Trialkoxysilane Grafting in Alcohols: A Simple Approach towards Modified Silica-Based Materials
Abstract
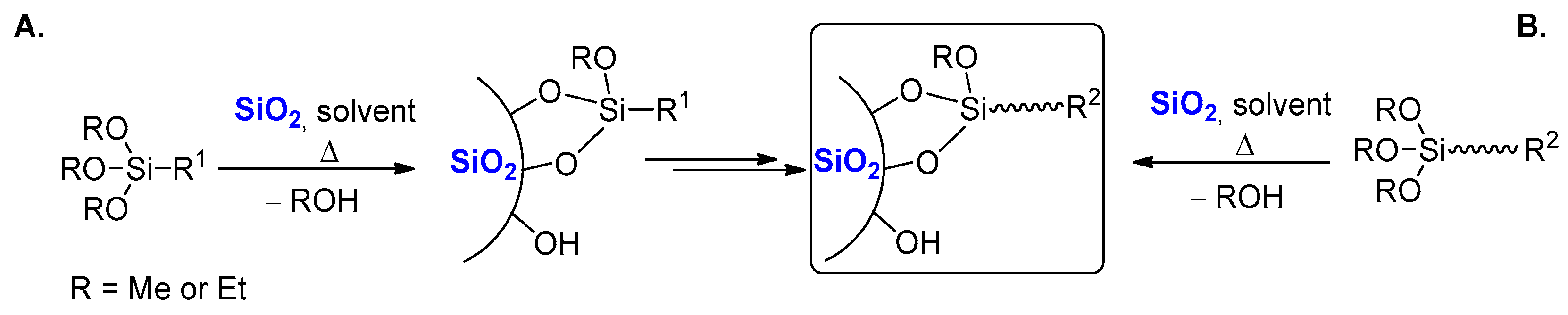
1. Introduction
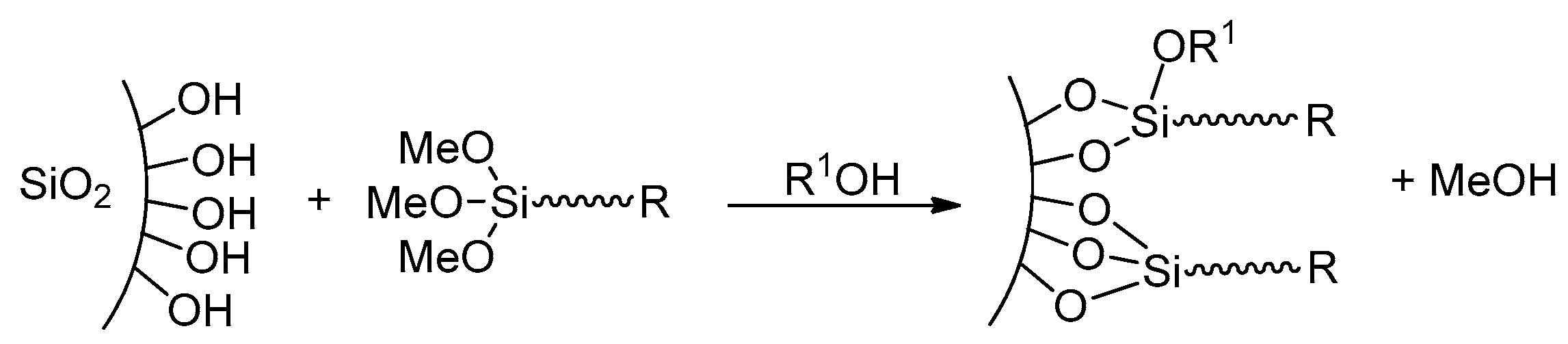
2. Results and Discussion
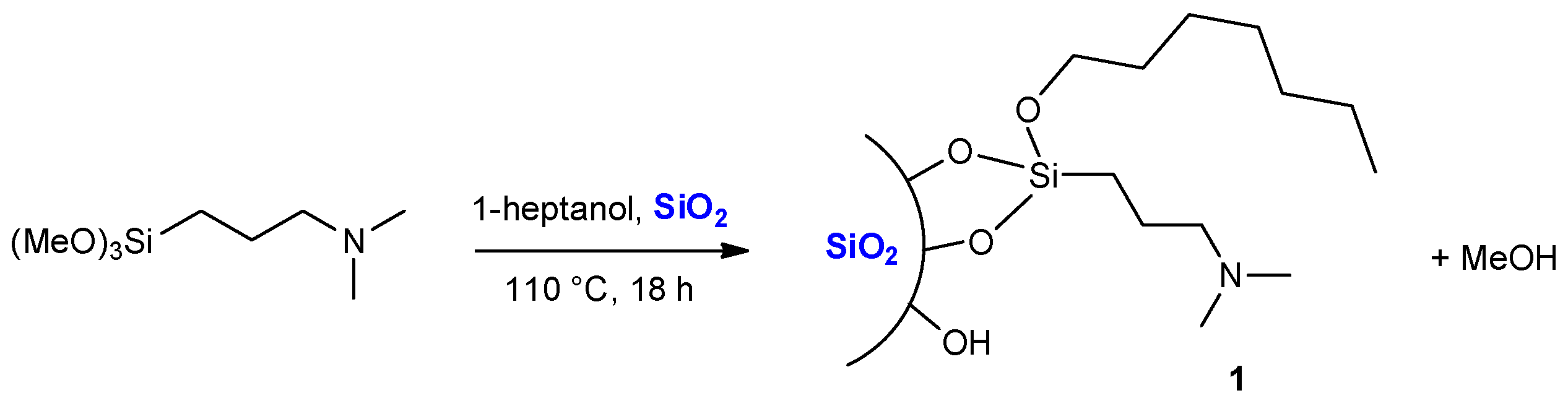
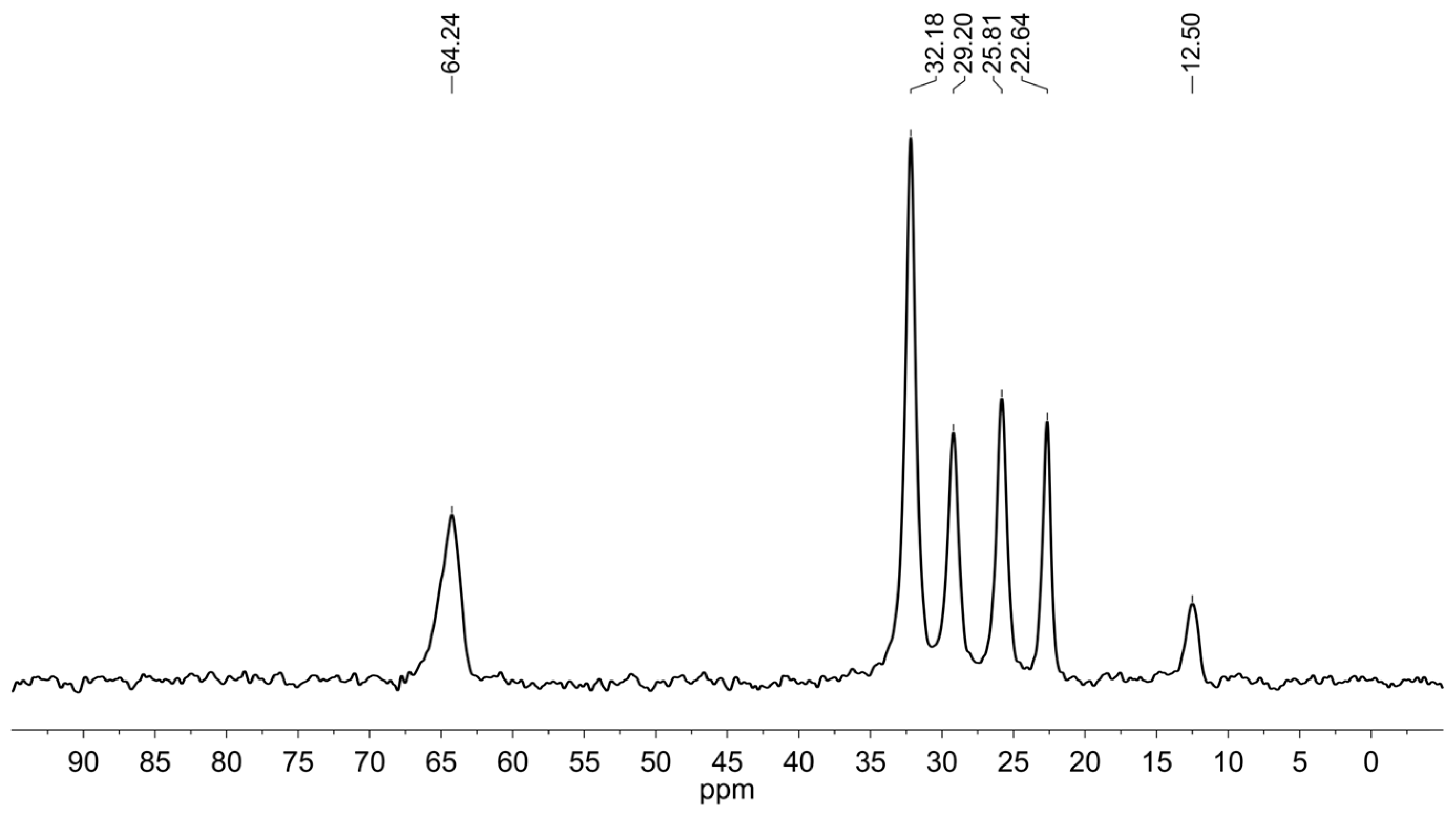
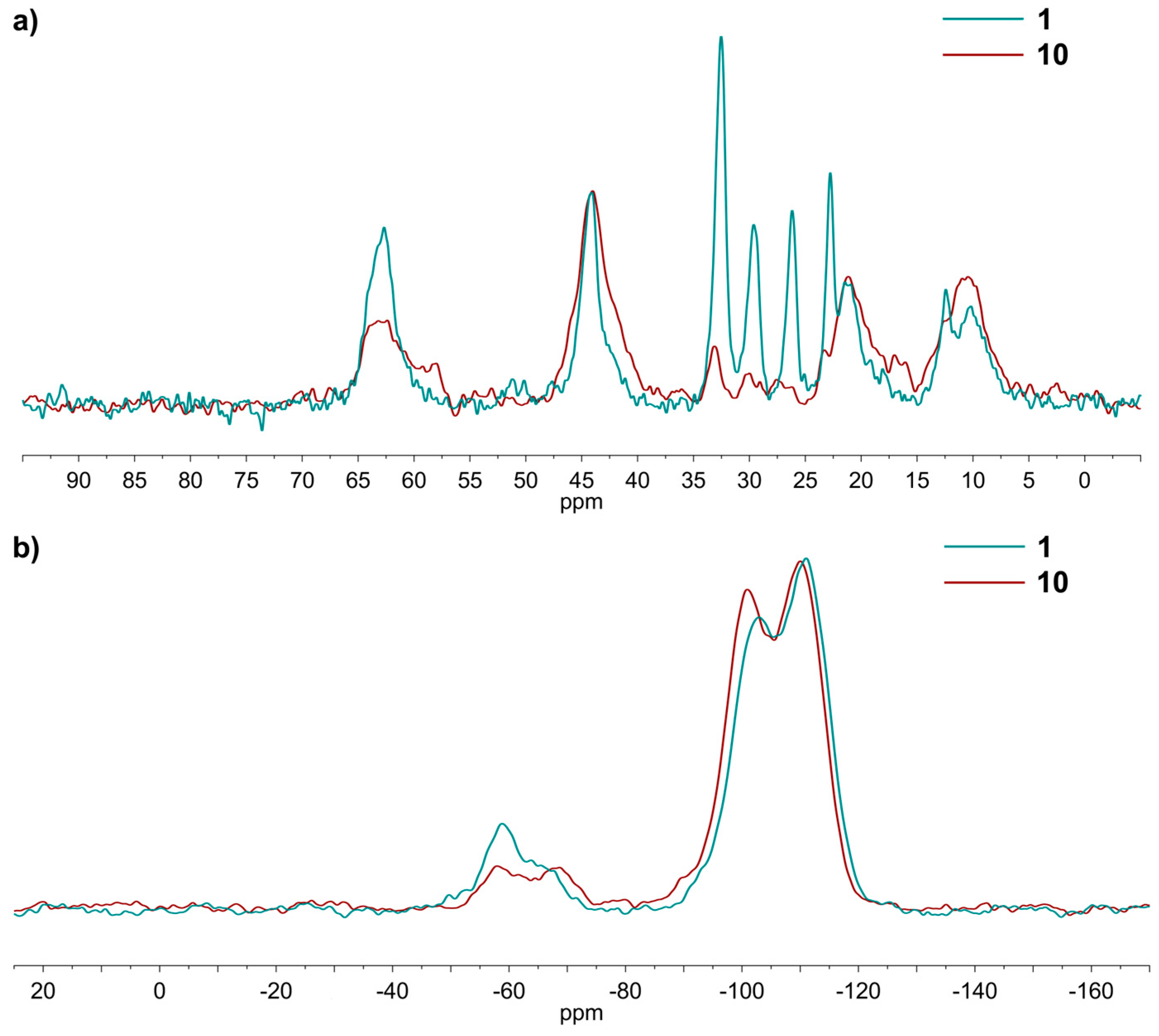
2.1. Grafting of (N,N-dimethyl-3-aminopropyl)trimethoxysilane in Heptanol as a Benchmark Reaction
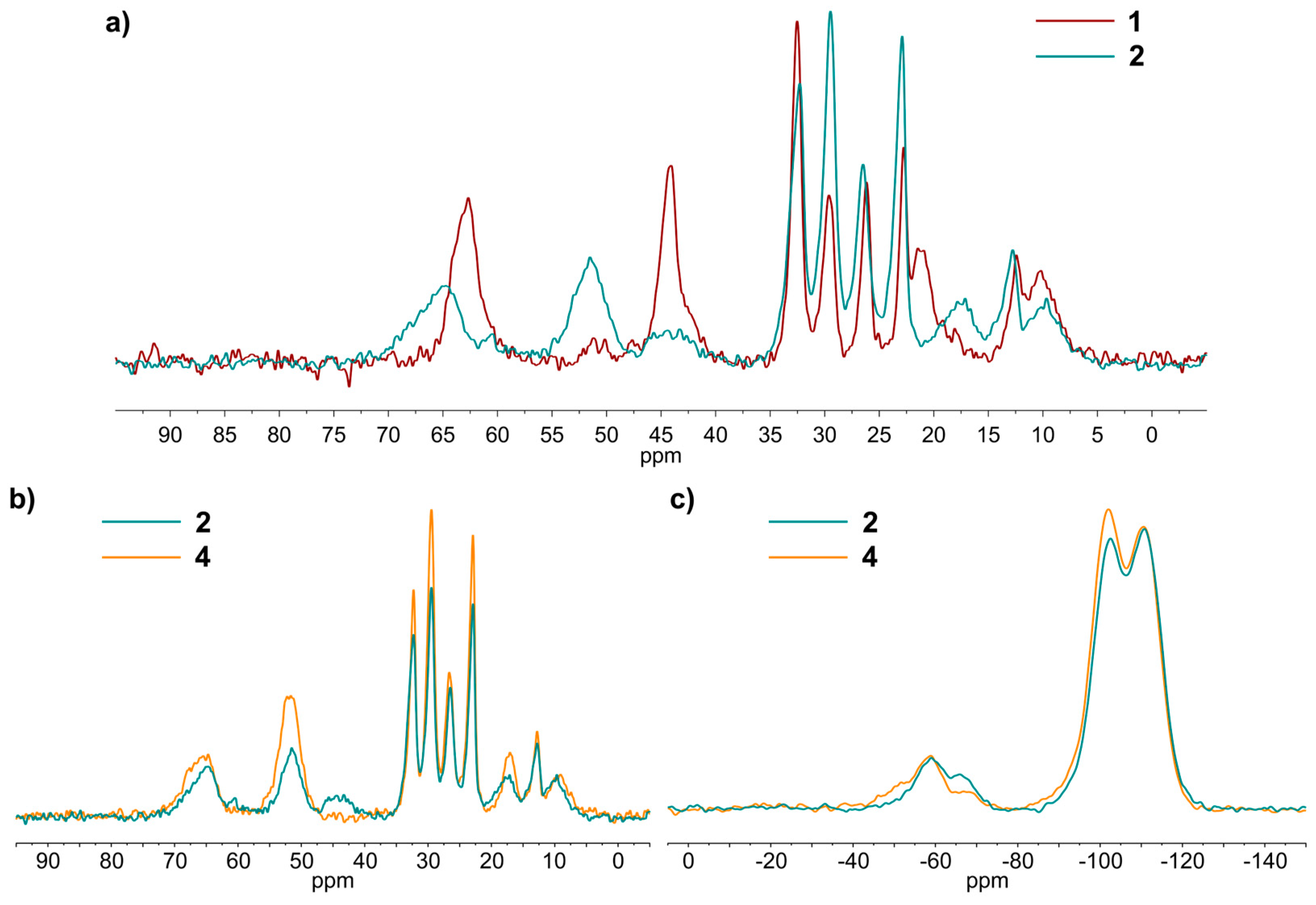
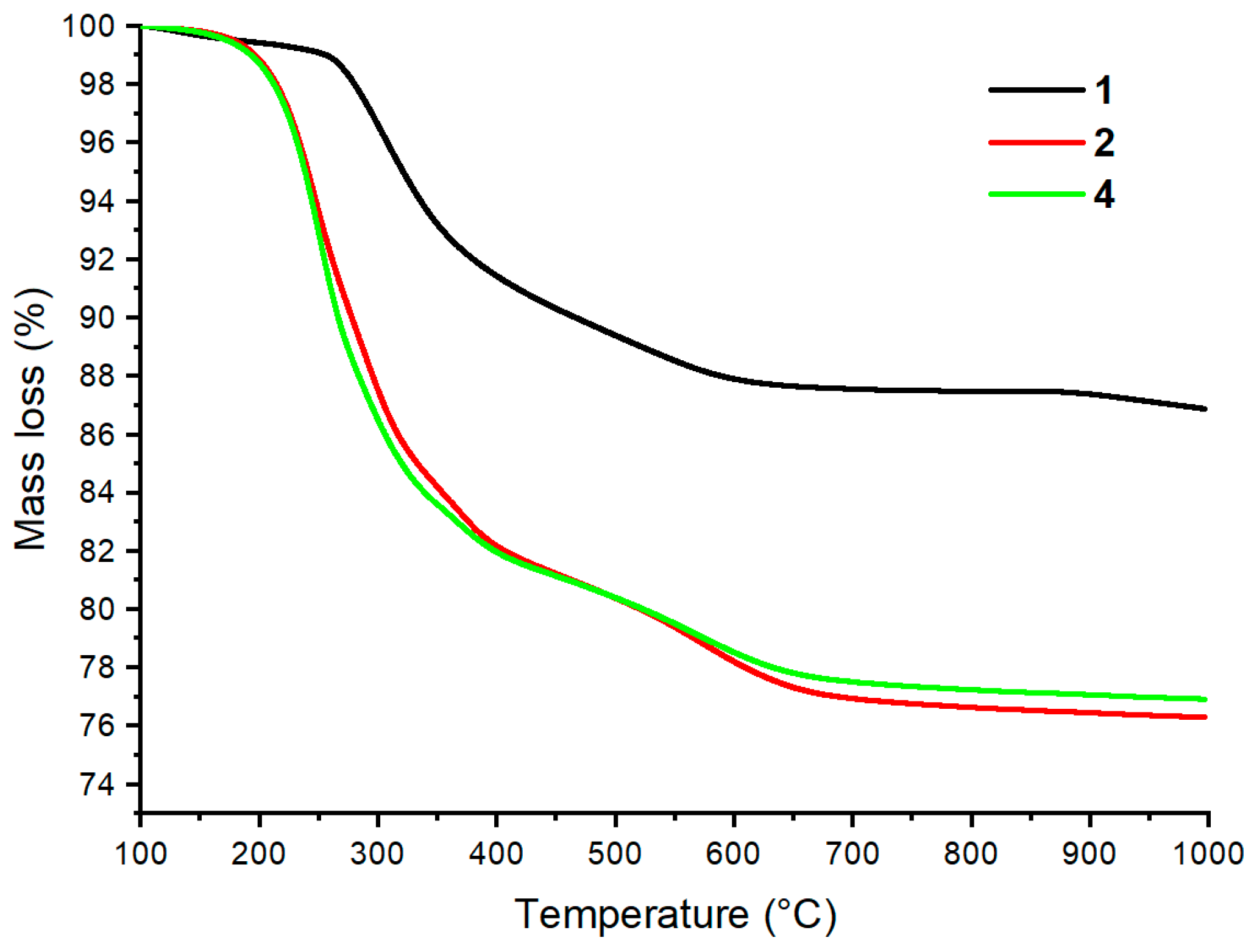
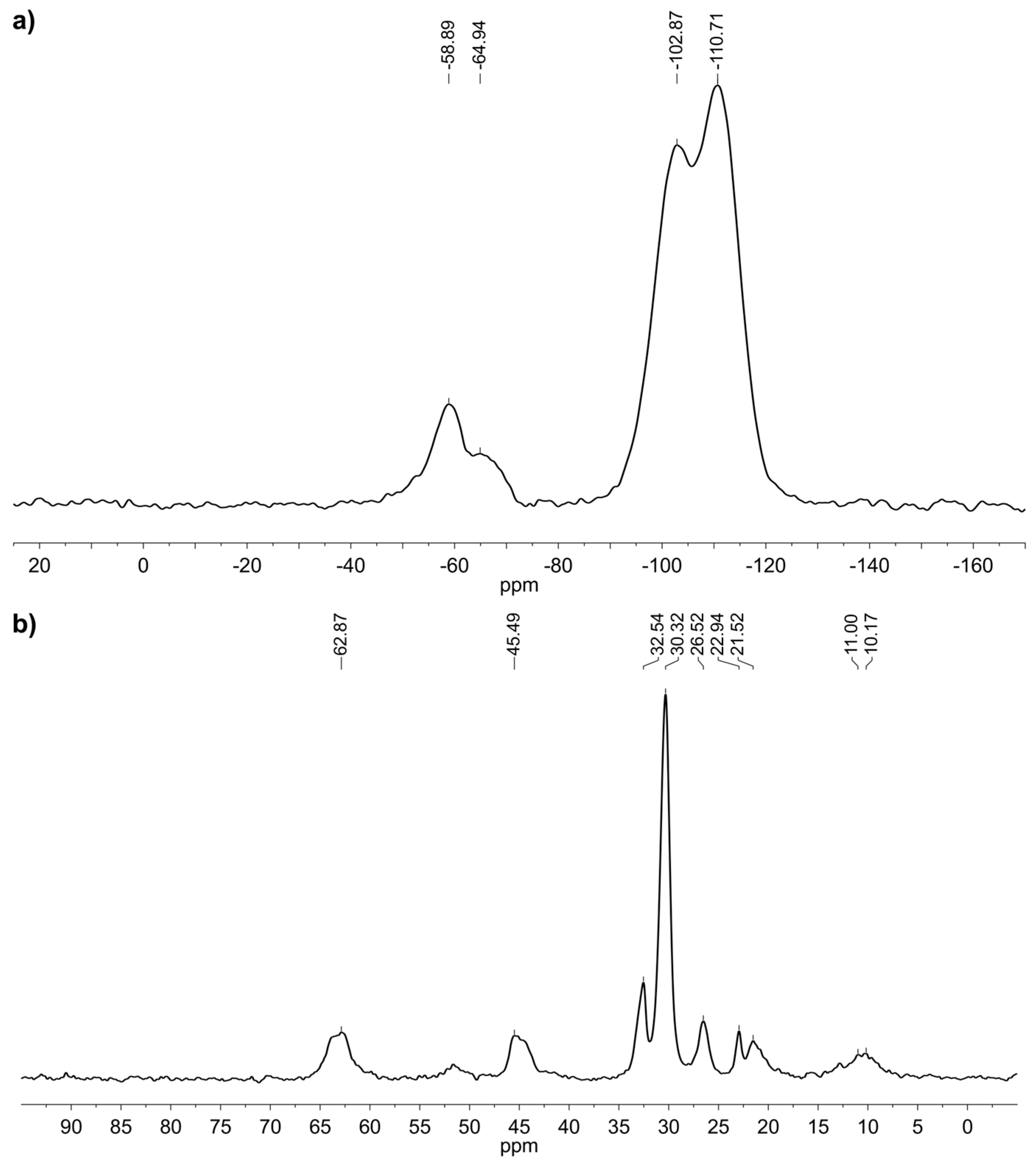
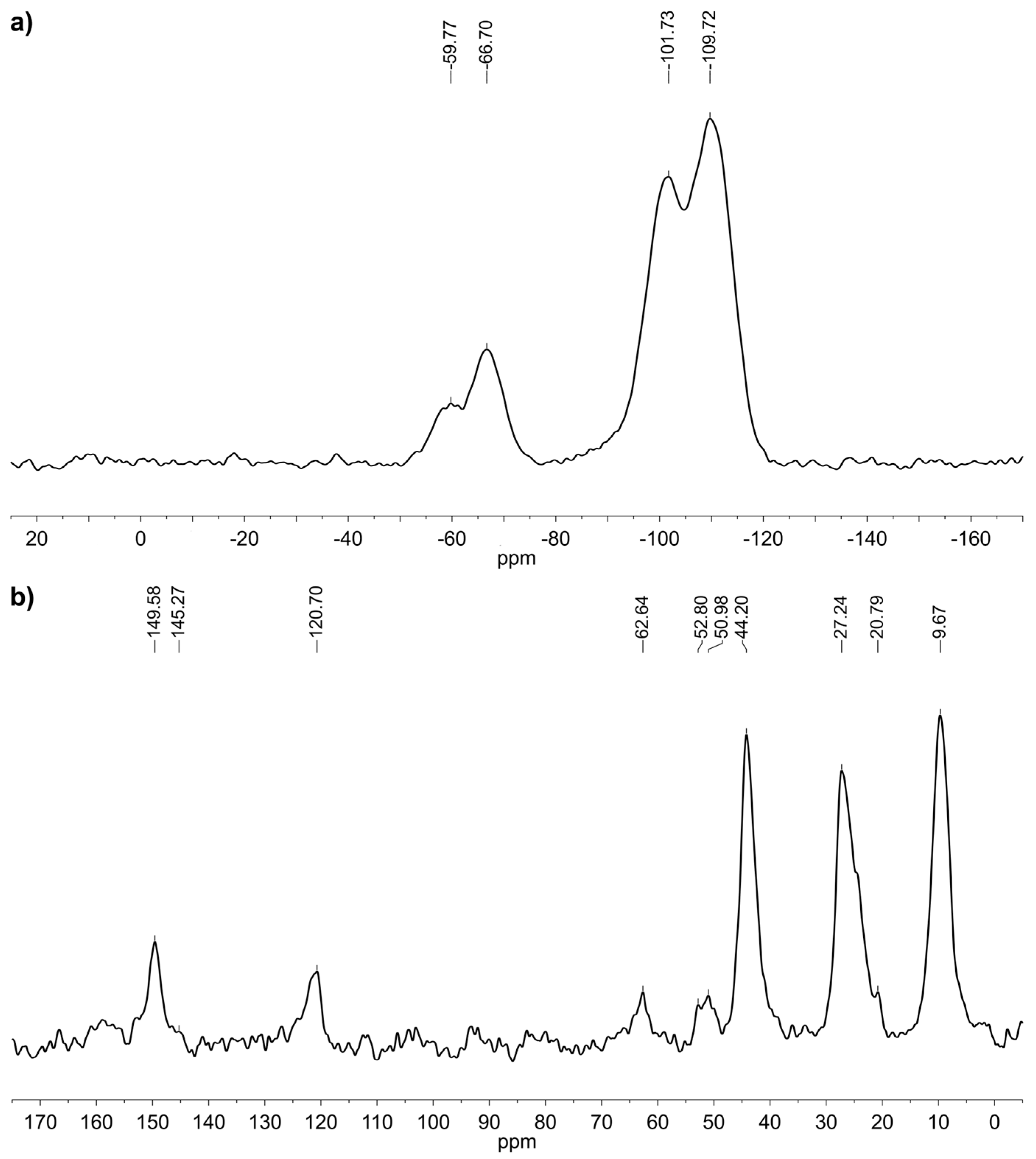
2.2. Grafting of Trialkoxysilanes in the Presence of Different Alcohols
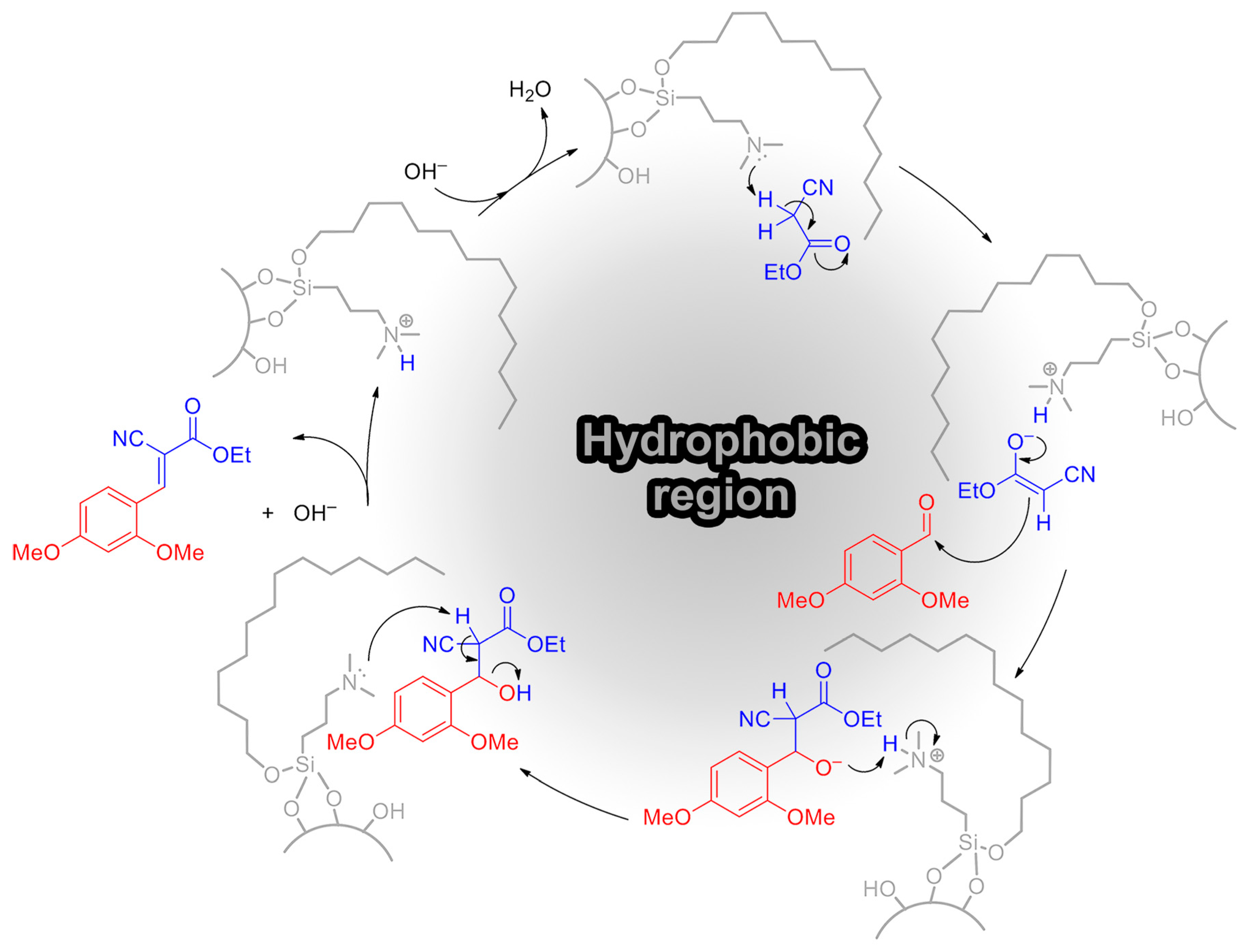
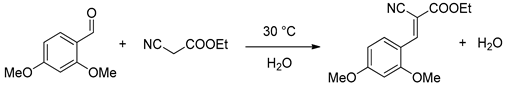
2.3. Catalytic Activity in Knoevenagel Reaction
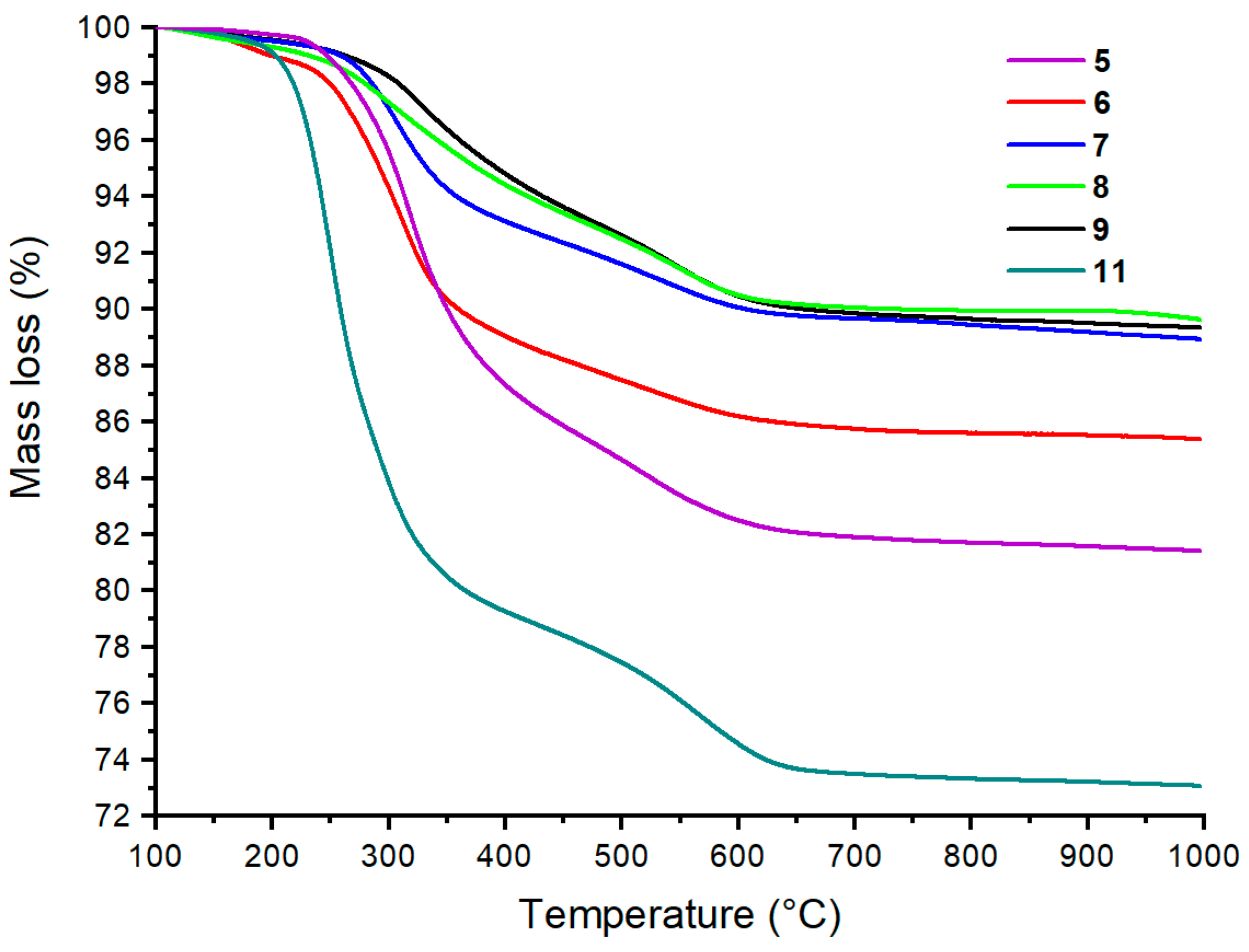
2.4. Dual-Functional Silica-Based Materials for Antifouling Applications
3. Materials and Methods
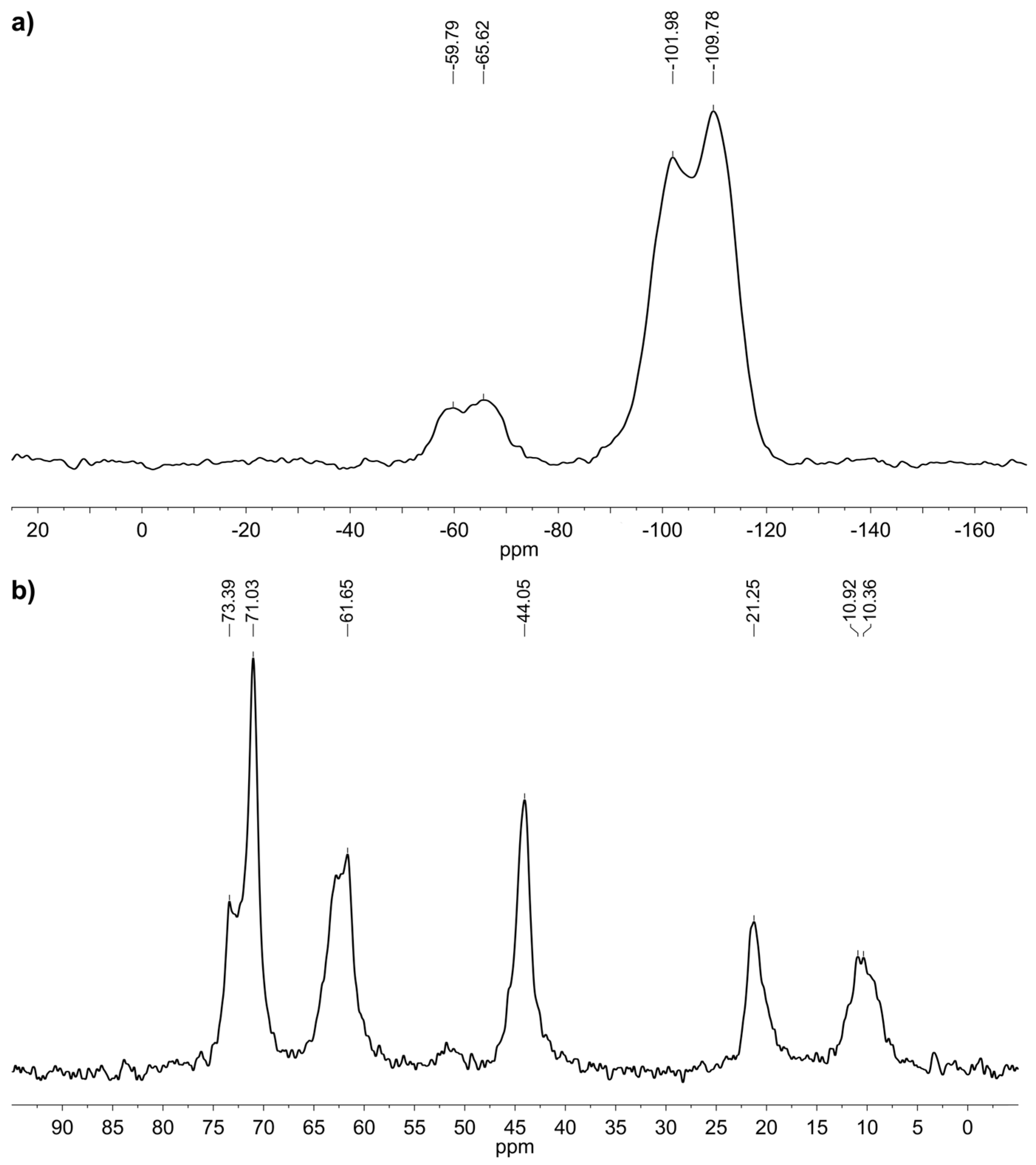
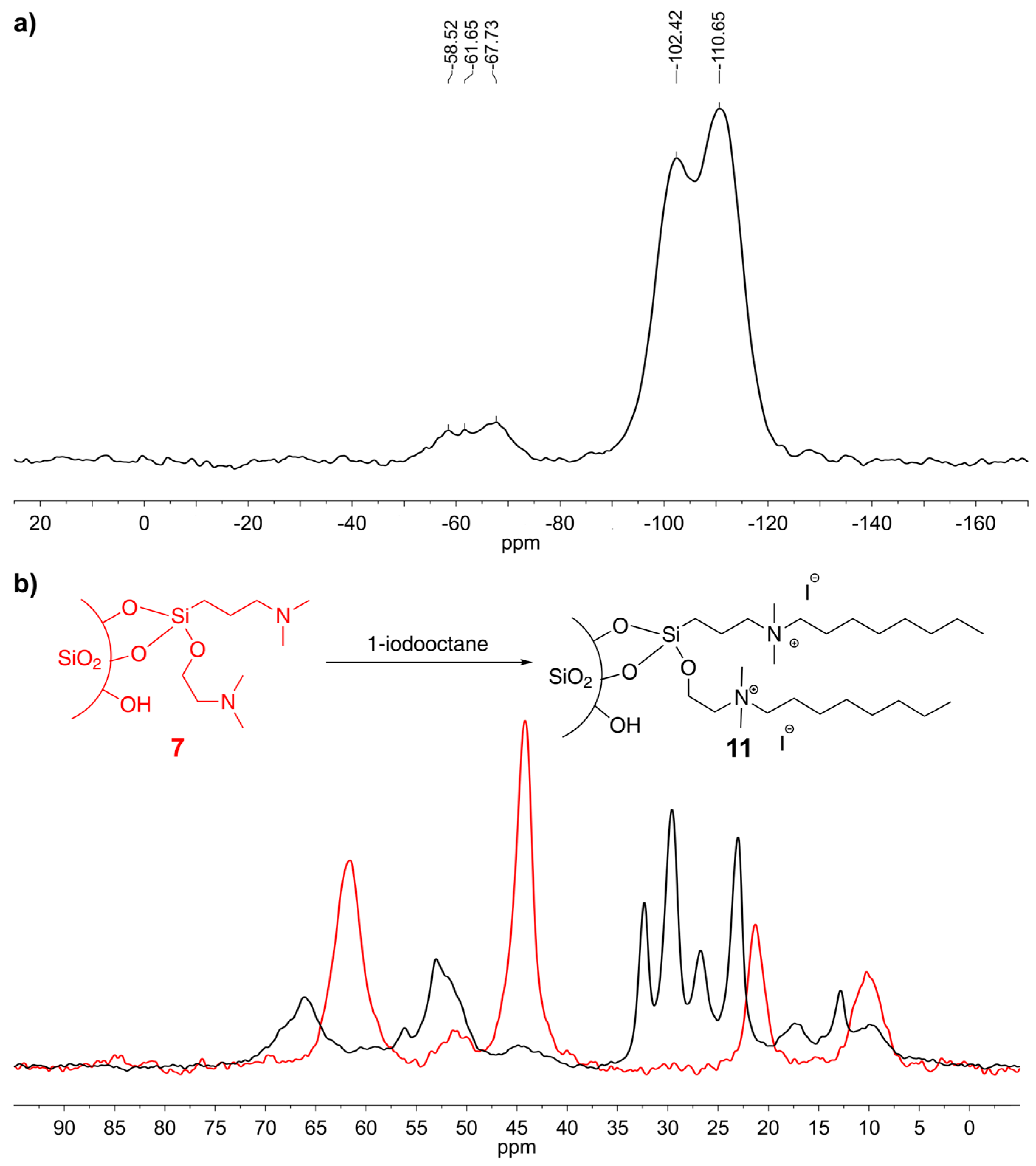
3.1. Spectroscopic and Analytical Methods
3.2. Preparation of Silica 1
