Adsorption Property and Morphology Evolution of C Deposited on HCP Co Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. C Adsorption on HCP Co Surfaces
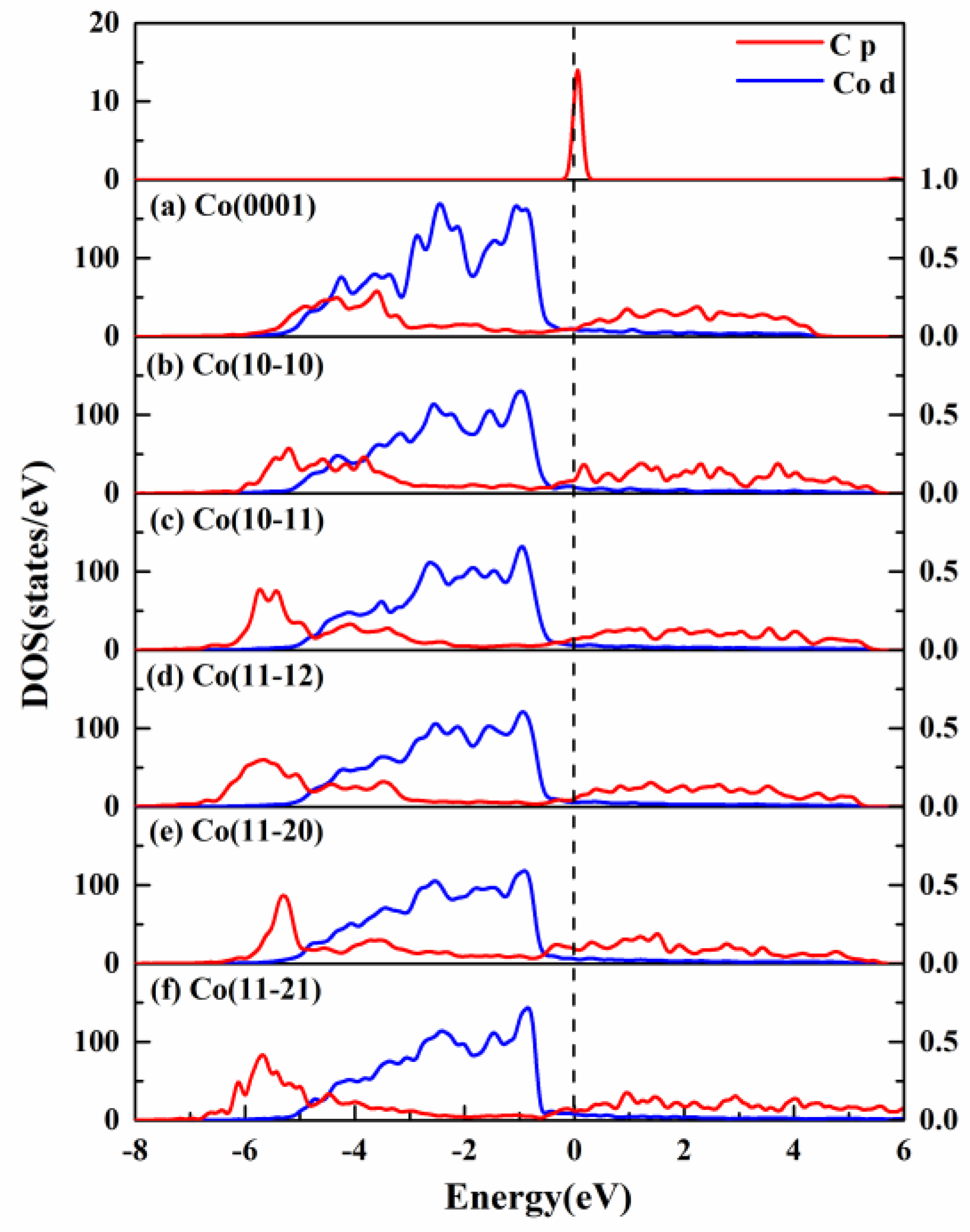
2.2. Electronic Property of C Atom on HCP Co Surfaces
2.3. C Diffusion Behavior on HCP Co Surfaces
2.4. Morphology Evolution of HCP Co Nanoparticles Induced by Deposited C
3. Conclusions
4. Models and Methods
4.1. Surface Models
4.2. Computational Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Li, Y.; Zhu, H.; Lyu, Y.; Ding, Y. A review of Co/Co2C-based catalysts in Fischer-Tropsch synthesis: From fundamental understanding to industrial applications. Chem. Commun. 2023, 59, 3827–3837. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hou, B.; Liu, Y.; Jia, L.; Ma, Z.; Wang, J.; Wang, Q.; Li, D. Higher alcohols synthesis via Fischer–Tropsch reaction at hcp-Co@Co2C interface. Fuel 2023, 341, 127500. [Google Scholar] [CrossRef]
- van Koppen, L.M.; Dugulan, A.I.; Hensen, E.J.M.; Bezemer, G.L. Tuning stability of titania-supported Fischer-Tropsch catalysts: Impact of surface area and noble metal promotion. Catal. Today 2024, 429, 114471. [Google Scholar] [CrossRef]
- Ghogia, A.C.; Nzihou, A.; Serp, P.; Soulantica, K.; Pham Minh, D. Cobalt catalysts on carbon-based materials for Fischer-Tropsch synthesis: A review. Appl. Catal. A Gen. 2021, 609, 117906. [Google Scholar] [CrossRef]
- Suo, Y.; Yao, Y.; Zhang, Y.; Xing, S.; Yuan, Z.-Y. Recent advances in cobalt-based Fischer-Tropsch synthesis catalysts. J. Ind. Eng. Chem. 2022, 115, 92–119. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer−Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Hofer, L.J.E.; Sterling, E.; McCartney, J.T. Structure of carbon deposited from carbon monoxide on iron, cobalt and nickel. J. Phys. Chem. C 1955, 59, 1153–1155. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Tan, K.F.; Xu, J.; Chang, J.; Borgna, A.; Saeys, M. Carbon deposition on Co catalysts during Fischer-Tropsch synthesis: A computational and experimental study. J. Catal. 2010, 274, 121–129. [Google Scholar] [CrossRef]
