Dobinin K Displays Antiplasmodial Activity through Disruption of Plasmodium falciparum Mitochondria and Generation of Reactive Oxygen Species
Abstract
1. Introduction
2. Results
2.1. Dobinin K Is a Potent Antiplasmodial Compound In Vitro
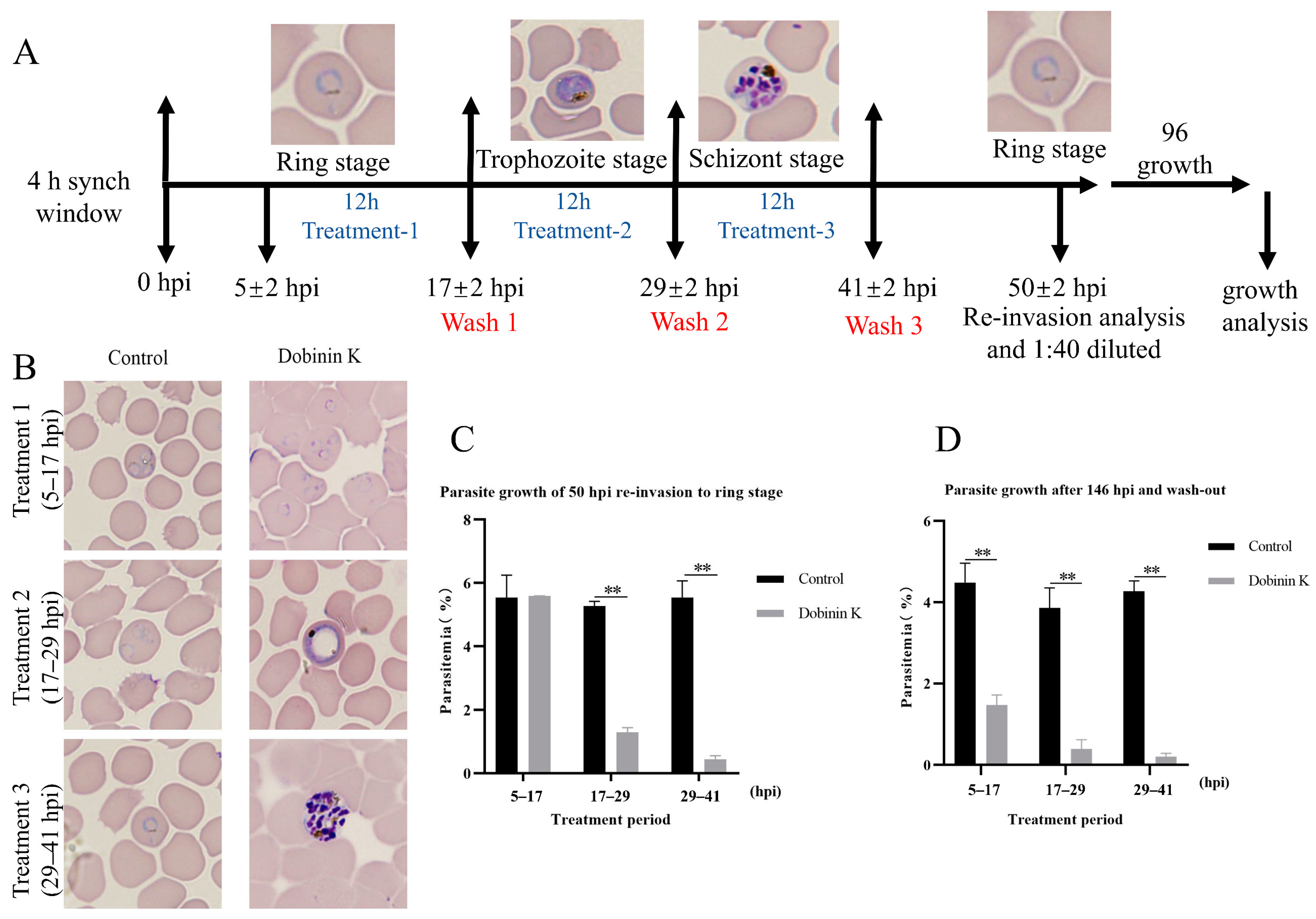
2.2. Dobinin K Suppresses the Growth of P. falciparum at the Trophozoite and Schizont Stages
2.3. Target Prediction of Dobinin K Based on Transcriptome Analysis
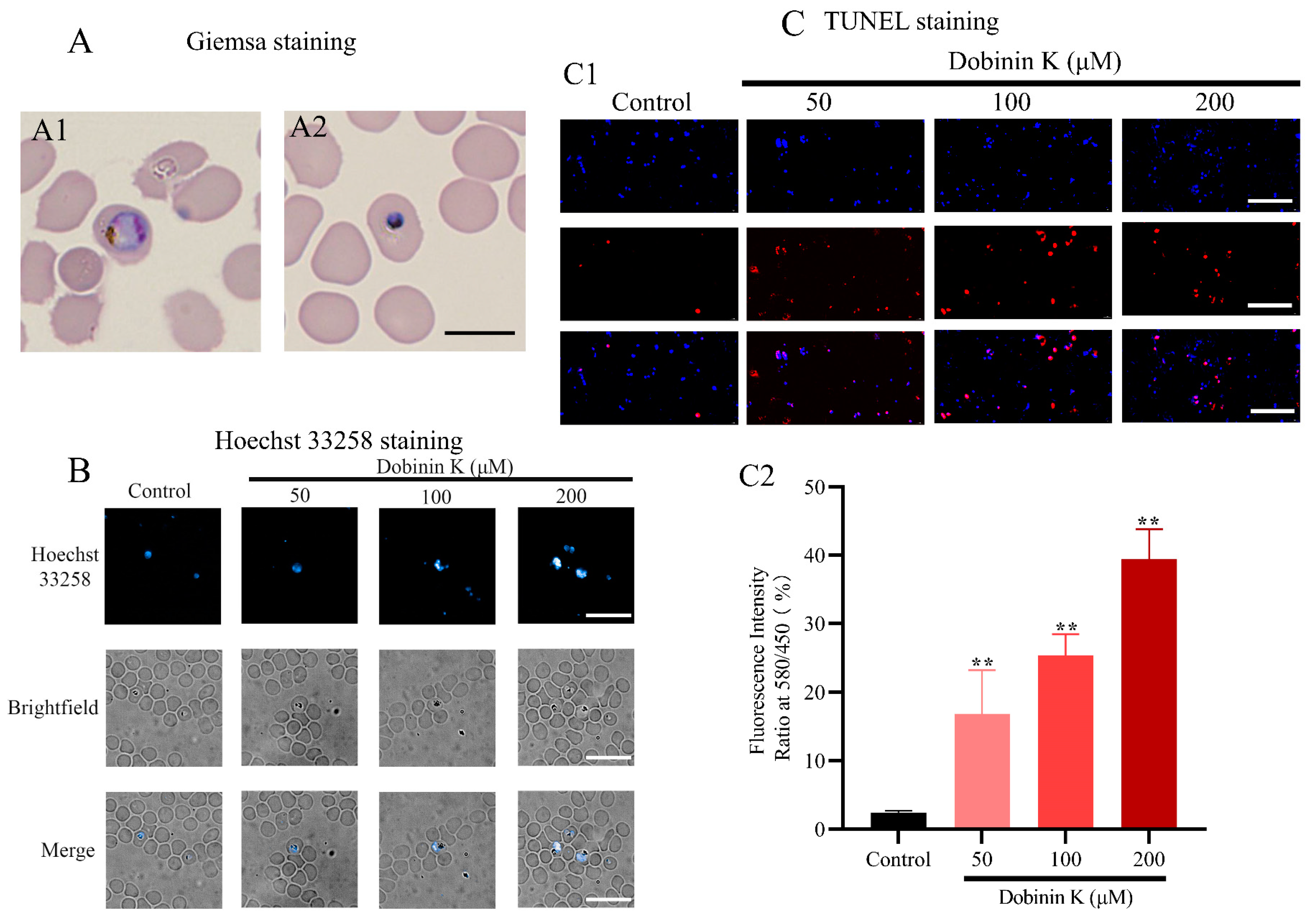
2.4. Dobinin K Induces Apoptosis of P. falciparum and Increases Intracellular ROS Levels
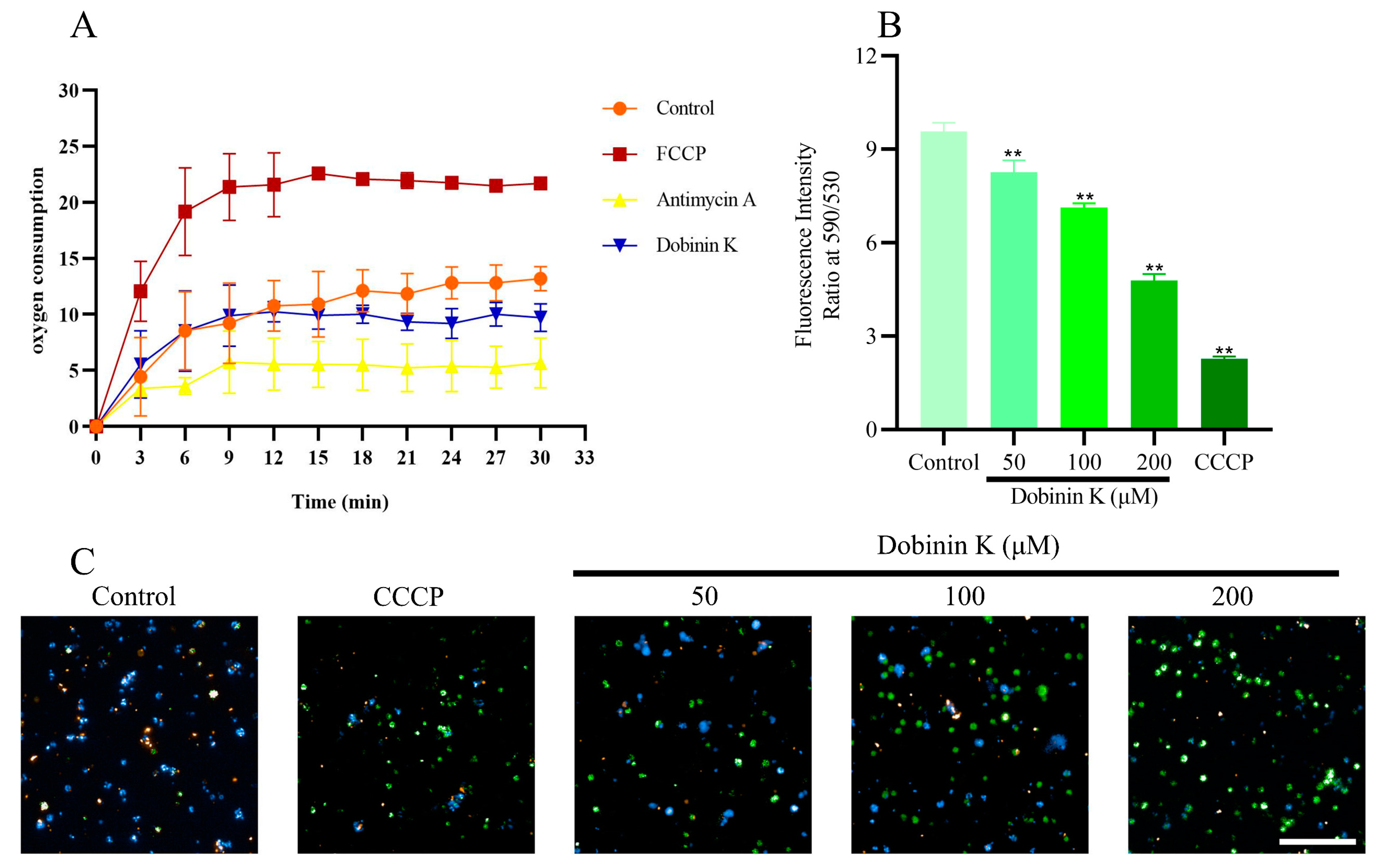
2.5. Dobinin K Decreases Oxygen Consumption and Disrupts Membrane Potential (Δψm) of P. falciparum Mitochondria
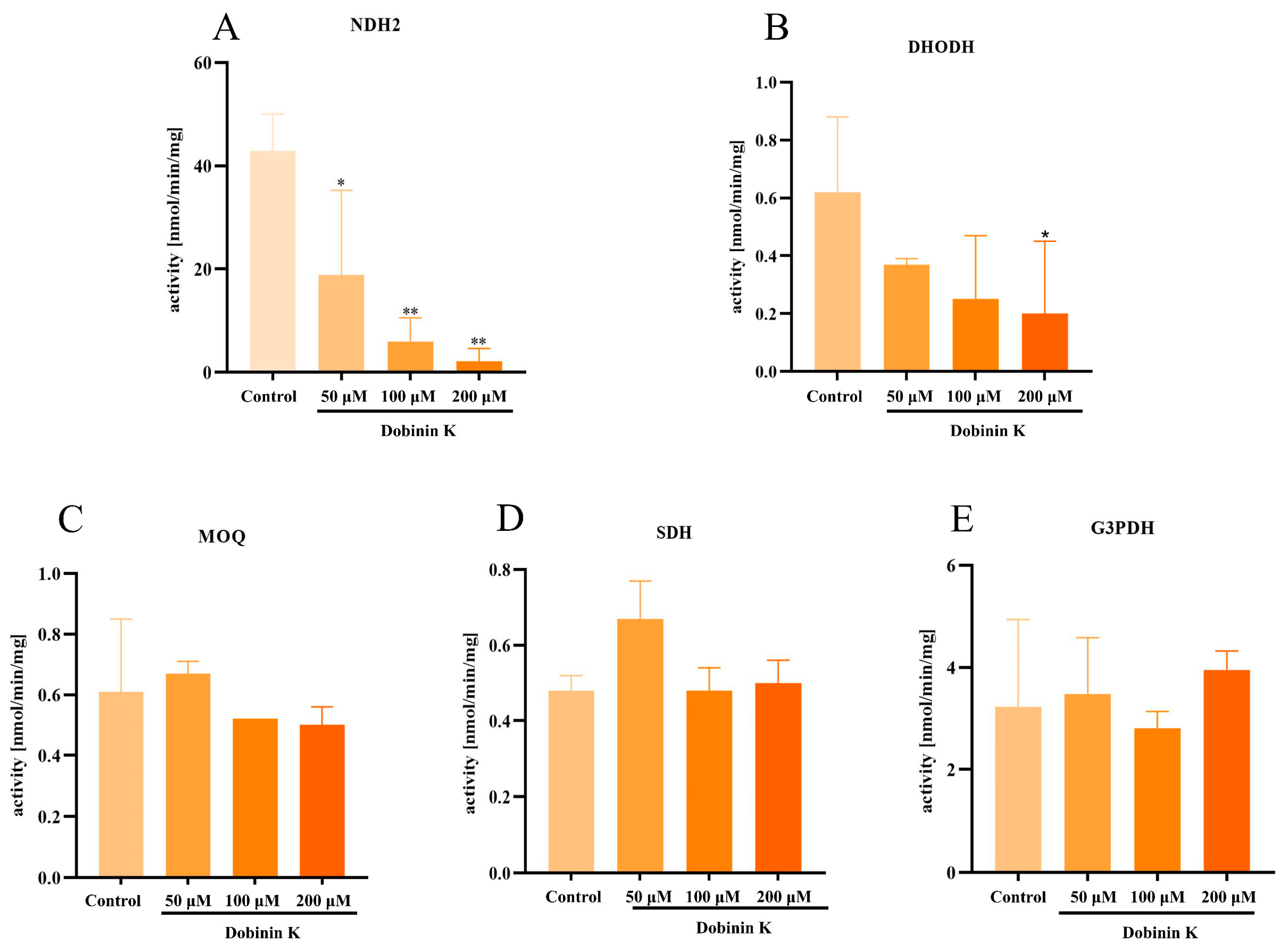
2.6. Dobinin K Effectively Inhibits P. falciparum NDH2 Activity
2.7. Molecular Docking Study of Dobinin K with PfNDH2 and PfDHODH
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. P. falciparum Culture
4.3. Concentration-Dependent Parasite Inhibition Assay
4.4. Antiplasmodial Activity Determination of the Combination with Dobinin K and Chloroquine against Dd2 Strain
4.5. Time-Dependent Parasite Inhibition Assay
4.6. Stage-Specific Parasite Inhibition Assay
4.7. Isolation of P. falciparum and Mitochondria
4.8. Determination of ROS Production
4.9. Detection of Oxygen Consumption
4.10. Measurement of Mitochondrial Membrane Potential
4.11. Hoechst 33258 Staining Assay
4.12. TUNEL Assay
4.13. Measurement of Enzyme Activities in the ETC
4.14. Transcriptomic Analysis
4.15. Molecular Docking Study