3.3. Preparation of Silica 2
3.4. Preparation of Silica 4
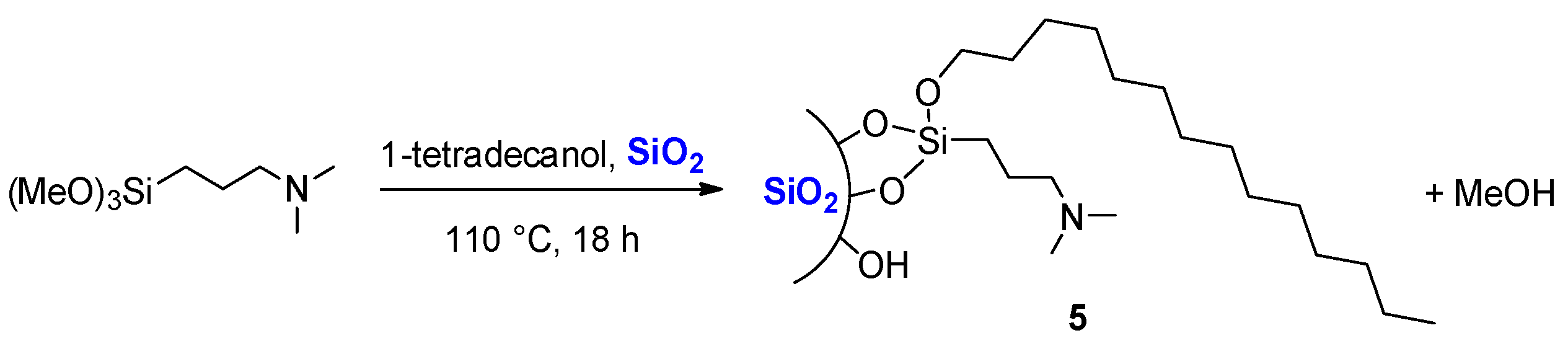
3.5. Preparation of Silica 5
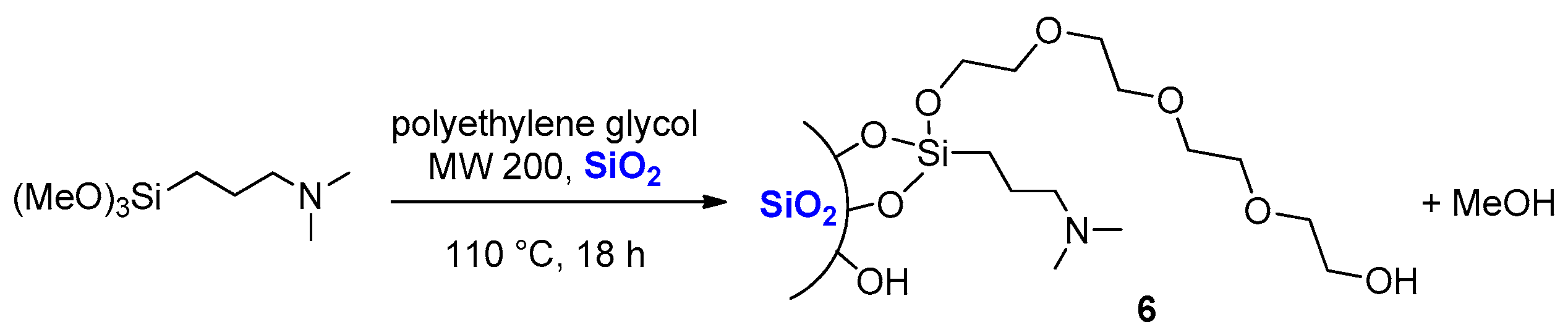
3.6. Preparation of Silica 6
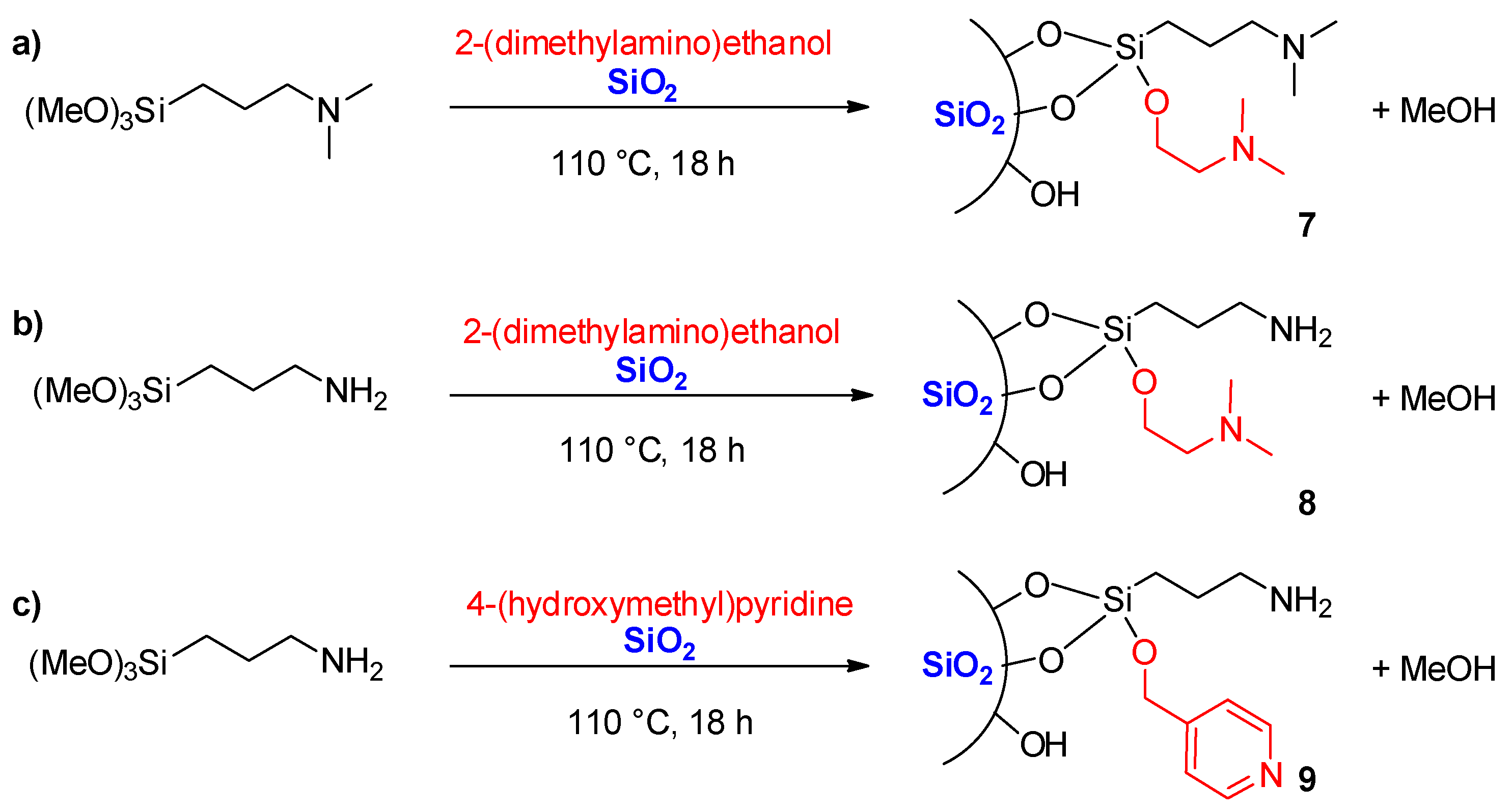
3.7. Preparation of Silica 7
3.8. Preparation of Silica 8
3.9. Preparation of Silica 9
3.10. Preparation of Silica 11
3.11. Typical Knoevenagel Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Zhang, S.; Duan, Y.; Song, X.; Chang, M.; Feng, W.; Chen, Y. Silicon-containing nanomedicine and biomaterials: Materials chemistry, multi-dimensional design, and biomedical application. Chem. Soc. Rev. 2024, 53, 1167–1315. [Google Scholar] [PubMed]
- Costa, J.A.S.; de Jesus, R.A.; Santos, D.O.; Neris, J.B.; Figueiredo, R.T.; Paranhos, C.M. Synthesis, functionalization, and environmental application of silica-based mesoporous materials of the M41S and SBA-n families: A review. J. Environ. Chem. Eng. 2021, 9, 105259. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Benhaim, A.; Itzhaik Alkotzer, Y.; Zelinger, E.; Yaakov, N.; Mechrez, G. In situ Fabrication of Multi-Walled Carbon Nanotubes/Silica Hybrid Colloidosomes by Pickering Emulsion Templating Using Trialkoxysilanes of Opposite Polarity. Polymers 2019, 11, 1480. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Shaily. Synthesis and catalytic applications of organo-functionalized MCM-41 catalyst: A review. Appl. Organomet. Chem. 2023, 37, e7007. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F.; Zaghrioui, M.; Mabkhot, Y.N. Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals. Molecules 2014, 19, 247–262. [Google Scholar] [CrossRef]
- Rath, D.; Rana, S.; Parida, K.M. Organic amine-functionalized silica-based mesoporous materials: An update of syntheses and catalytic applications. RSC Adv. 2014, 4, 57111–57124. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Yang, Z. An excellent universal catalyst support-mesoporous silica: Preparation, modification and applications in energy-related reactions. Int. J. Hydrogen Energy 2022, 47, 9537–9565. [Google Scholar] [CrossRef]
- Yokoi, T.; Kubota, Y.; Tatsumi, T. Amino-functionalized mesoporous silica as base catalyst and adsorbent. Appl. Catal. A Gen. 2012, 421–422, 14–37. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Shi, T.; Yang, H.; Zhang, C.; Qi, W.; Li, C.; Liu, R.; He, W.; Liu, Y. Waterborne robust superhydrophobic PFDTES@TiO2-PU coating with stable corrosion resistance, long-term environmental adaptability, and delayed icing functions on Al–Li alloy. J. Mater. Res. Technol. 2024, 32, 3357–3370. [Google Scholar] [CrossRef]
- Calabrese, C.; Liotta, L.F.; Soumoy, L.; Aprile, C.; Giacalone, F.; Gruttadauria, M. New Hybrid Organic-inorganic Multifunctional Materials Based on Polydopamine-like Chemistry. Asian J. Org. Chem. 2021, 10, 2932–2943. [Google Scholar] [CrossRef]
- Campisciano, V.; Salvo, A.M.P.; Liotta, L.F.; Spinella, A.; Giacalone, F.; Gruttadauria, M. Cross-Linked Polyamine from Imidazolium-Based Materials: A Simple Route to Useful Catalytic Materials. Eur. J. Org. Chem. 2018, 2018, 1352–1358. [Google Scholar] [CrossRef]
- Campisciano, V.; Taormina, B.; Spinella, A.; Liotta, L.F.; Giacalone, F.; Gruttadauria, M. First Evidence of Tris(catecholato)silicate Formation from Hydrolysis of an Alkyl Bis(catecholato)silicate. Molecules 2022, 27, 2521. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Campisciano, V.; Viseras Iborra, C.; Liotta, L.F.; Sánchez-Polo, M.; Riela, S.; Gruttadauria, M. New Mussel Inspired Polydopamine-Like Silica-Based Material for Dye Adsorption. Nanomaterials 2020, 10, 1416. [Google Scholar] [CrossRef]
- Presentato, A.; La Greca, E.; Consentino, L.; Alduina, R.; Liotta, L.F.; Gruttadauria, M. Antifouling Systems Based on a Polyhedral Oligomeric Silsesquioxane-Based Hexyl Imidazolium Salt Adsorbed on Copper Nanoparticles Supported on Titania. Nanomaterials 2023, 13, 1291. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Schlecht, C.A.; Maurer, J.A. Functionalization of glass substrates: Mechanistic insights into the surface reaction of trialkoxysilanes. RSC Adv. 2011, 1, 1446–1448. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green. Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M.; Dong, W.; Le, Z.; Zhang, D.; Henderson, I.M. A 29Si, 1H, and 13C Solid-State NMR Study on the Surface Species of Various Depolymerized Organosiloxanes at Silica Surface. Nanoscale Res. Lett. 2019, 14, 160. [Google Scholar] [CrossRef]
- Cui, J.; Chatterjee, P.; Slowing, I.I.; Kobayashi, T. In Situ 29Si solid-state NMR study of grafting of organoalkoxysilanes to mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2022, 339, 112019. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Mossuto Marculescu, A.; Lo Meo, P.; Riela, S.; Noto, R. Hydrophobically Directed Aldol Reactions: Polystyrene-Supported L-Proline as a Recyclable Catalyst for Direct Asymmetric Aldol Reactions in the Presence of Water. Eur. J. Org. Chem. 2007, 2007, 4688–4698. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M. Water in Organocatalytic Reactions. In Comprehensive Enantioselective Organocatalysis; Wiley-VCH: Weinheim, Germany, 2013; pp. 673–717. [Google Scholar]
- Hyde, J.F. Silanol Derivatives of the Dimethyl Substituted Organosilicon Compounds. JACS 1953, 75, 2166–2167. [Google Scholar] [CrossRef]
- Kantor, S.W. The Hydrolysis of Methoxysilanes. Dimethylsilanediol. JACS 1953, 75, 2712–2714. [Google Scholar] [CrossRef]
