Healing Activity of Propolis of Stingless Bee (Scaptotrigona aff. postica), Reared in Monoculture of Açaí (Euterpe oleracea), in Induced Wounds in Rats
Abstract
1. Introduction
2. Results
2.1. Total Phenol Content Results
2.2. Flavonoid Content Results
2.3. Gravimetric Results
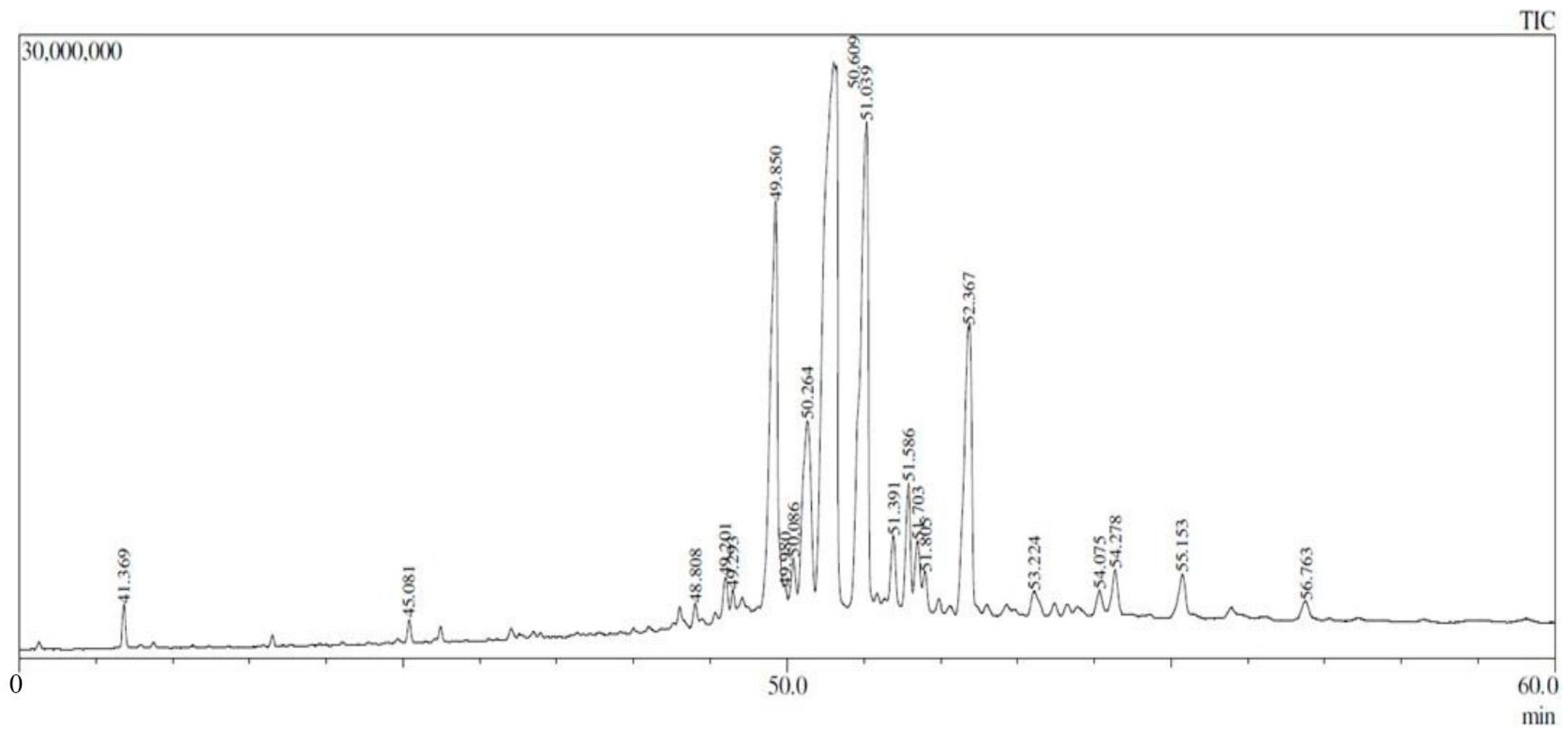
2.4. GC-MS Results
2.5. Macroscopic Results
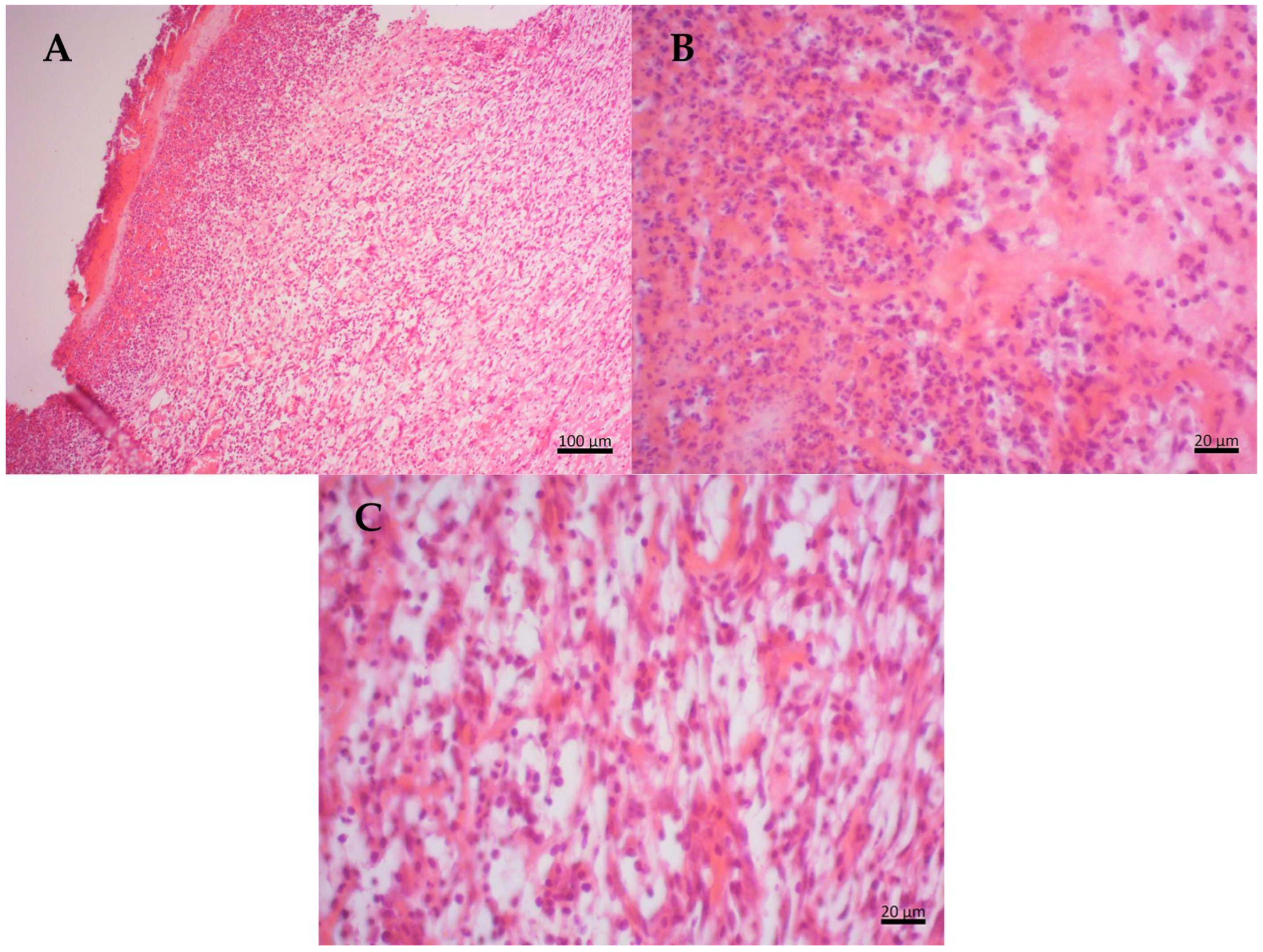
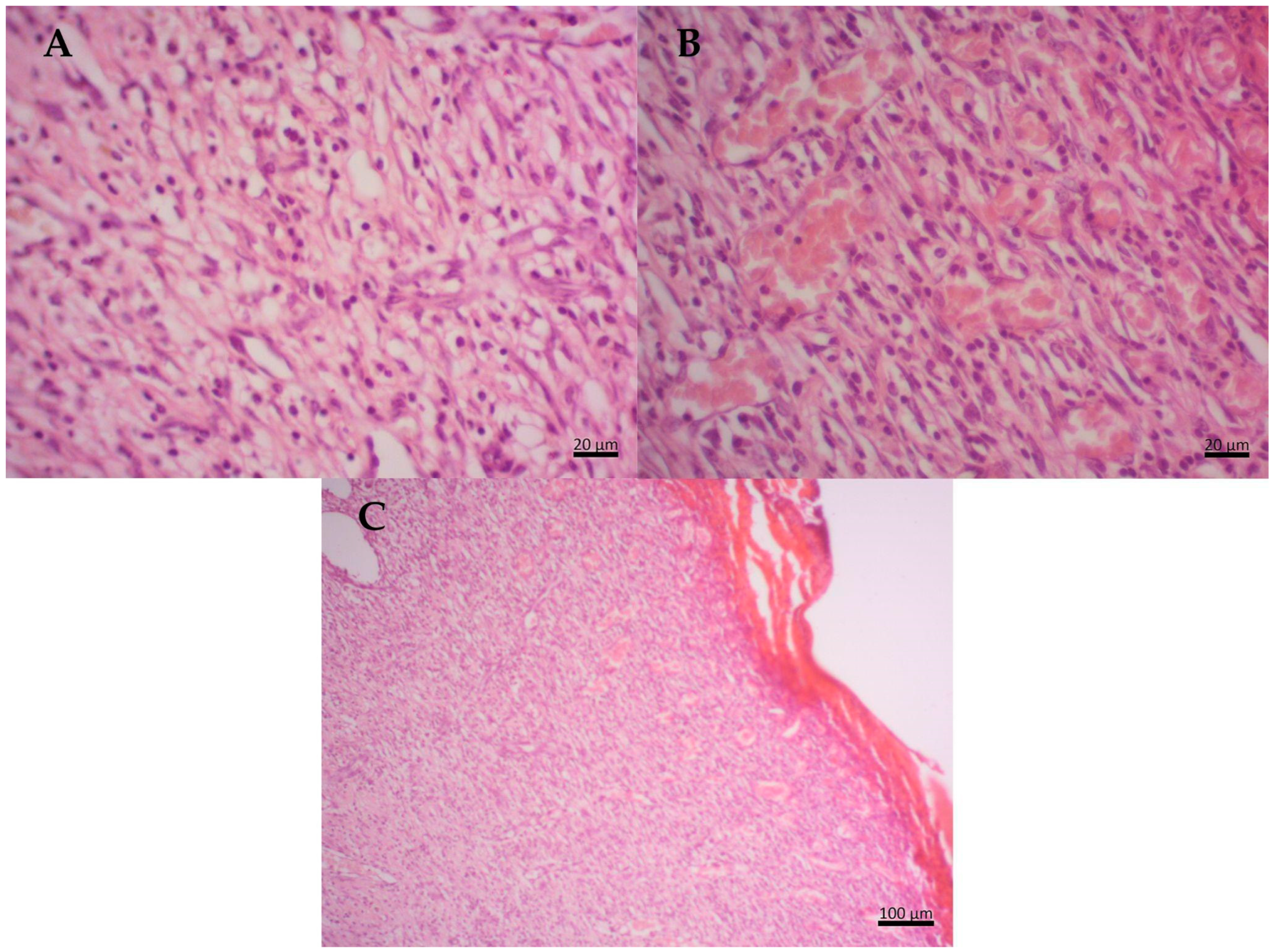
2.6. Histopathological Results
3. Discussion
4. Materials and Methods
4.1. Material Collection
4.2. Preparation of Physicochemical Analysis
4.3. Physicochemical Analysis
4.3.1. Quantification of Total Polyphenols
4.3.2. Quantification of Total Flavonoids
4.3.3. Determination of Ash Content
4.3.4. Loss on Drying at 105 °C
4.4. Preparation of Propolis Extract for GC-MS
Gas Chromatography
4.5. Preparation of Propolis-Based Cream
4.6. Animals