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, H.P.; Zhang, G.P.; Ma, L.N.; Su, P.; Zhang, Z.X.; Dai, B.Q.; Ye, Z.G. Effects and Mechanism of Action of Artemisinin on Mitochondria of Plasmodium berghei. Chin. J. Integr. Med. 2020, 26, 277–282. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Kavunga-Membo, H.; Ilombe, G.; Masumu, J.; Matangila, J.; Imponge, J.; Manzambi, E.; Wastenga, F.; Ngoyi, D.M.; Van, G.J.P.; Muyembe, J.J. Molecular identification of Plasmodium species in symptomatic children of Democratic Republic of Congo. Malar. J. 2018, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.E.; Fidock, D.A.; Bridgford, J.L. Plasmodium falciparum resistance to artemisinin-based combination therapies. Curr. Opin. Microbiol. 2022, 69, 102193. [Google Scholar] [CrossRef] [PubMed]
- Platon, L.; Leroy, D.; Fidock, D.A.; Ménard, D. Drug-induced stress mediates Plasmodium falciparum ring-stage growth arrest and reduces in vitro parasite susceptibility to artemisinin. Microbiol. Spectr. 2024, 12, e0350023. [Google Scholar] [CrossRef]
- Jeang, B.; Zhong, D.; Lee, M.C.; Atieli, H.; Yewhalaw, D.; Yan, G. Molecular surveillance of Kelch 13 polymorphisms in Plasmodium falciparum isolates from Kenya and Ethiopia. Malar. J. 2024, 23, 36. [Google Scholar] [CrossRef]
- Dwivedi, M.K.; Shukla, R.; Sharma, N.K.; Manhas, A.; Srivastava, K.; Kumar, N.; Singh, P.K. Evaluation of ethnopharmacologically selected Vitex negundo L. for In vitro antimalarial activity and secondary metabolite profiling. J. Ethnopharmacol. 2021, 275, 114076. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, H.; Shen, L.; Li, H.X.; Dong, X.; Xiao, C.J.; Jiang, B. Dodelates A-E: Five dimeric eudesmane sesquiterpenoids from Dobinea delavayi. Bioorg Chem. 2020, 95, 103488. [Google Scholar] [CrossRef]
