The Optimization of the Hot Water Extraction of the Polysaccharide-Rich Fraction from Agaricus bisporus
Abstract
1. Introduction
2. Results and Discussion
2.1. Polysaccharides Concentrations and Aldehyde Group Content
2.2. Antioxidant Activity of Agaricus bisporus Extracts
2.3. Validation of Optimized Models
2.4. Summary
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Variables and the Extraction Process
3.2.2. Experimental Design
3.2.3. Total Carbohydrate Content Analysis Using Colorimetric Dubois Method
3.2.4. Quantification of Free Aldehyde Group—Reducing Sugars
3.2.5. DPPH Radical Scavenging Activity
3.2.6. ABTS Radical-Scavenging Activity
3.2.7. Hydroxyl Radical Scavenging Activity Assay
3.2.8. Model Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirón, I.J.; Linares, C.; Díaz, J. The Influence of Climate Change on Food Production and Food Safety. Environ. Res. 2023, 216, 114674. [Google Scholar] [CrossRef] [PubMed]
- Mirzabaev, A.; Olsson, L.; Kerr, R.B.; Pradhan, P.; Ferre, M.G.R.; Lotze-Campen, H. Climate Change and Food Systems. In Science and Innovations for Food Systems Transformation; von Braun, J., Afsana, K., Fresco, L.O., Hassan, M.H.A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 511–529. ISBN 978-3-031-15703-5. [Google Scholar]
- Turan, Y.; Berber, D.; Sesal, N.C. Could Insects Be an Alternative Food Source? A Comprehensive Review. Nutr. Rev. 2024; nuae019, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Noguera, N.H.; Lima, D.C. Chapter 7—Plant Life-Associated Natural Products: Algae and Mushrooms. In Natural Plant Products in Inflammatory Bowel Diseases; do Nascimento, R.d.P., da Fonseca Machado, A.P., Rodriguez-Nogales, A., Leal, R.F., Real Martinez, C.A., Galvez, J., Marostica, M.R., Jr., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 173–213. ISBN 978-0-323-99111-7. [Google Scholar]
- e Moura, M.A.F.; Martins, B.d.A.; de Oliveira, G.P.; Takahashi, J.A. Alternative Protein Sources of Plant, Algal, Fungal and Insect Origins for Dietary Diversification in Search of Nutrition and Health. Crit. Rev. Food Sci. Nutr. 2023, 63, 10691–10708. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, R. A Review on Nutritional Advantages of Edible Mushrooms and Its Industrialization Development Situation in Protein Meat Analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, R.C.; Antonio, A.L.; Queiroz, M.-J.R.P.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Bioactive Compounds and Antioxidant Properties of Three Edible Mushrooms from Poland. Food Biosci. 