4.6.1. Experimental Procedures of Induced Wounds
4.6.2. Macroscopic Evaluation
4.7. Microscopic Evaluation
4.7.1. Sample Collection
4.7.2. Embedding in Paraffin
4.8. Histopathology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dos Santos, M.J.; do Amaral Carneiro Vianna, L.; Gamba, M.A. The effect of propolis cream in healing chronic ulcers. Acta Paul. Enferm. 2007, 20, 199–204. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.V. Propolis: Chemical diversity and challenges in quality control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- Menezes, H. Propolis: A review of the recent studies of its pharmacological properties. Arq. Inst. Biol. 2005, 72, 405–411. [Google Scholar] [CrossRef]
- Tran, C.T.N.; Brooks, P.R.; Bryen, T.J.; Williams, S.; Berry, J.; Tavian, F.; McKee, B.; Tran, T.D. Quality assessment and chemical diversity of Australian propolis from Apis mellifera bees. Sci. Rep. 2022, 12, 13574. [Google Scholar] [CrossRef]
- Sanches, M.A. Ação da Própolis de Scaptotrigona aff. Postica (Latreille, 1807) (Hymenoptera, Apidae, Meliponini) em Diferentes Linhagens de Células Tumorais; Universidade Federal de Viçosa: Minas Gerais, Brazil, 2014; Available online: https://locus.ufv.br//handle/123456789/274 (accessed on 9 August 2024).
- Lavinas, F.C.; Macedo, E.H.B.; Sá, G.B.; Amaral, A.C.F.; Silva, J.R.; Azevedo, M.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S.; et al. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farmacogn. 2019, 29, 389–399. [Google Scholar] [CrossRef]
- Pattanayak, S.P.; Sunita, P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (Lf) Ettingsh. J. Ethnopharmacol. 2008, 120, 241–247. [Google Scholar] [CrossRef]