- Shen, Y.; Cui, S.J.; Chen, H.; Shen, L.; Wang, M.; Dong, X.; Xiao, C.J.; Jiang, B. Antimalarial Eudesmane Sesquiterpenoids from Dobinea delavayi. J. Nat. Prod. 2020, 83, 927–936. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Komatsuya, K.; Sakura, T.; Shiomi, K.; Ōmura, S.; Hikosaka, K.; Nozaki, T.; Kita, K.; Inaoka, D.K. Siccanin Is a Dual-Target Inhibitor of Plasmodium falciparum Mitochondrial Complex II and Complex III. Pharmaceuticals 2022, 15, 903. [Google Scholar] [CrossRef]
- Van, N.D.D.; Du, T.F.; Green, K.; Palm, D.; Snoep, J.L. A detailed kinetic model of glycolysis in Plasmodium falciparum-infected red blood cells for antimalarial drug target identification. J. Biol. Chem. 2023, 299, 105111. [Google Scholar]
- Fisher, G.M.; Cobbold, S.A.; Jezewski, A.; Carpenter, E.F.; Arnold, M.; Cowell, A.N.; Tjhin, E.T.; Saliba, K.J.; Skinner-Adams, T.S.; Lee, M.C.S.; et al. The Key Glycolytic Enzyme Phosphofructokinase Is Involved in Resistance to Antiplasmodial Glycosides. mBio 2020, 11, 2842-20. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, W.; Diao, Y.; Sun, H.; Li, H.; Zhu, L.; Zhou, H.; Zhao, Z. Synthesis, Design, and Structure–Activity Relationship of the Pyrimidone Derivatives as Novel Selective Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase. Molecules 2018, 23, 1254. [Google Scholar] [CrossRef] [PubMed]
- Painter, H.J.; Morrisey, J.M.; Mather, M.W.; Orchard, L.M.; Luck, C.; Smilkstein, M.J.; Riscoe, M.K.; Vaidya, A.B.; Llinás, M. Atypical Molecular Basis for Drug Resistance to Mitochondrial Function Inhibitors in Plasmodium falciparum. Antimicrob. Agents Chemother. 2021, 65, 2143-20. [Google Scholar] [CrossRef]