- Zhong, L.; Yu, F.; An, Y.; Zhao, Y.; Sun, Y.; Li, Z.; Lin, T.; Lin, Y.; Qi, X.; Dai, Y.; et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 2016, 538, 84–87. [Google Scholar] [CrossRef]
- Shen, X.; Luo, D.; Ma, C.; Suo, H.; Yan, L.; Zhang, T.; Liu, X.; Wen, X.; Li, Y.; Yang, Y. Carburized cobalt catalyst for the Fischer–Tropsch synthesis. Catal. Sci. Technol. 2021, 11, 6564–6572. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; van Santen, R.A.; Hensen, E.J.M. Carbon-Induced Surface Transformations of Cobalt. ACS Catal. 2015, 5, 596–601. [Google Scholar] [CrossRef][Green Version]
- Saib, A.M.; Moodley, D.J.; Ciobîcă, I.M.; Hauman, M.M.; Sigwebela, B.H.; Weststrate, C.J.; Niemantsverdriet, J.W.; van de Loosdrecht, J. Fundamental understanding of deactivation and regeneration of cobalt Fischer–Tropsch synthesis catalysts. Catal. Today 2010, 154, 271–282. [Google Scholar] [CrossRef]
- Cheng, K.; Subramanian, V.; Carvalho, A.; Ordomsky, V.V.; Wang, Y.; Khodakov, A.Y. The role of carbon pre-coating for the synthesis of highly efficient cobalt catalysts for Fischer–Tropsch synthesis. J. Catal. 2016, 337, 260–271. [Google Scholar] [CrossRef]
- Zhai, P.; Chen, P.-P.; Xie, J.; Liu, J.-X.; Zhao, H.; Lin, L.; Zhao, B.; Su, H.-Y.; Zhu, Q.; Li, W.-X.; et al. Carbon induced selective regulation of cobalt-based Fischer-Tropsch catalysts by ethylene treatment. Faraday Discuss. 2017, 197, 207–224. [Google Scholar] [CrossRef]
- Weststrate, C.J.; Ciobîcă, I.M.; Saib, A.M.; Moodley, D.J.; Niemantsverdriet, J.W. Fundamental issues on practical Fischer–Tropsch catalysts: How surface science can help. Catal. Today 2014, 228, 106–112. [Google Scholar] [CrossRef]
- Kistamurthy, D. Fundamental Understanding of Cobalt Fischer-Tropsch Synthesis Catalyst Deactivation. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2015. [Google Scholar]
