Effect of Roasting Level on the Development of Key Aroma-Active Compounds in Coffee
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Volatile Compounds
2.2. Principal Component Analysis (PCA)
2.3. Heat Map
3. Materials and Methods
3.1. Sample
3.2. Drying, Hulling, Roasting, and Milling of Coffee
3.3. Extraction of Volatile Compounds by SPME
3.4. Identification of Compounds by GC-MS
3.5. Identification of Aroma-Active Compounds
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bröhan, M.; Huybrighs, T.; Wouters, C.; Van der Bruggen, B. Influence of storage conditions on aroma compounds in coffee pads using static headspace GC–MS. Food Chem. 2009, 116, 480–483. [Google Scholar] [CrossRef]
- López-Galilea, I.; Fournier, N.; Cid, C.; Guichard, E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J. Agric. Food Chem. 2006, 54, 8560–8566. [Google Scholar] [CrossRef] [PubMed]
- Amalia, F.; Aditiawati, P.; Yusianto; Putri, S.P.; Fukusaki, E. Gas chromatography/mass spectrometry-based metabolite profiling of coffee beans obtained from different altitudes and origins with various postharvest processing. Metabolomics 2021, 17, 69. [Google Scholar] [CrossRef]
- Angeloni, S.; Mustafa, A.M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E. Characterization of the aroma profile and main key odorants of espresso coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Linforth, R.; Fisk, I.D.; Yang, N. Modifying Robusta coffee aroma by green bean chemical pre-treatment. Food Chem. 2019, 272, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bolka, M.; Emire, S. Effects of coffee roasting technologies on cup quality and bioactive compounds of specialty coffee beans. Food Sci. Nutr. 2020, 8, 6120–6130. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.L.; Debona, D.G.; Pinheiro, P.F.; de Oliveira, G.F.; ten Caten, C.S.; Moksunova, V.; Kopanina, A.V.; Vlasova, I.I.; Talskikh, A.I.; Yamamoto, H. Roasting process. In Quality Determinants in Coffee Production; Springer: Berlin/Heidelberg, Germany, 2020; pp. 303–372. [Google Scholar]
- Domingues, D.S.; Pauli, E.D.; de Abreu, J.E.; Massura, F.W.; Cristiano, V.; Santos, M.J.; Nixdorf, S.L. Detection of roasted and ground coffee adulteration by HPLC by amperometric and by post-column derivatization UV–Vis detection. Food Chem. 2014, 146, 353–362. [Google Scholar] [CrossRef]
- Figueroa, J.; Vargas, L. Evaluation of SDE, SFE and SPME/GC-MS for extraction and determination of aroma compounds from Vilcabamba—Ecuadorian roasted coffee. Química Nova 2016, 39, 712–719. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ito, O.; Ishizaki, S.; Kurobayashi, Y.; Fujita, A. Solid-phase aroma concentrate extraction (SPACE™): A new headspace technique for more sensitive analysis of volatiles. Flavour Fragr. J. 2004, 19, 183–187. [Google Scholar] [CrossRef]
- Sarrazin, C.; Le Quéré, J.-L.; Gretsch, C.; Liardon, R. Representativeness of coffee aroma extracts: A comparison of different extraction methods. Food Chem. 2000, 70, 99–106. [Google Scholar] [CrossRef]
- Petisca, C.; Pérez-Palacios, T.; Farah, A.; Pinho, O.; Ferreira, I.M. Furans and other volatile compounds in ground roasted and espresso coffee using headspace solid-phase microextraction: Effect of roasting speed. Food Bioprod. Process. 2013, 91, 233–241. [Google Scholar] [CrossRef]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef]