- Mounkoro, P.; Michel, T.; Blandin, S.; Golinelli-Cohen, M.P.; Davioud-Charvet, E.; Meunier, B. Investigating the mode of action of the redox-active antimalarial drug plasmodione using the yeast model. Free Radic. Biol. Med. 2019, 141, 269–278. [Google Scholar] [CrossRef]
- Berneburg, I.; Peddibhotla, S.; Heimsch, K.C.; Haeussler, K.; Maloney, P.; Gosalia, P.; Preuss, J.; Rahbari, M.; Skorokhod, O.; Valente, E.; et al. An Optimized Dihydrodibenzothiazepine Lead Compound (SBI-0797750) as a Potent and Selective Inhibitor of Plasmodium falciparum and P. vivax Glucose 6-Phosphate Dehydrogenase 6-Phosphogluconolactonase. Antimicrob. Agents Chemother. 2022, 66, e0210921. [Google Scholar] [CrossRef]
- Pal, C. Redox modulating small molecules having antimalarial efficacy. Biochem. Pharmacol. 2023, 218, 115927. [Google Scholar] [CrossRef]
- Shafi, S.; Gupta, S.; Jain, R.; Shoaib, R.; Munjal, A.; Maurya, P.; Kumar, P.; Kalam, N.A.; Singh, S. Tackling the emerging Artemisinin-resistant malaria parasite by modulation of defensive oxido-reductive mechanism via nitrofurantoin repurposing. Biochem. Pharmacol. 2023, 215, 115756. [Google Scholar] [CrossRef]
- Rathore, S.; Datta, G.; Kaur, I.; Malhotra, P.; Mohmmed, A. Disruption of cellular homeostasis induces organelle stress and triggers apoptosis like cell-death pathways in malaria parasite. Cell Death Dis. 2015, 6, e1803. [Google Scholar] [CrossRef]
