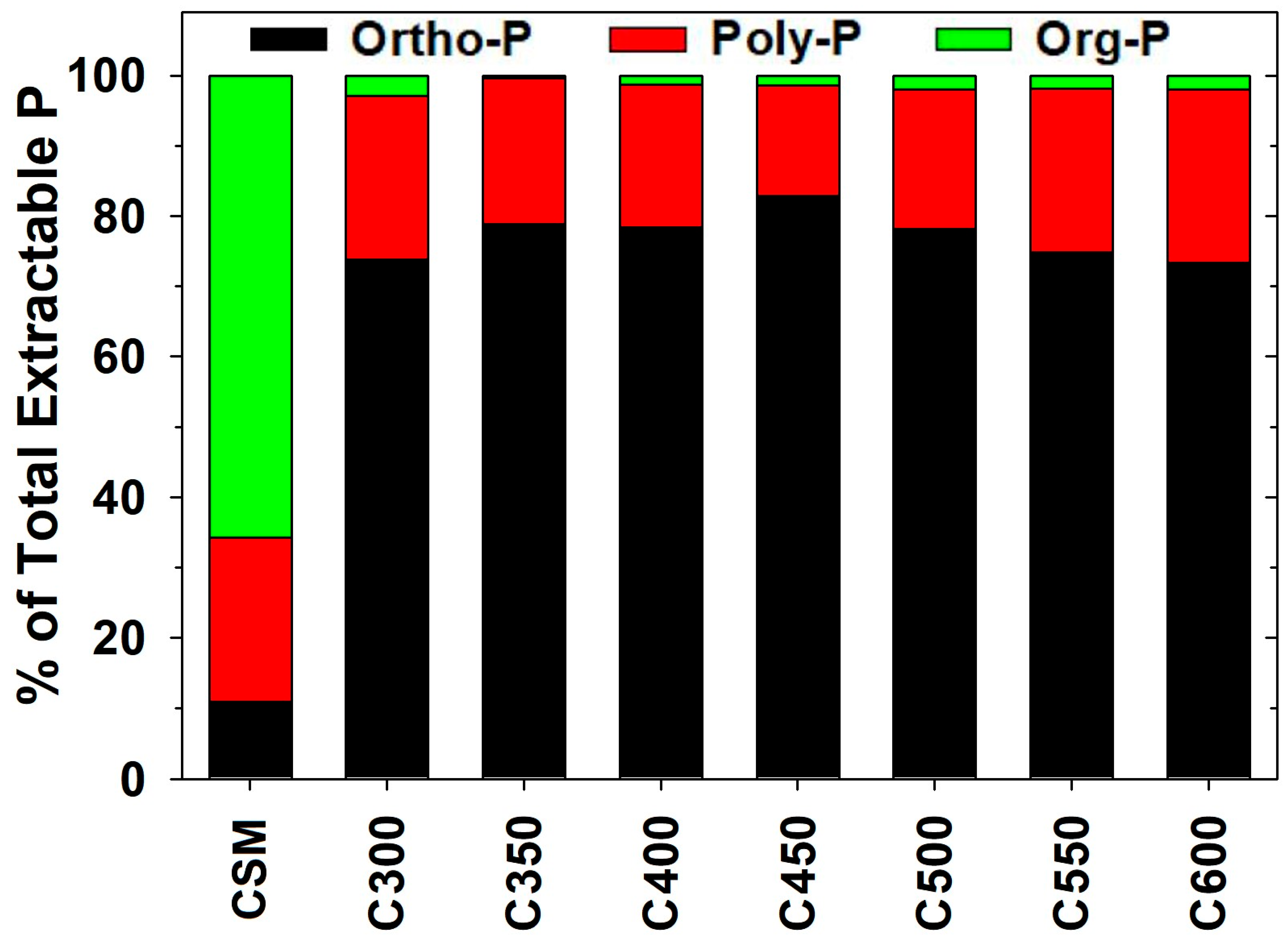
2.3. Lability-Based Chemical Fractionation of P in CSM-Derived Biochars
2.4. Batch Extractability-Based Lability and Bioavailability of P in CSM-Derived Biochars
3. Materials and Methods
3.1. Defatted Cottonseed Meal
3.2. Converting CSM to Biochar
3.3. General Characterization of CSM and the Derived Biochars
3.4. Chemical Characterization of P in CSM and the Derived Biochars
3.5. Experimental Quality Control and Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolek, Y.; Tekerek, H.; Hayat, K.; Bardak, A. Screening of cotton genotypes for protein content, oil and fatty acid composition. J. Agric. Sci. 2016, 8, 107–121. [Google Scholar] [CrossRef]
- Johnson, J.; MacDonald, S.; Meyer, L.; Stone, L. The World and United States Cotton Outlook for 2018/19. In Proceedings of the USDA’s 94th Annual Agricultural Outlook Forum, Arlington, VA, USA, 23 February 2018; Available online: https://econpapers.repec.org/paper/agsusao18/272725.htm (accessed on 18 October 2023).