- Risticevic, S.; Carasek, E.; Pawliszyn, J. Headspace solid-phase microextraction-gas chromatographic-time-of-flight mass spectrometric methodology for geographical origin verification of coffee. Anal. Chim. Acta 2008, 617, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Čajka, T.; Hajšlová, J.; Cochran, J.; Holadová, K.; Klimánková, E. Solid phase microextraction-comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry for the analysis of honey volatiles. J. Sep. Sci. 2007, 30, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Zhuang, H.; Ye, R.; Jin, Z.; Xu, X.; Xie, Z. Analysis of volatile compounds of Mesona Blumes gum/rice extrudates via GC–MS and electronic nose. Sens. Actuators B 2011, 160, 964–973. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. J. Agric. Food Chem. 2009, 57, 5823–5831. [Google Scholar] [CrossRef] [PubMed]
- Blank, I. Gas chromatography–olfactometry in food aroma analysis. In Techniques for Analyzing; CRC Press: Boca Raton, FL, USA, 2020; pp. 293–329. [Google Scholar]
- Buffo, R.A.; Cardelli-Freire, C. Coffee flavour: An overview. Flavour Fragr. J. 2004, 19, 99–104. [Google Scholar] [CrossRef]
- de Fatima Alpendurada, M. Solid-phase microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.A.; Klensporf-Pawlik, D.; Dziadas, M.; Jeleń, H.H. Identification of aroma active compounds of cereal coffee brew and its roasted ingredients. J. Agric. Food Chem. 2013, 61, 2648–2654. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Czerny, M.; Grosch, W. Sensory study of the character impact aroma compounds of a coffee beverage. Eur. Food Res. Technol. 2000, 211, 272–276. [Google Scholar] [CrossRef]
- Blank, I.; Sen, A.; Grosch, W. Potent odorants of the roasted powder and brew of Arabica coffee. Z Leb. Unters. Forsch 1992, 195, 239–245. [Google Scholar] [CrossRef]