- Shi, X.; Wei, M.; Xu, Z.; Liu, Y.; Zhang, M.; Lv, L.; Wang, Q. Vitamin C Inhibits Blood-Stage Plasmodium Parasites via Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 639944. [Google Scholar] [CrossRef]
- Cheema, H.S.; Prakash, O.; Pal, A.; Khan, F.; Bawankule, D.U.; Darokar, M.P. Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum. Parasitol. Int. 2014, 63, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Deepak, S.K.; Saurabh, K.; Ashish, K.; Mahendra, P.D.; Anirban, P.; Rajendra, S.B. Design and synthesis of novel glycyrrhetinic acid-triazole derivatives that exert anti-plasmodial activity inducing mitochondrial-dependent apoptosis in Plasmodium falciparum. New J. Chem. 2023, 47, 6967–6982. [Google Scholar]
- Lai, J.W.; Maah, M.J.; Tan, K.W.; Sarip, R.; Lim, Y.A.L.; Ganguly, R.; Khaw, L.T.; Ng, C.H. Dinuclear and mononuclear metal(II) polypyridyl complexes against drug-sensitive and drug-resistant Plasmodium falciparum and their mode of action. Malar. J. 2022, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Pidathala, C.; Amewu, R.; Pacorel, B.; Nixon, G.L.; Gibbons, P.; Hong, W.D.; Leung, S.C.; Berry, N.G.; Sharma, R.; Stocks, P.A.; et al. Identification, design and biological evaluation of bisaryl quinolones targeting Plasmodium falciparum type II NADH: Quinone oxidoreductase (PfNDH2). J Med Chem 2012, 55, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Y.; Li, X.; Li, J.; Wu, Y.; Yu, J.; Ge, J.; Huang, Z.; Jiang, L.; Rao, Y.; et al. Target Elucidation by Cocrystal Structures of NADH-Ubiquinone Oxidoreductase of Plasmodium falciparum (PfNDH2) with Small Molecule to Eliminate Drug-Resistant Malaria. J. Med. Chem. 2017, 60, 1994–2005. [Google Scholar] [CrossRef]