- Stewart, L.; Rossi, J. Using Cotton Byproducts in Beef Cattle Diets; Bulletin 1311; University of Georgia Cooperative Extension: Athens, GA, USA, 2010. [Google Scholar]
- NCCA. Cotton: From Field to Fabric; National Cotton Council of America: Memphis, TN, USA, 2003; Available online: https://www.cotton.org/pubs/cottoncounts/fieldtofabric/ (accessed on 6 October 2023).
- Mitchell, C.C. Nutrient Content of Fertilizer Materials; ANR-174; Alabama Cooperative Extension System: Auburn, AL, USA, 2008. [Google Scholar]
- Ma, X.; Hu, J.; Shang, Q.; Liu, H.; Piao, X. Chemical composition, energy content and amino acid digestibility in cottonseed meals fed to growing pigs. J. Appl. Anim. Res. 2019, 47, 280–288. [Google Scholar] [CrossRef]
- USDA. Cotton-Oil Crops Yearbook; U.S. Department of Agriculture: Washington, DC, USA, 2023. Available online: https://www.ers.usda.gov/webdocs/DataFiles/52218/Cotton.xlsx?v=0 (accessed on 4 January 2024).
- USDA. Cottonseed and Cottonseed Products: U.S. Acreage; U.S. Department of Agriculture Economic Research Service: Washington, DC, USA, 2018. Available online: https://www.ers.usda.gov/webdocs/DataFiles/52218/Cotton.xlsx?v=0 (accessed on 6 October 2023).
- Peng, L.; Wu, D.; Wang, T.; Guo, J.; Jia, D. Cottonseed meal derived porous carbon prepared via the protease pretreatment and reduced activator dosage carbonization for supercapacitor. J. Chem. Phys. 2023, 159, 214702. [Google Scholar] [CrossRef] [PubMed]
- Putun, E.; Uzun, B.B.; Putun, A.E. Fixed-bed catalytic pyrolysis of cotton-seed cake: Effects of pyrolysis temperature, natural zeolite content and sweeping gas flow rate. Bioresour. Technol. 2006, 97, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Soni, A.; Kumar, S.; Singh, R. Characterization of liquid product obtained by pyrolysis of cottonseed de-oiled cake. J. Biobased Mater. Bioenergy 2014, 8, 338–343. [Google Scholar]
- Tan, C.F.; Kwan, S.H.; Lee, C.S.; Soh, Y.N.A.; Ho, Y.S.; Bi, X. Cottonseed meal protein isolate as a new source of alternative proteins: A proteomics perspective. Int. J. Mol. Sci. 2022, 23, 10105. [Google Scholar] [CrossRef]
- He, Z.; Guo, M.; Fortier, C.; Cao, X.; Schmidt-Rohr, K. Fourier transform infrared and solid state 13C nuclear magnetic resonance spectroscopic characterization of defatted cottonseed meal-based biochars. Mod. Appl. Sci. 2021, 15, 108–121. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Soil biochar amendment as a climate change mitigation tool: Key parameters and mechanisms involved. J. Environ. Manag. 2016, 181, 484–497. [Google Scholar] [CrossRef]
- Singh Yadav, S.P.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Ghimire, N.; Paudel, P.; Paudel, P.; Shrestha, J.; et al. Biochar application: A sustainable approach to improve soil health. J. Agri. Food Res. 2023, 11, 100498. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochars generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Uchimiya, M.; Hiradate, S. Pyrolysis temperature-dependent changes in dissolved phosphorus speciation of plant and manure biochars. J. Agric. Food Chem. 2014, 62, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, X.; Song, W.; Guo, M. Transformation of phosphorus in speciation and bioavailability during converting poultry litter to biochar. Front. Sustain. Food Syst. 2018, 2, 20. [Google Scholar] [CrossRef]
- Sun, K.; Qiu, M.; Han, L.; Jin, J.; Wang, Z.; Pan, Z.; Xing, B. Speciation of phosphorus in plant- and manure-derived biochars and its dissolution under various aqueous conditions. Sci. Total Environ. 2018, 634, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Chiu, P.; Imhoff, P.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512–513, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Caillat, S.; Vakkilainen, E. Large-scale biomass combustion plants: An overview. In Biomass Combustion Science, Technology and Engineering; Rosendahl, L., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 189–224. [Google Scholar]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar pH, electrical conductivity and liming potential. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–38. [Google Scholar]