- Hong, S.J.; Boo, C.G.; Yoon, S.; Jeong, H.; Jo, S.M.; Youn, M.Y.; Kim, J.K.; Kim, Y.J.; Shin, E.-C. Impact of roasting conditions on physicochemical, taste, volatile, and odor-active compound profiles of Coffea arabica L. (cv. Yellow Bourbon) using electronic sensors and GC–MS-O using a multivariate approach. Food Chem. X 2024, 21, 101119. [Google Scholar] [CrossRef] [PubMed]
- Tatsuka, K.; Suekane, S.; Sakai, Y.; Sumitani, H. Volatile constituents of kiwi fruit flowers: Simultaneous distillation and extraction versus headspace sampling. J. Agric. Food Chem. 1990, 38, 2176–2180. [Google Scholar] [CrossRef]
- Takeoka, G.; Perrino, C.; Buttery, R. Volatile constituents of used frying oils. J. Agric. Food Chem. 1996, 44, 654–660. [Google Scholar] [CrossRef]
- Kim, J.S. Einfluss der Temperatur beim Rösten von Sesam auf Aroma und antioxidative Eigenschaften des Öls. Ph.D. Thesis, TU Berlin, Berlin, Germany, 2001. [Google Scholar]
- Shimoda, M.; Shiratsuchi, H.; Nakada, Y.; Wu, Y.; Osajima, Y. Identification and sensory characterization of volatile flavor compounds in sesame seed oil. J. Agric. Food Chem. 1996, 44, 3909–3912. [Google Scholar] [CrossRef]
- Shimoda, M.; Shibamoto, T. Isolation and identification of headspace volatiles from brewed coffee with an on-column GC/MS method. J. Agric. Food Chem. 1990, 38, 802–804. [Google Scholar] [CrossRef]
- Yeo, H.C.; Shibamoto, T. Microwave-induced volatiles of the Maillard model system under different pH conditions. J. Agric. Food Chem. 1991, 39, 370–373. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Majcher, M.; Pikul, J.; Bielińska, S.; Czauderna, M.; Wójtowski, J. The effect of Camelina sativa cake diet supplementation on sensory and volatile profiles of ewe’s milk. Afr. J. Biotechnol. 2011, 10, 7245–7252. [Google Scholar]