- Boysen, K.E.; Matuschewski, K. Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase. J. Biol. Chem. 2011, 286, 32661–32671. [Google Scholar] [CrossRef]
- Lunev, S.; Bosch, S.S.; Batista, F.A.; Wrenger, C.; Groves, M.R. Crystal structure of truncated aspartate transcarbamoylase from Plasmodium falciparum. Acta Crystallogr. F Struct. Biol. Commun. 2016, 72, 523–533. [Google Scholar] [CrossRef]
- Dickerman, B.K.; Elsworth, B.; Cobbold, S.A.; Nie, C.Q.; McConville, M.J.; Crabb, B.S.; Gilson, P.R. Identification of inhibitors that dually target the new permeability pathway and dihydroorotate dehydrogenase in the blood stage of Plasmodium falciparum. Sci. Rep. 2016, 6, 37502. [Google Scholar] [CrossRef][Green Version]
- Sheokand, P.K.; Pradhan, S.; Maclean, A.E.; Mühleip, A.; Sheiner, L. Plasmodium falciparum Mitochondrial Complex III, the Target of Atovaquone, Is Essential for Progression to the Transmissible Sexual Stages. Int. J. Mol. Sci. 2024, 25, 9239. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.D.; Siregar, J.E.; Mollard, V.; Vega-Rodríguez, J.; Syafruddin, D.; Matsuoka, H.; Matsuzaki, M.; Toyama, T.; Sturm, A.; Cozijnsen, A.; et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science 2016, 352, 349–353. [Google Scholar] [CrossRef]
- Alruwaili, M.; Elahi, R.; van Schalkwyk, D.; Sutherland, C.; Shapiro, T.; Prigge, S.; Sullivan, D. Creating and Validating Ligase Primers to Detect Single Nucleotide Polymorphisms Associated with Atovaquone Resistance in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 2023, 108, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.S.; Fidock, D.A. Elucidating Mechanisms of