- Muto, N.A.; de Sousa Leite, R.O.; Pereira, D.S.; Rogez, H.L.G.; Venturieri, G.C. Impact of the Introduction of Stingless Bee Colonies (Scaptotrigona aff. postica) on the Productivity of Acai (Euterpe Oleracea). Rev. Verde De Agroecol. E Desenvolv. Sustentável 2020, 15, 265–273. [Google Scholar] [CrossRef]
- Campbell, A.J.; Da Silva Silva, F.D.; Maués, M.M.; Leão, K.L.; Carvalheiro, L.G.; Moreira, E.F.; Mertens, F.; De Freitas Konrad, M.L.; de Queiroz, J.A.L.; Menezes, C. Forest conservation maximises açaí palm pollination services and yield in the Brazilian Amazon. J. Appl. Ecol. 2023, 60, 1964–1976. [Google Scholar] [CrossRef]
- González, M.; Guzman, B.; Rudyk, R.; Romano, E.; Molina, M.A. Spectrophotometric determination of phenolic compounds in propolis. Lat. Am. J. Pharm. 2003, 22, 243–248. [Google Scholar]
- Ministério da Agricultura, Pecuária e Abastecimento do Brazil, 2001. Instrução Normativa 21/2001. Available online: https://sistemasweb.agricultura.gov.br/sislegis (accessed on 9 August 2024).