2015, 11, 48–55. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Guan, J.; Chen, J.; Xu, X. Mushroom Polysaccharides with Potential in Anti-Diabetes: Biological Mechanisms, Extraction, and Future Perspectives: A Review. Front. Nutr. 2022, 9, 1087826. [Google Scholar] [CrossRef]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the Health Effects and Bioactive Components in Agaricus Bisporus Mushrooms: A Scoping Review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef]
- Barzee, T.; Cao, L.; Pan, Z.; Zhang, R. Fungi for Future Foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Pk, M.M.U.; Islam, M.S.; Pervin, R.; Dutta, S.; Talukder, R.I.; Rahman, M. Optimization of Extraction of Antioxidant Polysaccharide from Pleurotus ostreatus (Jacq.) P. Kumm and Its Cytotoxic Activity against Murine Lymphoid Cancer Cell Line. PLoS ONE 2019, 14, e0209371. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liao, K.; Wu, Y.; Pan, Q.; Wu, L.; Jiao, H.; Guo, D.; Li, B.; Liu, B. Optimization, Characterization, Sulfation and Antitumor Activity of Neutral Polysaccharides from the Fruit of Borojoa Sorbilis Cuter. Carbohydr. Polym. 2016, 151, 364–372. [Google Scholar] [CrossRef]
- Huang, S.-Q.; Li, J.-W.; Wang, Z.; Pan, H.-X.; Chen, J.-X.; Ning, Z.-X. Optimization of Alkaline Extraction of Polysaccharides from Ganoderma lucidum and Their Effect on Immune Function in Mice. Molecules 2010, 15, 3694–3708. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, K.; Jia, F.; Zhao, X.; Cui, G.; Guo, F.; Zhu, R. Characterization and Biological Activity of Taishan Pinus Massoniana Pollen Polysaccharide In Vitro. PLoS ONE 2015, 10, e0115638. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kim, S.Y.; Kim, Y.T.; Kim, E.-A.; Lee, S.-H.; Ko, S.-C.; Wijesinghe, W.A.J.P.; Samarakoon, K.W.; Kim, Y.-S.; Cho, J.H.; et al. In Vitro and in Vivo Antioxidant Activities of Polysaccharide Purified from Aloe Vera (Aloe Barbadensis) Gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic Activity of Armillaria Mellea Polysaccharides: Joint Ultrasonic and Enzyme Assisted Extraction. Ultrason. Sonochem. 2023, 95, 106370. [Google Scholar] [CrossRef]
- Mounika, A.; Ilangovan, B.; Mandal, S.; Shraddha Yashwant, W.; Priya Gali, S.; Shanmugam, A. Prospects of Ultrasonically Extracted Food Bioactives in the Field of Non-Invasive Biomedical Applications—A Review. Ultrason. Sonochem. 2022, 89, 106121. [Google Scholar] [CrossRef]