- Blacher, S.; Sahouli, B.; Heinrichs, B.; Lodewyckx, P.; Pirard, R.; Pirard, J.P. Micropore size distributions of activated carbons. Langmuir 2000, 16, 6754–6756. [Google Scholar] [CrossRef]
- Qiu, G.; Guo, M. Quality of poultry litter-based granular activated carbon. Bioresour. Technol. 2010, 101, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, D.; Żyszka-Haberecht, B.; Kafka, A.; Lipok, J. Determination of phosphorus compounds in plant tissues: From colorimetry to advanced instrumental analytical chemistry. Plant Methods 2022, 18, 22. [Google Scholar] [CrossRef]
- Han, Y.W. Removal of phytic acid from soybean and cottonseed meals. J. Agric. Food Chem. 1988, 36, 1181–1183. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Fang, D.D.; Zeng, L.; Jenkins, J.N.; McCarty, J.C. Effects of inter-species chromosome substitution on cottonseed mineral and protein nutrition profiles. Agron. J. 2020, 112, 3963–3974. [Google Scholar] [CrossRef]
- Huang, R.; Fang, C.; Lu, X.; Jiang, R.; Tang, Y. Transformation of phosphorus during (hydro)thermal treatments of solid biowastes: Reaction mechanisms and implications for P reclamation and recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. [Google Scholar] [CrossRef]
- Tiyapongpattana, W.; Pongsakul, P.; Shiowatana, J.; Nacapricha, D. Sequential extraction of phosphorus in soil and sediment using a continuous-flow system. Talanta 2004, 62, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F., Jr.; Ferguson, J.D. Laboratory procedures for characterizing manure phosphorus. J. Environ. Qual. 2000, 29, 508–514. [Google Scholar] [CrossRef]
- Dou, Z.; Ramberg, C.F.; Toth, J.D.; Wang, Y.; Sharpley, A.N.; Boyd, S.E.; Chen, C.R.; Williams, D.; Xu, Z.H. Phosphorus speciation and sorption-desorption characteristics in heavily manured soils. Soil Sci. Soc. Am. J. 2009, 73, 93–101. [Google Scholar] [CrossRef]
- Turner, B.L.; Leytem, A.B. Phosphorus compounds in sequential extracts of animal manures: chemical speciation and a novel fractionation procedure. Environ. Sci. Technol. 2004, 38, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Jiang, H. Migration of phosphorus in sewage sludge during different thermal treatment processes. ACS Sustain. Chem. Eng. 2014, 2, 1411–1419. [Google Scholar] [CrossRef]
- Khourchi, S.; Delaplace, P.; Bargaz, A. Polyphosphate fertilizer use efficiency strongly relies on soil physicochemical properties and root-microbial activities. Geoderma 2022, 429, 116281. [Google Scholar] [CrossRef]
- Christiansen, N.H.; Sørensen, P.; Labouriau, R.; Christensen, B.T.; Rubaek, G.H. Characterizing phosphorus availability in waste products by chemical extractions and plant uptake. J. Plant Nutr. Soil Sci. 2020, 183, 416–428. [Google Scholar] [CrossRef]
- McCray, J.M.; Wright, A.L.; Luo, Y.; Ji, S. Soil phosphorus forms related to extractable phosphorus in the Everglades agricultural area. Soil Sci. 2012, 177, 31–38. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, 8th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Scheel, T.; Dorfler, C.; Kalbitz, K. Precipitation of dissolved organic matter by Aluminum stabilizes carbon in acidic forest soils. Soil Sci. Soc. Am. J. 2007, 71, 64–74. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Method for Chemical Analysis of Wood Charcoal. The American Society of Testing and Materials: Philadelphia, PA, USA, 1990.