- Paravisini, L.; Gourrat-Pernin, K.; Gouttefangeas, C.; Moretton, C.; Nigay, H.; Dacremont, C.; Guichard, E. Identification of compounds responsible for the odorant properties of aromatic caramel. Flavour Fragr. J. 2012, 27, 424–432. [Google Scholar] [CrossRef]
- Blagojević, P.; Radulović, N.; Palić, R.; Stojanović, G. Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J. Agric. Food Chem. 2006, 54, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Palá-Paúl, J.; Copeland, L.M.; Brophy, J.J.; Goldsack, R.J. Essential oil composition of two variants of Prostanthera lasianthos Labill. from Australia. Biochem. Syst. Ecol. 2006, 34, 48–55. [Google Scholar] [CrossRef]
- Shimoda, M.; Shigematsu, H.; Shiratsuchi, H.; Osajima, Y. Comparison of the odor concentrates by SDE and adsorptive column method from green tea infusion. J. Agric. Food Chem. 1995, 43, 1616–1620. [Google Scholar] [CrossRef]
- Ames, J.M.; Guy, R.C.; Kipping, G.J. Effect of pH and temperature on the formation of volatile compounds in cysteine/reducing sugar/starch mixtures during extrusion cooking. J. Agric. Food Chem. 2001, 49, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Suriyaphan, O.; Cadwallader, K. Aroma components of cooked tail meat of American lobster (Homarus americanus). J. Agric. Food Chem. 2001, 49, 4324–4332. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.; Kowalczyk, A.; Coroneo, V.; Russo, M.T.; Dessì, S.; Cabras, P. Chemical composition and antioxidant, antimicrobial, and antifungal activities of the essential oil of Achillea ligustica All. J. Agric. Food Chem. 2005, 53, 10148–10153. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Macku, C.; Shibamoto, T. Isolation and identification of headspace volatiles formed in heated butter. J. Agric. Food Chem. 1991, 39, 1972–1975. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Identification of potent odorants in Japanese green tea (Sen-cha). J. Agric. Food Chem. 1999, 47, 5169–5172. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, J.; Song, H.; Zou, T.; Liu, Y.; Zhang, S. Formation mechanism of volatile and non-volatile compounds in peptide–xylose Maillard reaction. Food Res. Int. 2013, 54, 683–690. [Google Scholar] [CrossRef]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Identification of potent odourants in wine and brewed coffee using gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2011, 1218, 7487–7498. [Google Scholar] [CrossRef] [PubMed]
- Ayseli, M.T.; Kelebek, H.; Selli, S. Elucidation of aroma-active compounds and chlorogenic acids of Turkish coffee brewed from medium and dark roasted Coffea arabica beans. Food Chem. 2021, 338, 127821. [Google Scholar] [CrossRef] [PubMed]
- Flament, I. Coffee Flavor Chemistry; Wiley: Hoboken, NJ, USA, 2002; p. 424. [Google Scholar]
- Edwards, W., Jr.; Reeves, L.H. Furfuryl Formate. J. Am. Chem. Soc. 1942, 64, 1583–1584. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.-P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on the volatile fraction of coffee beans: I. Green coffee. J. Food Compos. Anal. 2007, 20, 289–296. [Google Scholar] [CrossRef]
- Nkondjock, A. Coffee consumption and the risk of cancer: An overview. Cancer Lett. 2009, 277, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Aguilar, G.; Bompard, E.; Rafinon, A.; Anthony, F. Comportement agronomique et résistance aux principaux déprédateurs des lignées de Sarchimor et Catimor (Coffea arabica L.) au Costa Rica. Plant. Rech. Dev. 1997, 5, 312–321. [Google Scholar]