- Devi, P.V.; Islam, J.; Narzary, P.; Sharma, D.; Sultana, F. Bioactive Compounds, Nutraceutical Values and Its Application in Food Product Development of Oyster Mushroom. J. Future Foods 2024, 4, 335–342. [Google Scholar] [CrossRef]
- Siwulski, M.; Budka, A.; Rzymski, P.; Gąsecka, M.; Kalač, P.; Budzyńska, S.; Magdziak, Z.; Niedzielski, P.; Mleczek, P.; Mleczek, M. Worldwide Basket Survey of Multielemental Composition of White Button Mushroom Agaricus Bisporus. Chemosphere 2020, 239, 124718. [Google Scholar] [CrossRef]
- Nweze, C.C.; Rasaq, N.O.; Istifanus, B.I. Ameliorating Effect of Agaricus Bisponus and Pleurotus Ostreatus Mixed Diet on Alloxan-Induced Hyperglycemic Rats. Sci. Afr. 2020, 7, e00209. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-Glucans from Edible and Medicinal Mushrooms: Characteristics, Physicochemical and Biological Activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Jing, Y.; Gao, Y.; Wang, W.; Cheng, Y.; Lu, P.; Ma, C.; Zhang, Y. Optimization of the Extraction of Polysaccharides from Tobacco Waste and Their Biological Activities. Int. J. Biol. Macromol. 2016, 91, 188–197. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of Polysaccharides from Edible Mushrooms: Emerging Technologies and Recent Advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef] [PubMed]
- FAOStat Report: Mushrooms and Truffles. Available online: https://data.un.org/Data.aspx?d=FAO&f=itemCode%3A449 (accessed on 13 September 2024).
- Kalac, P. Edible Mushrooms: Chemical Composition and Nutritional Value, 1st ed.; Academic Press: London, UK, 2016; ISBN 978-0-12-804455-1. [Google Scholar]
- Sławińska, A.; Jablonska-Rys, E.; Stachniuk, A. High-Performance Liquid Chromatography Determination of Free 548 Sugars and Mannitol in Mushrooms Using Corona Charged Aerosol Detection. Food Anal. Methods 2020, 14, 549. [Google Scholar] [CrossRef]
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional Value of Mushrooms Widely Consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically Active Polysaccharide from Edible Mushrooms: A Review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural Antioxidants from Some Fruits, Seeds, Foods, Natural Products, and Associated Health Benefits: An Update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Ionita, P. The Chemistry of DPPH· Free Radical and Congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC): New Horizons in Relating Dietary Antioxidants/Bioactives and Health Benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jiao, H.; Sun, J.; Okoye, C.O.; Zhang, H.; Li, Y.; Lu, X.; Wang, Q.; Liu, J. Structure-Activity Relationships of Bioactive Polysaccharides Extracted from Macroalgae towards Biomedical Application: A Review. Carbohydr. Polym. 