- Pierzynski, G. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; Southern Cooperative Series Bulletin No. # 396; North Carolina State University: Raleigh, NC, USA, 2000. [Google Scholar]


| CSM | C300 | C350 | C400 | C450 | C500 | C550 | C600 | |
|---|---|---|---|---|---|---|---|---|
| Pyrolysis T, °C | 300 | 350 | 400 | 450 | 500 | 550 | 600 | |
| Yield ‡, % | 100 | 53.3 | 46.7 | 40.8 | 36.0 | 35.0 | 33.4 | 32.2 |
| Fixed C, % | - | 43.7 | 52.3 | 61.8 | 66.2 | 68.7 | 70.8 | 70.1 |
| Volatile matter, % | - | 43.9 | 33.6 | 22.4 | 15.6 | 12.4 | 9.7 | 9.3 |
| OC, % | 46.5 | 55.6 | 36.8 | 30.2 | 25.9 | 25.6 | 25.3 | 25.1 |
| Mineral ash, % | 6.57 | 12.4 | 14.1 | 15.8 | 18.2 | 18.9 | 19.5 | 20.6 |
| pH † | - | 9.1 | 9.6 | 10.1 | 10.4 | 10.3 | 10.3 | 10.3 |
| EC †, dS m−1 | - | 2.20 | 2.26 | 2.99 | 3.53 | 3.28 | 3.39 | 3.24 |
| TN, g kg−1 | 72.3 | 89.8 | 71.7 | 58.7 | 53.3 | 50.1 | 46.7 | 42.1 |
| TP, g kg−1 | 12.7 | 23.7 | 27.1 | 31.3 | 33.9 | 34.9 | 37.3 | 34.6 |
| S, g kg−1 | 4.21 | 3.04 | 2.75 | 2.15 | 2.05 | 1.75 | 1.35 | 0.98 |
| K, g kg−1 | 15.5 | 29.6 | 33.5 | 37.6 | 42.4 | 43.6 | 46.4 | 47.7 |
| Na, g kg−1 | 2.27 | 4.29 | 4.81 | 5.57 | 6.18 | 6.36 | 6.62 | 6.99 |
| Ca, g kg−1 | 2.06 | 3.85 | 4.48 | 5.04 | 5.70 | 5.85 | 6.15 | 6.37 |
| Mg, g kg−1 | 6.23 | 12.1 | 13.4 | 15.3 | 17.0 | 17.6 | 18.6 | 19.2 |
| Fe, g kg−1 | 0.087 | 0.166 | 0.190 | 0.215 | 0.238 | 0.249 | 0.257 | 0.270 |
| Zn, g kg−1 | 0.059 | 0.109 | 0.124 | 0.146 | 0.163 | 0.169 | 0.171 | 0.188 |
| IP | OP | |||
|---|---|---|---|---|
| g kg−1 | % of TP | g kg−1 | % of TP | |
| CSM | 0.962 ± 0.003 | 7.57 ± 0.18 a | 11.75 ± 0.023 | 92.43 ± 0.44 a |
| C300 | 18.60 ± 0.12 | 87.35 ± 0.77 d | 5.14 ± 0.18 | 12.65 ± 1.04 d |
| C350 | 23.60 ± 0.048 | 86.77 ± 0.44 d | 3.60 ± 0.12 | 13.23 ± 0.70 d |
| C400 | 26.72 ± 0.14 | 85.35 ± 1.26 d | 4.59 ± 0.39 | 14.65 ± 1.41 d |
| C450 | 30.29 ± 0.048 | 89.20 ± 0.29 d | 3.67 ± 0.098 | 10.80 ± 0.44 d |
| C500 | 25.56 ± 0.071 | 73.13 ± 0.35 c | 9.39 ± 0.12 | 26.87 ± 0.49 c |
| C550 | 21.09 ± 0.024 | 56.58 ± 0.33 b | 16.19 ± 0.12 | 43.42 ± 0.62 b |
| C600 | 19.41 ± 0.071 | 56.10 ± 1.52 b | 15.19 ± 0.53 | 43.90 ± 1.83 b |
| Water-Soluble P | NaHCO3-Extractable P | NaOH-Extractable P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pir | Pix | Po | Pir | Pix | Po | Pir | Pix | Po | |