- Moodley, D.; Van de Loosdrecht, J.; Saib, A.; Overett, M.; Datye, A.; Niemantsverdriet, J. Carbon deposition as a deactivation mechanism of cobalt-based Fischer–Tropsch synthesis catalysts under realistic conditions. Appl. Catal. A Gen. 2009, 354, 102–110. [Google Scholar] [CrossRef]
- Chen, C.; Hou, B.; Liu, Y.; Jia, L.; Ma, Z.; Wang, J.; Wang, Q.; Li, D. Carbon species on the surface of carbon-coated catalysts and their effects on Fischer-Tropsch synthesis products. Fuel 2023, 341, 127381. [Google Scholar] [CrossRef]
- Qin, H.; Kang, S.; Wang, Y.; Liu, H.; Ni, Z.; Huang, Y.; Li, Y.; Li, X. Lignin-Based Fabrication of Co@C Core–Shell Nanoparticles as Efficient Catalyst for Selective Fischer–Tropsch Synthesis of C5+ Compounds. ACS Sustain. Chem. Eng. 2016, 4, 1240–1247. [Google Scholar] [CrossRef]
- Lu, W.; Wang, J.; Ma, Z.; Chen, C.; Liu, Y.; Hou, B.; Li, D.; Wang, B. Classifying and understanding the role of carbon deposits on cobalt catalyst for Fischer-Tropsch synthesis. Fuel 2023, 332, 126115. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, B.; Chen, C.; Jia, L.; Ma, Z.; Wang, Q.; Li, D. Carbon coated cobalt catalysts for direct synthesis of middle n-alkanes from syngas. Fuel 2022, 327, 124889. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, C.; Mao, Y.; Hu, P. Quantitative Determination of CC Coupling Mechanisms and Detailed Analyses on the Activity and Selectivity for Fischer-Tropsch Synthesis on Co (0001): Microkinetic Modelling with Coverage Effects. ACS Catal. 2019, 9, 5957–5973. [Google Scholar] [CrossRef]
- Khodakov, A.Y. Fischer-Tropsch synthesis: Relations between structure of cobalt catalysts and their catalytic performance. Catal. Today 2009, 144, 251–257. [Google Scholar] [CrossRef]
- Leng, J.; Yang, X.; Zhong, M.; Liu, L.; Fan, Y.; Chen, Y.; Yang, P. Structure sensitivity in the photocatalytic reduction of CO2 with Co3O4 catalysts. Appl. Surf. Sci. 2023, 640, 158242. [Google Scholar] [CrossRef]
- van Santen, R.A.; Ghouri, M.M.; Shetty, S.; Hensen, E.M.H. Structure sensitivity of the Fischer–Tropsch reaction; molecular kinetics simulations. Catal. Sci. Technol. 2011, 1, 891. [Google Scholar] [CrossRef]