Drug-Resistant Plasmodium falciparum. Cell Host Microbe 2019, 26, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tan, Y.Z.; Wicht, K.J.; Erramilli, S.K.; Dhingra, S.K.; Okombo, J.; Vendome, J.; Hagenah, L.M.; Giacometti, S.I.; Warren, A.L.; et al. Structure and drug resistance of the Plasmodium falciparum transporter PfCRT. Nature 2019, 576, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Gunjan, S.; Sharma, T.; Yadav, K.; Chauhan, B.S.; Singh, S.K.; Siddiqi, M.I.; Tripathi, R. Artemisinin Derivatives and Synthetic Trioxane Trigger Apoptotic Cell Death in Asexual Stages of Plasmodium. Front. Cell Infect. Microbiol. 2018, 8, 256. [Google Scholar] [CrossRef]
- Reyser, T.; Paloque, L.; Nguyen, M.; Augereau, J.M.; Fuchter, M.J.; Lopez, M.; Arimondo, P.B.; Hassell-Hart, S.; Spencer, J.; DiStefano, L.; et al. Epidrugs as Promising Tools to Eliminate Plasmodium falciparum Artemisinin-Resistant and Quiescent Parasites. Pharmaceutics 2023, 15, 2440. [Google Scholar] [CrossRef]
- Peng, Y.; Yin, D.; Li, X.; Wang, K.; Li, W.; Huang, Y.; Liu, X.; Ren, Z.; Yang, X.; Zhang, Z.; et al. Integration of transcriptomics and metabolomics reveals a novel gene signature guided by FN1 associated with immune response in oral squamous cell carcinoma tumorigenesis. J. Cancer Res. Clin. Oncol. 2023, 149, 6097–6113. [Google Scholar] [CrossRef]




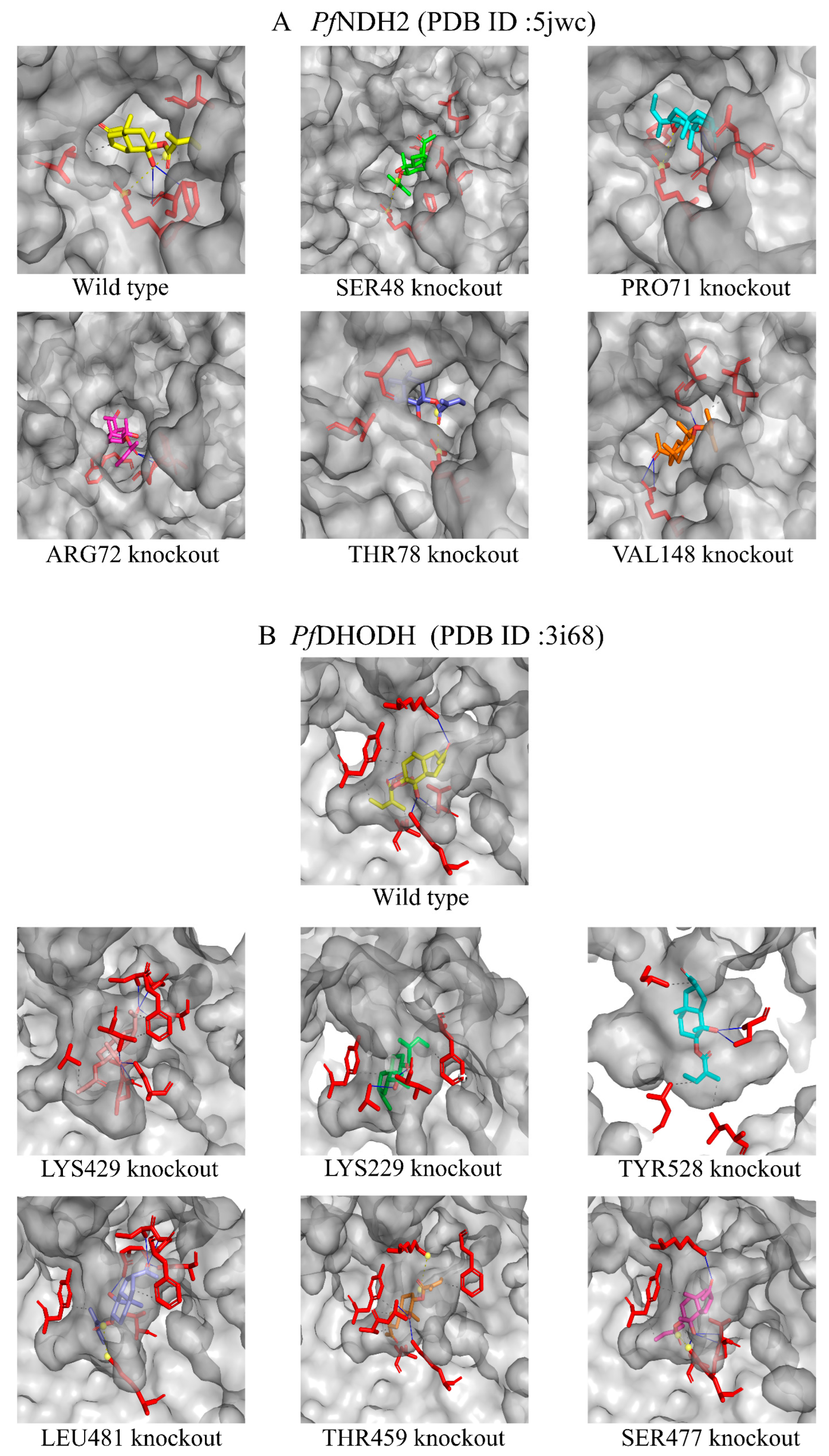
| Residue No. | Amino Acids | Force of Interaction | Binding Energies after Mutated Residues |
|---|---|---|---|
| 71 | PRO | Hydrophobic Interactions | −7.3 |
| 148 | VAL | Hydrophobic Interactions | −6.9 |
| 78 | THR | Hydrophobic Interactions | −6.8 |