2024, 324, 121533. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, J.; Wen, C.; Sedem Dzah, C.; Chidimma Juliet, I.; Duan, Y.; Zhang, H. Recent Advances in Agaricus Bisporus Polysaccharides: Extraction, Purification, Physicochemical Characterization and Bioactivities. Process Biochem. 2020, 94, 39–50. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from Fungi: A Review on Their Extraction, Purification, Structural Features, and Biological Activities. Food Chem. X 2022, 15, 100414. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Lapienis, G. Densitometric Determination of Carbohydrates: Application to Purification and Molecular Weight Determination of Polysaccharide from Hericium E*rinaceum Mushroom. Food Res. Int. 2010, 43, 988–995. [Google Scholar] [CrossRef]
- Drakhshan, A.; Rezaeian, S.; Pourianfar, H.R. Advances and Challenges in Polysaccharide Extraction from the Woody Structure of Fruiting Bodies of Reishi Mushroom, Ganoderma Lucidium. Chem. Rev. Lett. 2024, 7, 454–465. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Rattmann, Y.D.; Malquevicz-Paiva, S.M.; Carbonero, E.R.; Córdova, M.M.; Baggio, C.H.; Santos, A.R.S.; Gorin, P.A.J.; Iacomini, M. Agaricus Bisporus Fucogalactan: Structural Characterization and Pharmacological Approaches. Carbohydr. Polym. 2013, 92, 184–191. [Google Scholar] [CrossRef]
- Li, S.; Liu, M.; Zhang, C.; Tian, C.; Wang, X.; Song, X.; Jing, H.; Gao, Z.; Ren, Z.; Liu, W.; et al. Purification, in Vitro Antioxidant and in Vivo Anti-Aging Activities of Soluble Polysaccharides by Enzyme-Assisted Extraction from Agaricus Bisporus. Int. J. Biol. Macromol. 2018, 109, 457–466. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Zhou, X. Complex Enzyme-Assisted Extraction, Purification, and Antioxidant Activity of Polysaccharides from the Button Mushroom, Agaricus Bisporus (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 987–996. [Google Scholar] [CrossRef]
- Sangthong, S.; Pintathong, P.; Pongsua, P.; Jirarat, A.; Chaiwut, P. Polysaccharides from Volvariella Volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy. J. Fungi 2022, 8, 572. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lam, H.-H.; Nguyen, T.-M.-T.; Do, T.-A.-S.; Dinh, T.-H.; Dang-Bao, T. Quantification of Total Sugars and Reducing Sugars of Dragon Fruit-Derived Sugar-Samples by UV-Vis Spectrophotometric Method. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 012041. [Google Scholar] [CrossRef]