- Cheng, H.W.; Wang, S.; Chen, G.; Liu, Z.; Caracciolo, D.; Madiou, M.; Shan, S.; Zhang, J.; He, H.; Che, R.; et al. Insights into Heterogeneous Catalysts under Reaction Conditions by In Situ/Operando Electron Microscopy. Adv. Energy Mater. 2022, 12, 2202097. [Google Scholar] [CrossRef]
- Navarro, V.; van Spronsen, M.A.; Frenken, J.W. In situ observation of self-assembled hydrocarbon Fischer-Tropsch products on a cobalt catalyst. Nat. Chem. 2016, 8, 929–934. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.E.; Bremmer, G.M.; Aly, M.; Navarro, V.; Thybaut, J.W.; Kooyman, P.J.; Saeys, M. Shape of Cobalt and Platinum Nanoparticles Under a CO Atmosphere: A Combined In Situ TEM and Computational Catalysis Study. ACS Catal. 2019, 9, 7449–7456. [Google Scholar] [CrossRef]
- Golder, K.M.; Wintterlin, J. In Situ/Operando STM of the Fischer–Tropsch Synthesis on a Co (105) Surface—A Study to Bridge the Materials Gap between Single-Crystal Models and Supported Catalysts. ACS Catal. 2022, 12, 7199–7209. [Google Scholar] [CrossRef]
- Qin, C.; Hou, B.; Wang, J.; Wang, Q.; Wang, G.; Yu, M.; Chen, C.; Jia, L.; Li, D. Crystal-Plane-Dependent Fischer–Tropsch Performance of Cobalt Catalysts. ACS Catal. 2018, 8, 9447–9455. [Google Scholar] [CrossRef]
- Qin, C.; Hou, B.; Wang, J.; Ma, Z.; Chen, C.; Jia, L.; Li, D.; Ding, M. Tailoring Carbon Deposits on a Single-Crystal Cobalt Catalyst for Fischer–Tropsch Synthesis without Further Reduction: The Role of Surface Carbon and Penetrating Carbon. ACS Catal. 2023, 13, 8551–8560. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Li, D.; Fan, M.; Wang, B.; Ling, L.; Zhang, R. Crystallographic dependence of carbon deposition in the Fischer-Tropsch synthesis over cobalt catalysts: HCP versus FCC. Chem. Eng. J. 2024, 491, 151965. [Google Scholar] [CrossRef]