- Marzullo, P.; Gruttadauria, M.; D’Anna, F. Quaternary Ammonium Salts-Based Materials: A Review on Environmental Toxicity, Anti-Fouling Mechanisms and Applications in Marine and Water Treatment Industries. Biomolecules 2024, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Siopa, F.; Figueiredo, T.; Frade, R.F.M.; Neto, I.; Meirinhos, A.; Reis, C.P.; Sobral, R.G.; Afonso, C.A.M.; Rijo, P. Choline-Based Ionic Liquids: Improvement of Antimicrobial Activity. ChemistrySelect 2016, 1, 5909–5916. [Google Scholar] [CrossRef]
- Kang, J.-K.; Kim, S.-B. Synthesis of quaternized mesoporous silica SBA-15 with different alkyl chain lengths for selective nitrate removal from aqueous solutions. Microporous Mesoporous Mater. 2020, 295, 109967. [Google Scholar] [CrossRef]






 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Cat. Amount (mg) | Time (h) | Water (mL) | Yield (%) |
| entry 1 | 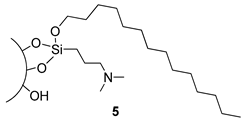 | 32 | 1 | 1 | >99 |
| entry 2 | 15 | 2 | 1 | 95 | |
| entry 3 | 15 | 2 | 3 | 61 | |
| entry 4 | 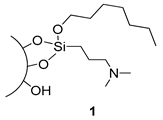 | 15 | 2 | 1 | 49 |
| entry 5 |  | 33.5 | 1 | 1 | 59 |
| entry 6 | 16 | 2 | 1 | 24 | |
| entry 7 | 16 | 2 | 3 | 28 | |