- Eweys, A.S.; Zhao, Y.-S.; Darwesh, O.M. Improving the Antioxidant and Anticancer Potential of Cinnamomum Cassia via Fermentation with Lactobacillus Plantarum. Biotechnol. Rep. 2022, 36, e00768. [Google Scholar] [CrossRef]
- Darwesh, O.; Eweys, A.; Zhao, Y.-S.; Matter, I. Application of Environmental-Safe Fermentation with Saccharomyces Cerevisiae for Increasing the Cinnamon Biological Activities. Bioresour. Bioprocess. 2023, 10, 12. [Google Scholar] [CrossRef]
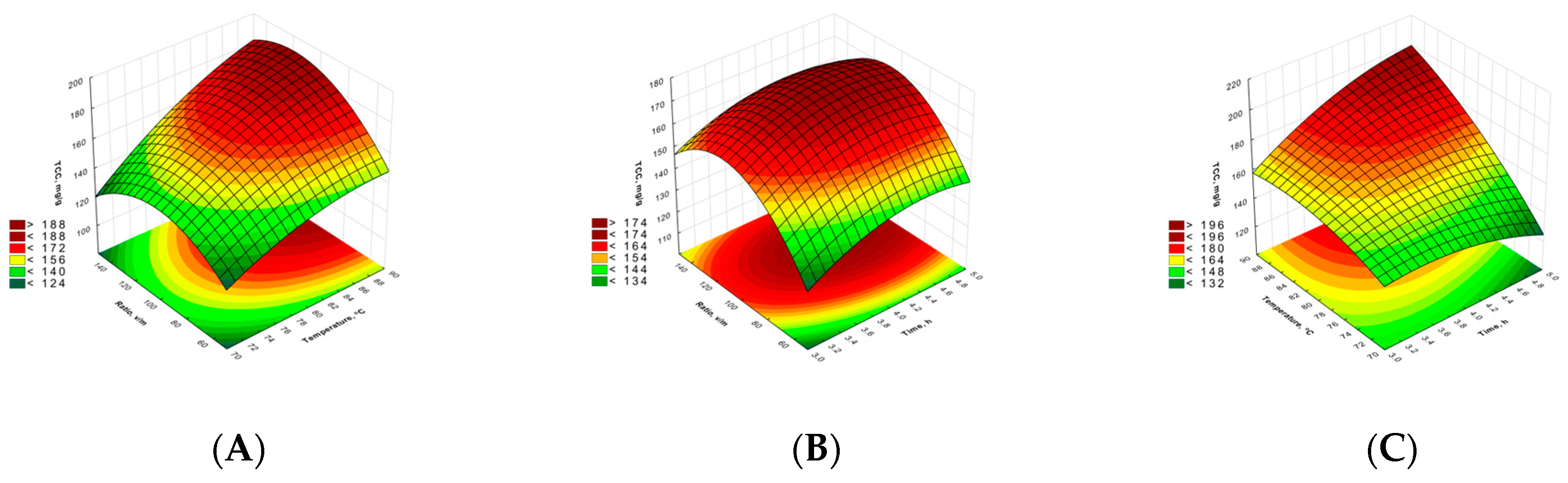
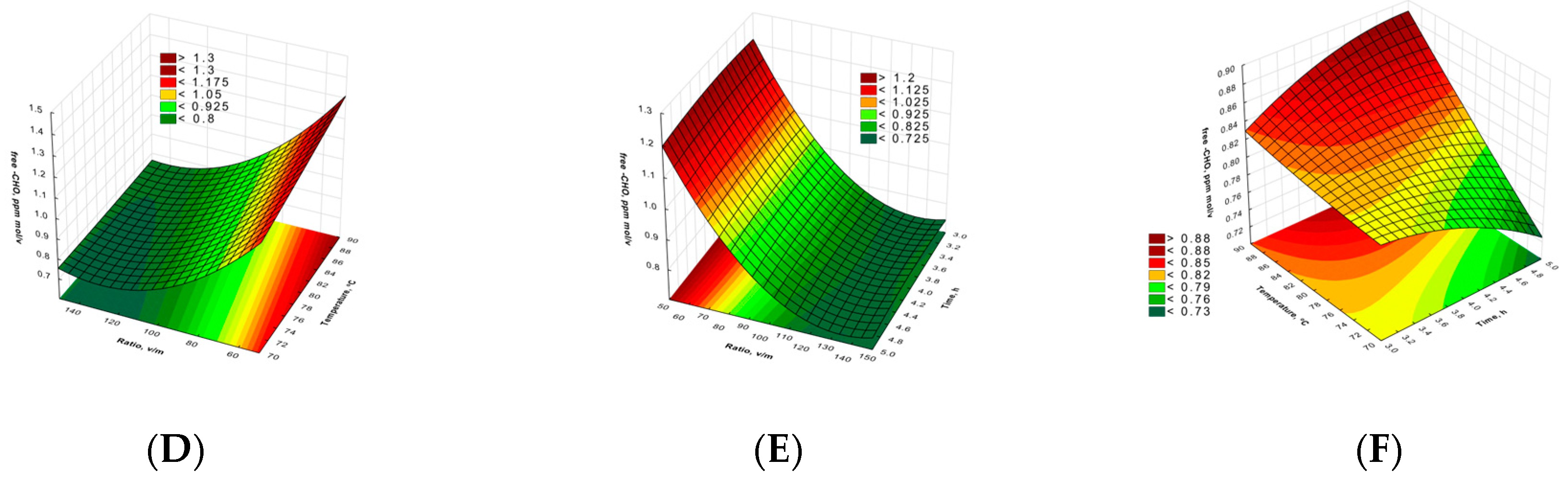
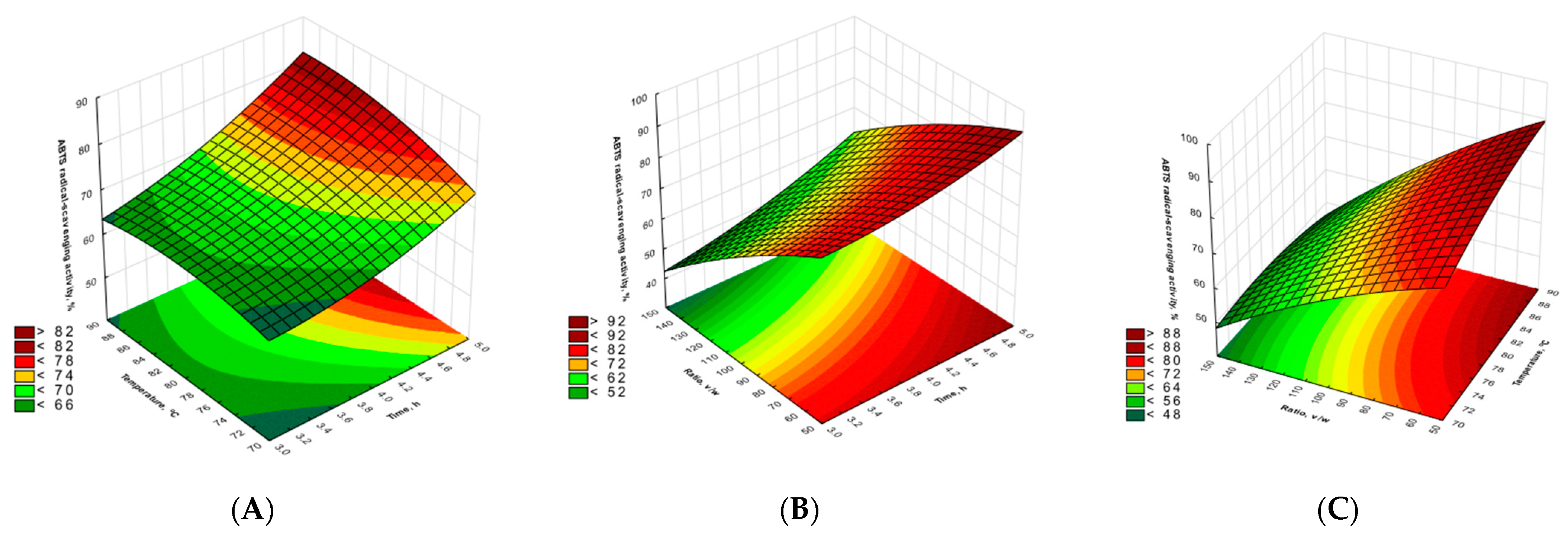
| TCC | RS | |
|---|---|---|
| Intercept | ||
| β0 | 172.181 | 0.824 |
| Linear | ||
| β1 | 11.782 | p > 0.01 |
| β2 | 44.536 | 0.093 |
| β3 | 17.613 | −0.465 |
| Quadratic | ||
| β11 | −16.371 | p > 0.01 |
| β22 | −15.496 | p > 0.01 |
| β33 | −43.625 | 0.329 |
| Mixed | ||
| β12 | 30.164 | 0.0699 |
| β13 | p > 0.01 | p > 0.01 |
| β23 | 17.128 | −0.083 |
| R2 | 0.915 | 0.957 |
| Predicted Response | ||||||
|---|---|---|---|---|---|---|
| TCC | Maximal, mg/g | ±95% CI | RS | Minimal, µmol/mL | ±95% CI | |
| Time, h | 5.0 | 202.51 | 196.38–207.91 | 4.78 | 0.68 | 0.658–0.717 |
| Temperature, °C | 90.0 | 70.45 | ||||
| Ratio, w/v | 118.59 | 128.82 | ||||
| DPPH | ABTS | H2O2 | |
|---|---|---|---|
| Intercept | |||
| β0 | p > 0.01 | 70.303 | 74.716 |
| Linear | |||
| β1 | −0.926 | 14.773 | p > 0.01 |
| β2 | −1.888 | 4.949 | p > 0.01 |
| β3 | 0.765 | −36.289 | 9.449 |
| Quadratic | |||
| β11 | 1.628 | p > 0.01 | p > 0.01 |
| β22 | 2.159 | p > 0.01 | p > 0.01 |
| β33 | p > 0.01 | p > 0.01 | p > 0.01 |
| Mixed | |||
| β12 | p > 0.01 | p > 0.01 | 12.927 |
| β13 | −1.144 | p > 0.01 | p > 0.01 |
| β23 | p > 0.01 | −7.045 | p > 0.01 |
| R2 | 0.661 | 0.937 | 0.41 |
| Predicted Response | |||
|---|---|---|---|
| ABTS | Maximal, % | ±95% CI | |
| Time, h | 4.96 | 94.69 | 91.82–97.57 |
| Temperature, °C | 82.39 | ||
| Ratio, w/v | 51.01 | ||
| Dependent Variables | Predicted Value | Experimental Value | %CV |
|---|---|---|---|
| TCC, mg/g | 202.51 | 209.98 | 8.79 |
| RS, µmol/mL | 0.68 | 0.61 | 9.68 |
| ABTS, % | 94.69 | 95.99 | 6.01 |
| Run | Independent Variables | TCC, mg/g | -CHO, µmol/mL | DPPH | ABTS | H2O2 | ||