- Zhang, D.; Yi, P.; Lai, X.; Peng, L.; Li, H. Active machine learning model for the dynamic simulation and growth mechanisms of carbon on metal surface. Nat. Commun. 2024, 15, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, L.; Liu, H.; Wang, B.; Li, D.; Fan, M. Crystal facet dependence of carbon chain growth mechanism over the Hcp and Fcc Co catalysts in the Fischer-Tropsch synthesis. Appl. Catal. B. 2020, 269, 118847. [Google Scholar] [CrossRef]
- Corral Valero, M.; Raybaud, P. Stability of Carbon on Cobalt Surfaces in Fischer–Tropsch Reaction Conditions: A DFT Study. J. Phys. Chem. C 2014, 118, 22479–22490. [Google Scholar] [CrossRef]
- Petersen, M.A.; van den Berg, J.-A.; Ciobica, I.M.; van Helden, P. Revisiting CO Activation on Co Catalysts: Impact Sites from DFT of Step and Kink. ACS Catal. 2017, 7, 1984–1992. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comp. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Bader, R. Atoms in molecules: A quantum theory. Int. Ser. Monogr. Chem. 1990, 22, 9–15. [Google Scholar]
- Weststrate, C.J.; Kızılkaya, A.C.; Rossen, E.T.R.; Verhoeven, M.W.G.M.; Ciobîcă, I.M.; Saib, A.M.; Niemantsverdriet, J.W. Atomic and Polymeric Carbon on Co(0001): Surface Reconstruction, Graphene Formation, and Catalyst Poisoning. J. Phys. Chem. C 2012, 116, 11575–11583. [Google Scholar] [CrossRef]
- Liu, J.-X.; Su, H.-Y.; Sun, D.-P.; Zhang, B.-Y.; Li, W.-X. Crystallographic Dependence of CO Activation on Cobalt Catalysts: HCP versus FCC. J. Am. Chem. Soc. 2013, 135, 16284–16287. [Google Scholar] [CrossRef]
- Chen, W.; Kimpel, T.F.; Song, Y.; Chiang, F.-K.; Zijlstra, B.; Pestman, R.; Wang, P.; Hensen, E.J.M. Influence of Carbon Deposits on the Cobalt-Catalyzed Fischer-Tropsch Reaction: Evidence of a Two-Site Reaction Model. ACS Catal. 2018, 8, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- de la Pena O’Shea, V.A.; Moreira Ide, P.; Roldan, A.; Illas, F. Electronic and magnetic structure of bulk cobalt: The alpha, beta, and epsilon-phases from density functional theory calculations. J. Chem. Phys. 2010, 133, 024701. [Google Scholar] [CrossRef]