- SCA. Coffee Standards; SCA: Santa Ana, CA, USA, 2018. [Google Scholar]
- Franca, A.S.; Oliveira, L.S.; Oliveira, R.C.; Agresti, P.C.M.; Augusti, R. A preliminary evaluation of the effect of processing temperature on coffee roasting degree assessment. J. Food Eng. 2009, 92, 345–352. [Google Scholar] [CrossRef]
- Li, X.; Zeng, X.; Song, H.; Xi, Y.; Li, Y.; Hui, B.; Li, H.; Li, J. Characterization of the aroma profiles of cold and hot break tomato pastes by GC-O-MS, GC × GC-O-TOF-MS, and GC-IMS. Food Chem. 2023, 405, 134823. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the volatile compounds in fresh and thermally treated watermelon juice via headspace-gas chromatography-ion mobility spectrometry and comprehensive two-dimensional gas chromatography-olfactory-mass spectrometry analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
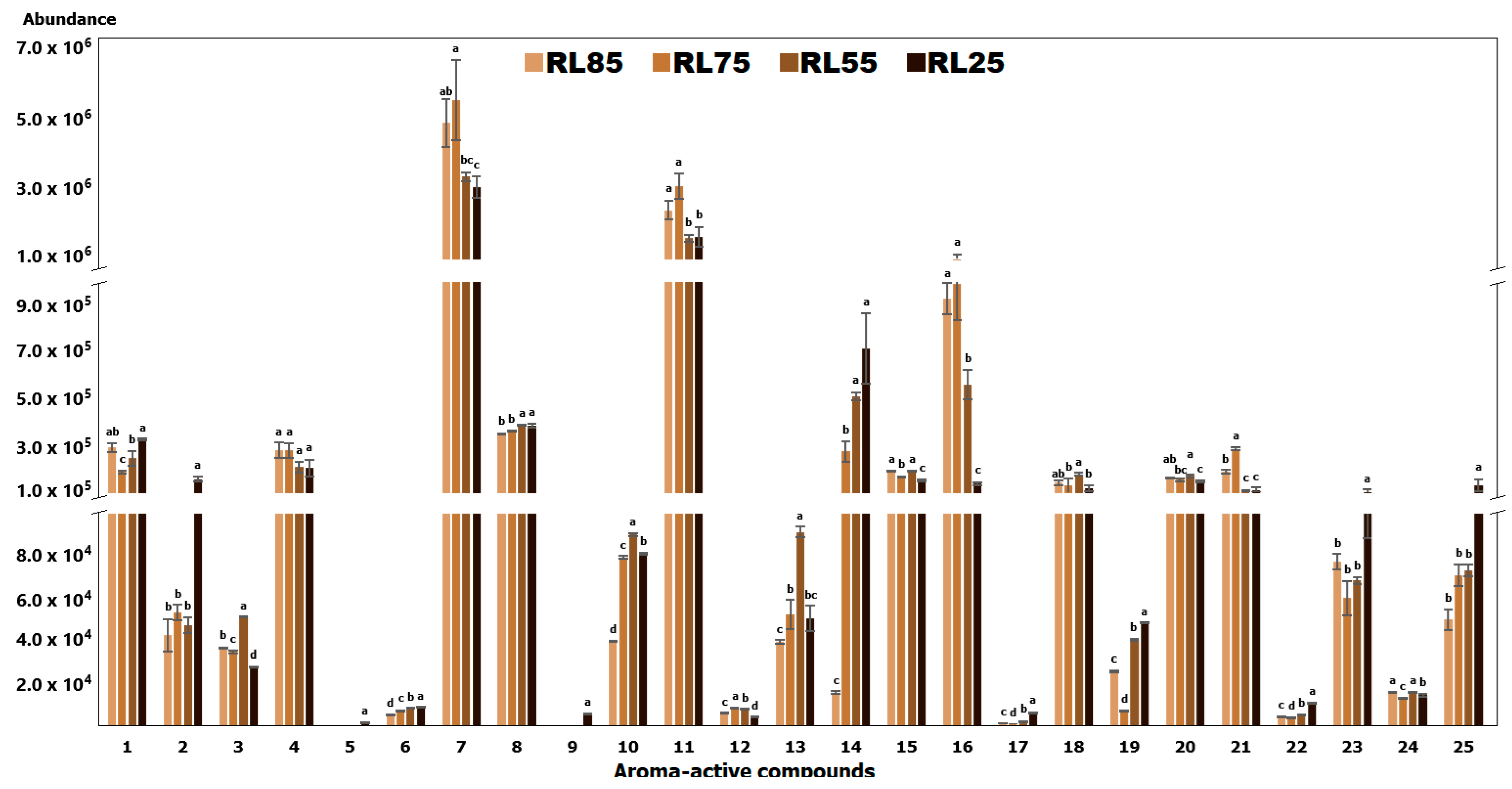
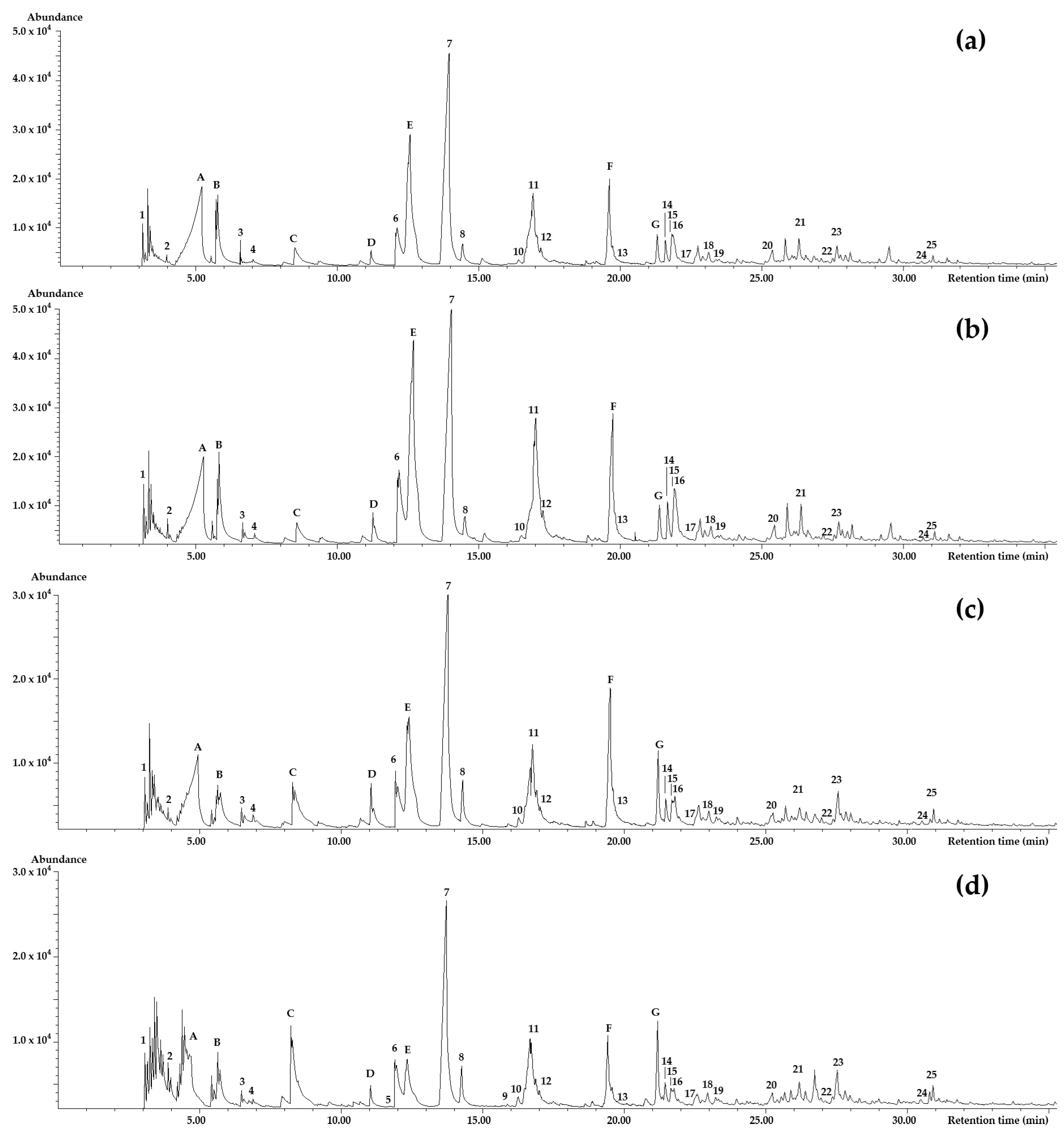
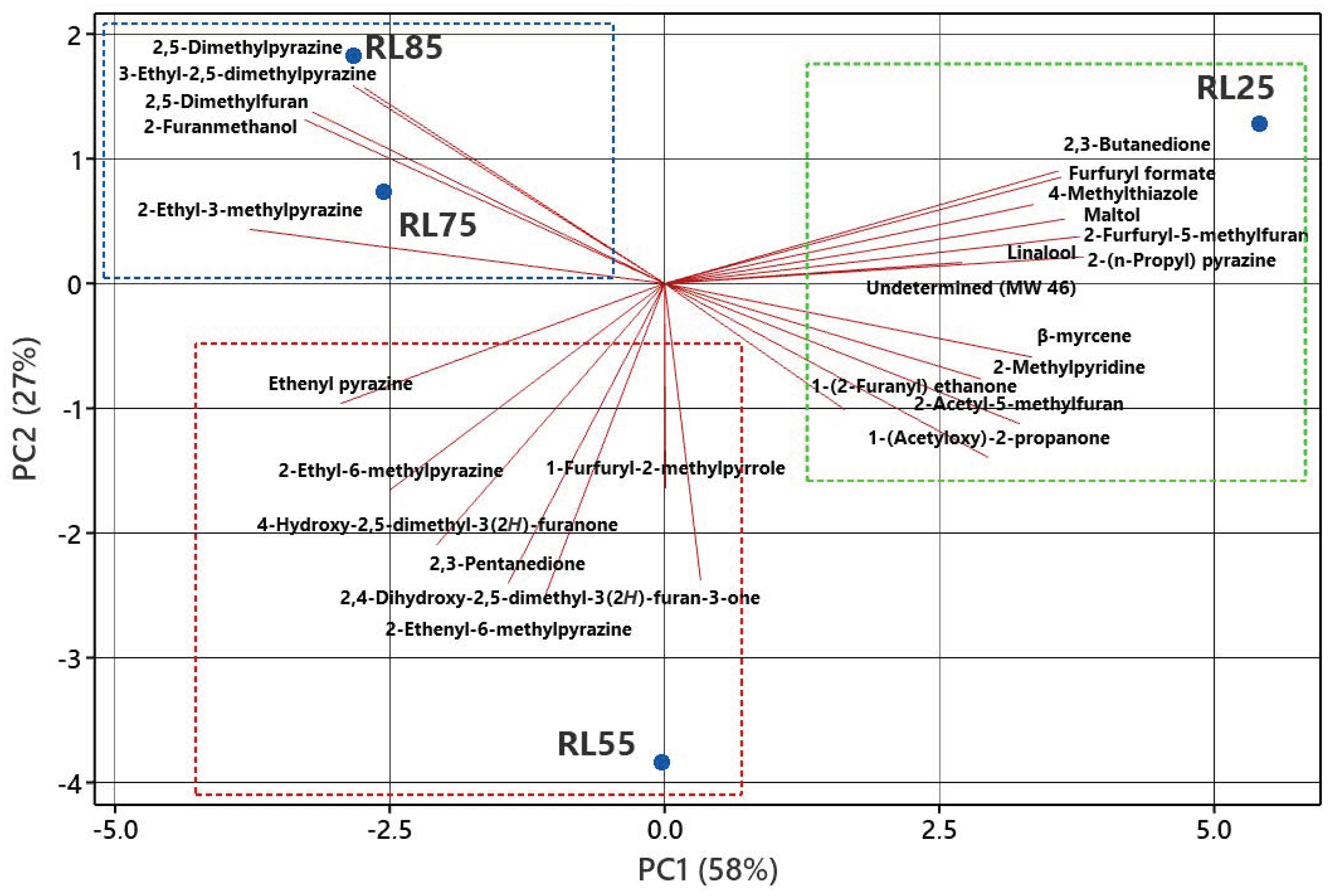
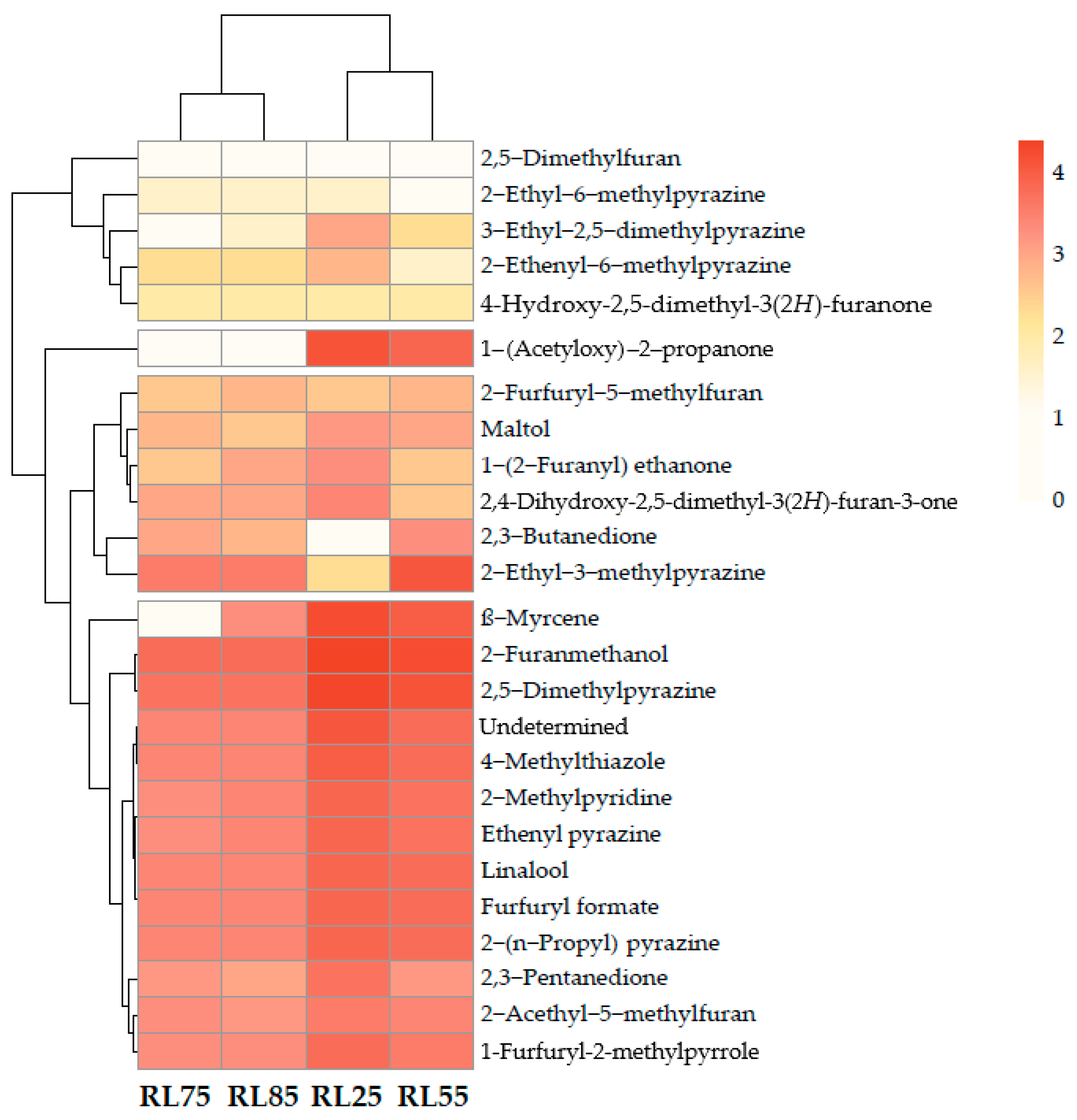

| Peak No. | Compound Name | (5%-Phenyl)-methylpolysiloxane | Polyethylene Glycol | Odor Description | Odor Intensity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-LRI | R-LRI | C-LRI | R-LRI | RL85 | RL75 | RL55 | RL25 | |||||
| 1 | Undetermined (MW 46) | 588 | -- | 938 | -- | Rotten potato | -- | -- | -- | 3 | ||
| 2 | 2,3-Butanedione | 609 | 599 | [14] | 976 | 977 | [25] | Sweet burnt | 1 | 1 | 1 | 2 |
| 3 | 2,3-Pentanedione | 659 | 697 | [14] | 1057 | 1060 | [25] | Sweet | -- | -- | 1 | -- |
| 4 | 2,5-Dimethylfuran | 675 | 695 | [26] | 952 | 946 | [27] | Roasted poultry | 1 | 1 | 1 | 1 |