- De Funari, C.S. Análise de Própolis da Serra do Japi, Determinação de sua Origem Botânica e Avaliação de sua Contribuição em Processos de Cicatrização. Master’s Thesis, Faculdade de Ciências Farmacêuticas-USP, São Paulo, Brazil, 2005. [Google Scholar] [CrossRef]
- Rodríguez-Arce, E.; Saldías, M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Funari, C.S.; Ferro, V.O. Propolis analysis. Food. Sci. Technol. 2006, 26, 171–178. [Google Scholar] [CrossRef]
- Cabral, I.S.R.; Oldoni, T.L.C.; Prado, A.; Bezerra, R.M.N.; de Alencar, S.M.; Ikegaki, M. Composição fenólica, atividade antibacteriana e antioxidante da própolis vermelha brasileira. Quim. Nova 2009, 32, 1523–1527. [Google Scholar] [CrossRef]
- Singh, M.; Yerramilli, V.; Nagar, L. Antioxidant Assay and GC-MS Profiling of Methanolic Fraction of Parent Plant Parts and Calli of Coccinia grandis L. J. Indian bot. Soc. 2023, 101, 146–155. [Google Scholar] [CrossRef]
- Farooqi, S.S.; Naveed, S.; Qamar, F.; Sana, A.; Farooqi, S.H.; Sabir, N.; Sadia, H. Phytochemical analysis, GC-MS characterization and antioxidant activity of Hordeum vulgare seed extracts. Heliyon 2024, 10, e27297. [Google Scholar] [CrossRef] [PubMed]
- Umar, H.I.; Awonyemi, I.O.; Abegunde, S.M.; Igbe, F.O.; Siraj, B. In silico molecular docking of bioactive molecules isolated from Raphia taedigera seed oil as potential anti-cancer agents targeting vascular endothelial growth factor receptor-2. Chem. Afr. 2021, 4, 161–174. [Google Scholar] [CrossRef]
- Sudaryadi, I.; Oktaweni, F.; Pramono, I.E.; Fatikasary, K.W.; Widiawati, H. Bioactive compound profile of propolis product from Beekeeping (Meliponiculture) in Turi Yogyakarta Indonesia. Conf. Ser. Earth Environ. Sci. 2023, 1200, 012002. [Google Scholar] [CrossRef]
- Vandana; Deora, G.S.; Bano, I.; Deora, V. GC-MS analysis of bioactive compounds from the methanolic leaf extract of Tephrosia villosa (Linn.) pers. an important medicinal plant of Indian Thar desert. Int. J. Botany Stud. 2021, 6, 693–699. [Google Scholar]
- Adeyinka, O.A.; Bankole, I.A.S.; John, R. Gc-Ms Bioprospecting of Phytochemicals in The Ethanolic Extract of Fresh Mangifera indica Leaves. J. Chem. Soc. Niger. 2022, 47, 798–806. [Google Scholar] [CrossRef]
- Aati, H.Y.; Anwar, M.; Al-Qahtani, J.; Al-Taweel, A.; Khan, K.-u.-R.; Aati, S.; Usman, F.; Ghalloo, B.A.; Asif, H.M.; Shirazi, J.H.; et al. Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk: An Unexplored Plant. Antibiotics 2022, 11, 1155. [Google Scholar] [CrossRef]
- Vieira, A.P.; dos Santos, N.R.; Borges, J.H.S.; Vincenzi, M.P.A.; Schmitz, W.O. Ação dos flavonóides na cicatrização por segunda intenção em feridas limpas induzidas cirurgicamente em ratos Wistar. Semin. Ciênc. Biol. Saúde 2008, 29, 65–74. [Google Scholar] [CrossRef][Green Version]