| entry 8 |  | 15 | 2 | 1 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzullo, P.; Campisciano, V.; Liotta, L.F.; D’Anna, F.; Giacalone, F.; Gruttadauria, M. Trialkoxysilane Grafting in Alcohols: A Simple Approach towards Modified Silica-Based Materials. Molecules 2024, 29, 4730. https://doi.org/10.3390/molecules29194730
Marzullo P, Campisciano V, Liotta LF, D’Anna F, Giacalone F, Gruttadauria M. Trialkoxysilane Grafting in Alcohols: A Simple Approach towards Modified Silica-Based Materials. Molecules. 2024; 29(19):4730. https://doi.org/10.3390/molecules29194730
Chicago/Turabian StyleMarzullo, Paola, Vincenzo Campisciano, Leonarda Francesca Liotta, Francesca D’Anna, Francesco Giacalone, and Michelangelo Gruttadauria. 2024. "Trialkoxysilane Grafting in Alcohols: A Simple Approach towards Modified Silica-Based Materials" Molecules 29, no. 19: 4730. https://doi.org/10.3390/molecules29194730
APA StyleMarzullo, P., Campisciano, V., Liotta, L. F., D’Anna, F., Giacalone, F., & Gruttadauria, M. (2024). Trialkoxysilane Grafting in Alcohols: A Simple Approach towards Modified Silica-Based Materials. Molecules, 29(19), 4730. https://doi.org/10.3390/molecules29194730