- Chen, Q.; Svenum, I.-H.; Qi, Y.; Gavrilovic, L.; Chen, D.; Holmen, A.; Blekkan, E.A. Potassium adsorption behavior on hcp cobalt as model systems for the Fischer-Tropsch synthesis: A density functional theory study. Phys. Chem. Chem. Phys. 2017, 19, 12246–12254. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Wang, Y.; Burke, K.; Perdew, J. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B. 1994, 50, 17953. [Google Scholar] [CrossRef]
- VandeVondele, J.; Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]



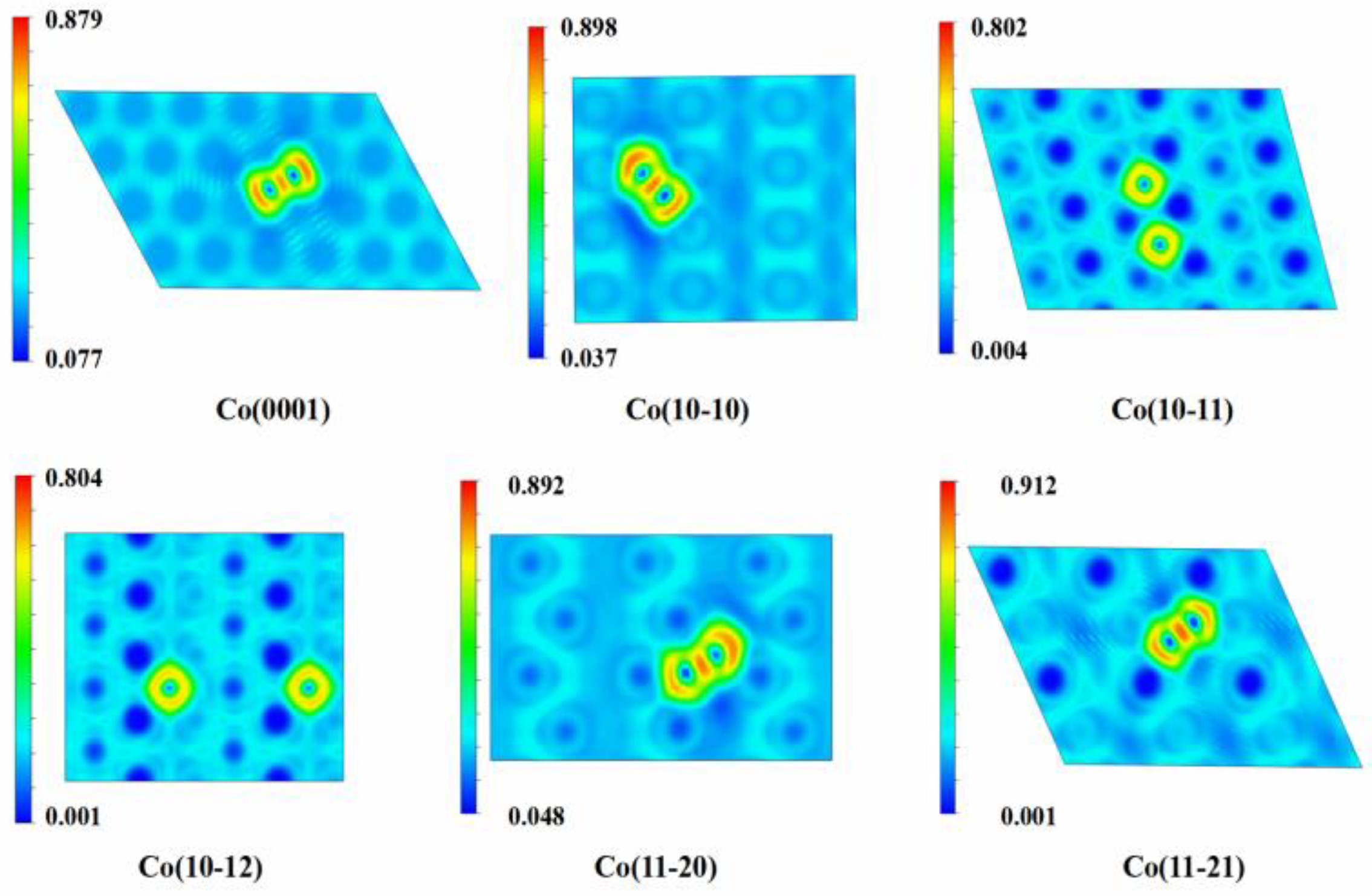


| Facet | NC | Adsorption Site | Eads/av (eV) | dC-Co (Å) | q |
|---|---|---|---|---|---|
| Co(0001) | 1 | H | −7.04 | 1.78 | 0.62 |
| 2,C2 dimer | H and F | −7.23 | 1.98 | 1.01 | |
| Co(10-10) | 1 | 4F | −7.13 | 1.87 | 0.68 |
| 2,C2 dimer | 4F and 3F | −7.35 | 2.09 | 1.05 | |
| Co(10-11) | 1 | 4F | −8.29 | 1.91 | 0.83 |
| 2,C + C | 4F and 4F | −8.14 | 1.90 | 1.60 | |
| Co(10-12) | 1 | 4F | −7.98 | 1.90 | 0.82 |
| 2,C + C | 4F and 4F | −7.94 | 1.91 | 1.61 | |
| Co(11-20) | 1 | 4F | −7.26 | 1.98 | 0.80 |
| 2,C2 dimer | 4F and 3F | −7.49 | 2.04 | 1.17 | |
| Co(11-21) | 1 | 4F | −7.73 | 1.90 | 0.82 |
| 2,C2 dimer | 4F and 3F2 | −7.78 | 2.01 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Shi, Y.; Rong, J.; Wang, Q.; Zhong, M. Adsorption Property and Morphology Evolution of C Deposited on HCP Co Nanoparticles. Molecules 2024, 29, 4760. https://doi.org/10.3390/molecules29194760
Liu L, Shi Y, Rong J, Wang Q, Zhong M. Adsorption Property and Morphology Evolution of C Deposited on HCP Co Nanoparticles. Molecules. 2024; 29(19):4760. https://doi.org/10.3390/molecules29194760
Chicago/Turabian StyleLiu, Lili, Yujia Shi, Jiamin Rong, Qiang Wang, and Min Zhong. 2024. "Adsorption Property and Morphology Evolution of C Deposited on HCP Co Nanoparticles" Molecules 29, no. 19: 4760. https://doi.org/10.3390/molecules29194760
APA StyleLiu, L., Shi, Y., Rong, J., Wang, Q., & Zhong, M. (2024). Adsorption Property and Morphology Evolution of C Deposited on HCP Co Nanoparticles. Molecules, 29(19), 4760. https://doi.org/10.3390/molecules29194760