- De Albuquerque-Júnior, R.L.C.; Barreto, A.L.S.; Pires, J.A.; Reis, F.P.; Lima, S.O.; Ribeiro, M.A.G.; Cardoso, J.C. Effect of Bovine Type-I Collagen-Based Films Containing Red Propolis on Dermal Wound Healing in Rodent Model. Int. J. Morphol. 2009, 27, 1105–1110. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardisation dun extrait de propolis et identification desprincipaux constituants. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Bezerra, K.K.S.; Bezerra, A.M.F.; Sousa, L.C.F.S.; da Silva Sousa, J.; da Silva, R.A.; Borges, M.D.G.B. Atividade antimicrobiana da própolis em lesões cutâneas. Agropecuária Científica No Semiárido 2013, 9, 17–23. [Google Scholar] [CrossRef]
- Krupp, T.; Dos Santos, B.D.; Gama, L.A.; Silva, J.R.; Arrais-Silva, W.W.; de Souza, N.C.; Américo, M.F.; Souto, P.C.S. Natural rubber-propolis membrane improves wound healing in a second-degree burning model. Int. J. Biol. Macromol. 2019, 131, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária (ANVISA). Farmacopeia Brasileira Volume 1, 5th ed.; ANVISA: Brasília, Brazil, 2010. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Raventós-Lamuela, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Da Silva, S.B.; Oliveira, A.; Ferreira, D.; Sarmento, B.; Pintado, M. Development and validation method for simultaneous quantification of phenolic compounds in natural extracts and nanosystems. Phytochem. Anal. 2013, 24, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Oksuz, H.; Duran, N.; Tamer, C.; Cetin, M.; Silici, S. Effect of propolis in the treatment of experimental Staphylococcus aureus keratitis in rabbits. Ophthalmic Res. 2005, 37, 328–334. [Google Scholar] [CrossRef]
- Gonçalves, A.R.; Filho, R.A.P. Desenvolvimento e Avaliação In Vitro e In Vivo de Emulsões Contendo Óleo de Canola e Ácidos Carboxílicos. Master’s Thesis, Universidade de São Paulo USP, São Paulo, Brazil, 2000. Available online: https://repositorio.usp.br/item/001321441 (accessed on 9 August 2024).
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic acid and wound healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef]



| Assay | Propolis Hydroethanolic Sample (%) | Coefficient of Variation (%) | Requirement (MAPA) |
|---|---|---|---|
| 1 Total phenol content | 5.04% | 0.60 | Min. 0.25% |
| 2 Flavonoid content | 1.91% | 0.15 | Min. 0.50% |
| Assay | Average | Coefficient of Variation (%) | Requirement (MAPA) |
|---|---|---|---|
| Loss due to desiccation | 7.8% | 0.10 | Max. 8% |
| Ash content | 3.19% | 0.15 | Max. 5% |
| Peak # | RT (min) | Area (%) | Name of Compound | Molecular Formula | MW (g/mol) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 38.307 | 0.28 | 1-heptacosanol | C27H56O | 396 | |
| 2 | 38.728 | 0.32 | heneicosane | C21H44 | 296 | |
| 3 | 41.369 | 0.59 | 1-heptacosanol | C27H56O | 396 | |
| 4 | 45.081 | 0.30 | thunbergol | C20H34O | 290 | |
| 5 | 48.808 | 0.29 | lanosta-8,24-dien-3-one | C30H48O | 424 | |
| 6 | 49.201 | 0.66 | lanosterol | C30H50O | 426 | |
| 7 | 49.293 | 0.28 | ursa-9(11),12-dien-3-one | C30H46O | 422 | |
| 8 | 49.850 | 13.16 | 4,4,6a,6b,8a,11,11,14b-octamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10 | C30H48O | 424 | [16] |
| 9 | 49.980 | 0.31 | stigmasta-5,24(28)-dien-3-ol, (3.beta.,24Z)- | C29H48O | 412 | |