|---|---|---|---|---|---|---|---|---|
| Time (X1), h | Temperature (X2), °C | Ratio w/v (X3) | % | |||||
| 1 | 3 | 70 | 100 | 134.42 ± 2.58 | 0.814 ± 0.018 | 3.45 ± 0.23 | 61.52 ± 1.47 | 77.82 ± 6.96 |
| 2 | 3 | 80 | 50 | 133.10 ± 3.88 | 1.236 ± 0.078 | 0.25 ± 0.19 | 86.72 ± 1.69 | 68.11 ± 2.08 |
| 3 | 3 | 80 | 150 | 153.37 ± 4.40 | 0.736 ± 0.047 | 3.18 ± 0.99 | 45.35 ± 0.76 | 74.32 ± 3.32 |
| 4 | 3 | 90 | 100 | 152.40 ± 9.51 | 0.808 ± 0.039 | 2.23 ± 0.99 | 57.58 ± 3.53 | 61.90 ± 10.98 |
| 5 | 4 | 70 | 50 | 122.10 ± 2.83 | 1.106 ± 0.035 | 2.39 ± 0.56 | 74.60 ± 3.13 | 54.99 ± 8.47 |
| 6 | 4 | 70 | 150 | 133.07 ± 2.41 | 0.754 ± 0.037 | 3.04 ± 0.97 | 46.21 ± 2.83 | 74.45 ± 2.65 |
| 7 | 4 | 80 | 100 | 172.18 ± 3.73 | 0.824 ± 0.053 | 0.52 ± 0.40 | 70.30 ± 2.69 | 74.72 ± 1.08 |
| 8 | 4 | 80 | 100 | 174.41 ± 3.61 | 0.870 ± 0.007 | 0.54 ± 0.44 | 67.88 ± 0.30 | 75.07 ± 1.31 |
| 9 | 4 | 80 | 100 | 169.96 ± 2.62 | 0.779 ± 0.031 | 0.50 ± 0.45 | 72.73 ± 0.61 | 74.37 ± 0.92 |
| 10 | 4 | 90 | 50 | 145.90 ± 2.41 | 1.312 ± 0.063 | 0.19 ± 0.07 | 91.57 ± 0.95 | 68.33 ± 3.57 |
| 11 | 4 | 90 | 150 | 180.27 ± 3.77 | 0.794 ± 0.023 | 0.31 ± 0.12 | 49.09 ± 2.71 | 82.41 ± 6.71 |
| 12 | 5 | 70 | 100 | 129.93 ± 5.40 | 0.746 ± 0.034 | 2.68 ± 0.51 | 78.99 ± 2.24 | 67.76 ± 5.17 |
| 13 | 5 | 80 | 50 | 133.29 ± 1.91 | 1.207 ± 0.021 | 0.40 ± 0.04 | 90.66 ± 2.75 | 77.17 ± 2.48 |
| 14 | 5 | 80 | 150 | 148.97 ± 8.79 | 0.718 ± 0.025 | 1.04 ± 0.31 | 57.73 ± 4.82 | 75.20 ± 1.64 |
| 15 | 5 | 90 | 100 | 208.24 ± 3.35 | 0.880 ± 0.015 | 1.48 ± 0.04 | 82.88 ± 1.73 | 77.69 ± 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, A.S.E.; Lukasiewicz, M. The Optimization of the Hot Water Extraction of the Polysaccharide-Rich Fraction from Agaricus bisporus. Molecules 2024, 29, 4783. https://doi.org/10.3390/molecules29194783
Khalil ASE, Lukasiewicz M. The Optimization of the Hot Water Extraction of the Polysaccharide-Rich Fraction from Agaricus bisporus. Molecules. 2024; 29(19):4783. https://doi.org/10.3390/molecules29194783
Chicago/Turabian StyleKhalil, Aya Samy Ewesys, and Marcin Lukasiewicz. 2024. "The Optimization of the Hot Water Extraction of the Polysaccharide-Rich Fraction from Agaricus bisporus" Molecules 29, no. 19: 4783. https://doi.org/10.3390/molecules29194783
APA StyleKhalil, A. S. E., & Lukasiewicz, M. (2024). The Optimization of the Hot Water Extraction of the Polysaccharide-Rich Fraction from Agaricus bisporus. Molecules, 29(19), 4783. https://doi.org/10.3390/molecules29194783