| 72 | ARG | Salt Bridges | −6.7 |
| 48 | SER | Hydrogen Bonds | −6.6 |
| Residue No. | Amino Acids | Force of Interaction | Binding Energies after Mutated Residues |
|---|---|---|---|
| 528 | TYR | Hydrophobic Interactions | −8.0 |
| 481 | LEU | Hydrophobic Interactions | −7.9 |
| 429 | LYS | Hydrogen Bonds | −8.6 |
| 477 | SER | Hydrogen Bonds | −8.0 |
| 229 | LYS | Hydrogen Bonds | −7.7 |
| 459 | THR | Hydrogen Bonds | −7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Liu, B.-C.; He, L.-F.; Xiao, C.-J.; Jiang, B.; Shen, L. Dobinin K Displays Antiplasmodial Activity through Disruption of Plasmodium falciparum Mitochondria and Generation of Reactive Oxygen Species. Molecules 2024, 29, 4759. https://doi.org/10.3390/molecules29194759
Sun H, Liu B-C, He L-F, Xiao C-J, Jiang B, Shen L. Dobinin K Displays Antiplasmodial Activity through Disruption of Plasmodium falciparum Mitochondria and Generation of Reactive Oxygen Species. Molecules. 2024; 29(19):4759. https://doi.org/10.3390/molecules29194759
Chicago/Turabian StyleSun, He, Bo-Chao Liu, Long-Fei He, Chao-Jiang Xiao, Bei Jiang, and Lei Shen. 2024. "Dobinin K Displays Antiplasmodial Activity through Disruption of Plasmodium falciparum Mitochondria and Generation of Reactive Oxygen Species" Molecules 29, no. 19: 4759. https://doi.org/10.3390/molecules29194759
APA StyleSun, H., Liu, B.-C., He, L.-F., Xiao, C.-J., Jiang, B., & Shen, L. (2024). Dobinin K Displays Antiplasmodial Activity through Disruption of Plasmodium falciparum Mitochondria and Generation of Reactive Oxygen Species. Molecules, 29(19), 4759. https://doi.org/10.3390/molecules29194759