| 10 | 50.086 | 1.01 | 6.beta.Bicyclo [4.3.0]nonane, 5.beta.-iodomethyl-1.beta.-isoprop | C15H25I | 332 | |
| 11 | 50.264 | 7.09 | beta.-Amyrin | C30H50O | 426 | [17] |
| 12 | 50.609 | 32.64 | lup-20(29)-en-3-one | C30H48O | 424 | [18] |
| 13 | 51.039 | 17.21 | lupeol | C30H50O | 426 | [19] |
| 14 | 51.391 | 1.37 | 9,19-cyclolanostan-3-ol, 24-methylene-, (3.beta.)- | C31H52O | 440 | |
| 15 | 51.586 | 2.54 | olean-12-en-3-ol, acetate, (3.beta.)- | C32H52O2 | 468 | [20] |
| 16 | 51.703 | 1.53 | 9,19-cyclolanostan-3-ol, 24-methylene-, (3.beta.)- | C32H52O | 440 | |
| 17 | 51.805 | 0.74 | lup-20(29)-en-3-one | C30H48O | 424 | |
| 18 | 52.367 | 9.93 | lup-20(29)-en-3-ol, acetate, (3.beta.)- | C32H52O | 468 | [21] |
| 19 | 53.224 | 0.85 | humulane-1,6-dien-3-ol | C15H26O | 222 | |
| 20 | 54.075 | 0.63 | 9,19-cyclolanost-25-en-3-ol, 24-methyl-, (3.beta.,24S)- | C31H52O | 440 | |
| 21 | 54.278 | 1.20 | acetic acid, 4,4,6a,6b,8a,11,12,14b-octamethyl-14-oxo-1,2,3,4,4 | C32H50O3 | 482 | |
| 22 | 55.153 | 1.20 | uvaol | C30H50O2 | 442 | |
| 23 | 56.763 | 0.49 | 9,19-cyclolanost-23-ene-3,25-diol, (3.beta.,23E)- | C30H50O2 | 442 | |
| 24 | 62.292 | 2.06 | olean-12-en-3-ol, acetate, (3.beta.)- | C32H52O2 | 468 | [22] |
| 25 | 63.937 | 1.07 | lup-20(29)-en-3-ol, acetate, (3.beta.)- | C32H52O2 | 468 | |
| 26 | 68.741 | 0.69 | octacosyl acetate | C30H60O2 | 452 | |
| 27 | 72.250 | 1.59 | olean-12-en-3-ol, acetate, (3.beta.)- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, S.R.L.; Teixeira, S.A.; Lima, G.O.; de Castro, J.N.R.S.; Teixeira, L.E.O.; Barros, C.A.R.; Pereira, D.S.; Hamoy, M.; Bahia, V.R.L.O.; Muto, N.A. Healing Activity of Propolis of Stingless Bee (Scaptotrigona aff. postica), Reared in Monoculture of Açaí (Euterpe oleracea), in Induced Wounds in Rats. Molecules 2024, 29, 4742. https://doi.org/10.3390/molecules29194742
Ferreira SRL, Teixeira SA, Lima GO, de Castro JNRS, Teixeira LEO, Barros CAR, Pereira DS, Hamoy M, Bahia VRLO, Muto NA. Healing Activity of Propolis of Stingless Bee (Scaptotrigona aff. postica), Reared in Monoculture of Açaí (Euterpe oleracea), in Induced Wounds in Rats. Molecules. 2024; 29(19):4742. https://doi.org/10.3390/molecules29194742
Chicago/Turabian StyleFerreira, Sara R. L., Suzanne A. Teixeira, Gabriella O. Lima, Jhennifer N. R. S. de Castro, Luís E. O. Teixeira, Carlos A. R. Barros, Daniel S. Pereira, Moisés Hamoy, Veronica R. L. O. Bahia, and Nilton A. Muto. 2024. "Healing Activity of Propolis of Stingless Bee (Scaptotrigona aff. postica), Reared in Monoculture of Açaí (Euterpe oleracea), in Induced Wounds in Rats" Molecules 29, no. 19: 4742. https://doi.org/10.3390/molecules29194742
APA StyleFerreira, S. R. L., Teixeira, S. A., Lima, G. O., de Castro, J. N. R. S., Teixeira, L. E. O., Barros, C. A. R., Pereira, D. S., Hamoy, M., Bahia, V. R. L. O., & Muto, N. A. (2024). Healing Activity of Propolis of Stingless Bee (Scaptotrigona aff. postica), Reared in Monoculture of Açaí (Euterpe oleracea), in Induced Wounds in Rats. Molecules, 29(19), 4742. https://doi.org/10.3390/molecules29194742