| % of Water-Soluble Pt | % of NaHCO3-Extractable Pt | % of NaOH-Extractable Pt | |||||||
| CSM | 10.9 ± 0.18 a | 20.3 ± 0.26 a | 68.8 ± 0.22 a | 12.8 ± 0.98 a | 49.8 ± 1.74 c,d | 37.4 ± 1.57 a | 8.36 ± 0.33 a | 37.3 ± 0.50 a | 54.4 ± 0.87 a |
| C300 | 36.3 ± 0.34 d | 57.7 ± 0.93 b | 6.02 ± 1.15 b | 32.9 ± 0.29 b | 53.2 ± 0.77 d | 13.9 ± 0.90 b | 40.3 ± 0.36 b,c,d | 59.7 ± 2.33 b | 0.00 ± 0.00 b |
| C350 | 35.7 ± 0.34 d | 62.0 ± 0.54 c,d | 2.35 ± 0.40 b | 41.8 ± 0.69 c | 55.8 ± 1.71 d | 2.47 ± 1.67 c | 42.2 ± 1.04 c,d | 57.8 ± 2.57 b | 0.00 ± 0.00 b |
| C400 | 32.5 ± 0.53 c | 64.5 ± 1.83 d,e | 2.97 ± 2.03 b | 49.5 ± 1.17 d | 46.3 ± 1.89 c | 4.15 ± 2.00 c | 39.9 ± 1.89 b | 60.1 ± 4.01 b | 0.00 ± 0.00 b |
| C450 | 28.9 ± 0.21 b | 66.2 ± 0.82 e | 4.91 ± 2.90 b | 61.4 ± 0.90 e | 34.7 ± 2.82 b | 3.91 ± 2.89 c | 40.5 ± 2.13 b,c | 59.5 ± 9.17 b | 0.00 ± 0.00 b |
| C500 | 29.1 ± 0.21 b | 67.9 ± 0.55 e | 2.94 ± 0.41 b | 62.0 ± 0.49 e | 32.7 ± 1.86 b | 5.29 ± 2.54 c | 35.9 ± 0.61 b | 64.1 ± 7.61 b | 0.00 ± 0.00 b |
| C550 | 30.4 ± 0.58 b,c | 67.2 ± 0.76 e | 2.49 ± 1.00 b | 72.5 ± 0.71 f | 24.2 ± 1.49 a | 3.37 ± 1.52 c | 36.4 ± 1.29 b,c | 63.6 ± 8.52 b | 0.00 ± 0.00 b |
| C600 | 35.9 ± 0.79 d | 61.7 ± 1.57 c | 2.33 ± 0.79 b | 72.0 ± 0.64 f | 24.7 ± 1.79 a | 3.28 ± 1.87 c | 38.9 ± 2.31 b,c | 61.1 ± 5.66 b | 0.00 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; He, Z.; Tian, J. Fractionation and Lability of Phosphorus Species in Cottonseed Meal-Derived Biochars as Influenced by Pyrolysis Temperature. Molecules 2024, 29, 303. https://doi.org/10.3390/molecules29020303
Guo M, He Z, Tian J. Fractionation and Lability of Phosphorus Species in Cottonseed Meal-Derived Biochars as Influenced by Pyrolysis Temperature. Molecules. 2024; 29(2):303. https://doi.org/10.3390/molecules29020303
Chicago/Turabian StyleGuo, Mingxin, Zhongqi He, and Jing Tian. 2024. "Fractionation and Lability of Phosphorus Species in Cottonseed Meal-Derived Biochars as Influenced by Pyrolysis Temperature" Molecules 29, no. 2: 303. https://doi.org/10.3390/molecules29020303
APA StyleGuo, M., He, Z., & Tian, J. (2024). Fractionation and Lability of Phosphorus Species in Cottonseed Meal-Derived Biochars as Influenced by Pyrolysis Temperature. Molecules, 29(2), 303. https://doi.org/10.3390/molecules29020303