| 5 | 4-Methylthiazole | 820 | 823 | [14] | 1286 | 1278 | [27] | Stinky (unpleasant smell) | -- | -- | -- | 3 |
| 6 | 2-Methylpyridine | 823 | 830 | [9] | 1225 | 1214 | [27] | Sulfurous | 1 | 1 | 2 | 3 |
| 7 | 2-Furanmethanol | 858 | 868 | [9] | 1667 | 1664 | [28] | Unpleasant roasted notes | 2 | 3 | 2 | 2 |
| 8 | 1-(Acetyloxy)-2-propanone | 870 | 870 | [15] | 1465 | 1477 | [17] | Medicinal note | 2 | 2 | 3 | 3 |
| 9 | Furfuryl formate | 906 | 907 | [9] | 1499 | 1497 | [29] | Blackcurrant | -- | -- | -- | 1 |
| 10 | 1-(2-Furanyl) ethanone | 908 | 910 | [9] | 1512 | 1512 | [30] | Blackcurrant | -- | -- | 2 | 2 |
| 11 | 2,5-Dimethylpyrazine | 913 | 917 | [9] | 1328 | 1320 | [27] | Roasted coffee | 2 | 3 | 2 | 2 |
| 12 | Ethenyl pyrazine | 927 | 938 | [9] | 1445 | 1438 | [28] | Toasted bread | 1 | 2 | 2 | 1 |
| 13 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 983 | 977 | [31] | 1562 | 1542 | [32] | Strong blackcurrant | 2 | 2 | 3 | 2 |
| 14 | β-myrcene | 993 | 994 | [33] | 1161 | 1156 | [34] | Blackcurrant | 1 | 2 | 2 | 3 |
| 15 | 2-Ethyl-6-methylpyrazine | 1001 | 1000 | [9] | 1393 | 1390 | [35] | Roasted hazelnut | 2 | -- | 2 | -- |
| 16 | 2-Ethyl-3-methylpyrazine | 1006 | 1004 | [9] | 1413 | 1402 | [27] | Roasted nutty | 2 | 2 | 1 | -- |
| 17 | 2-(n-Propyl) pyrazine | 1015 | 1010 | [36] | 1427 | 1428 | [2] | Rubber | -- | -- | 1 | 2 |
| 18 | 2-Ethenyl-6-methylpyrazine | 1028 | 1038 | [14] | 1489 | 1490 | [28] | Hazelnut | 2 | 2 | 3 | 2 |
| 19 | 2-Acetyl-5-methylfuran | 1037 | 1042 | [9] | 1627 | 1608 | [27] | Toasted | -- | -- | -- | 2 |
| 20 | 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | 1071 | 1061 | [37] | 2059 | 2060 | [37] | Caramel-like | 3 | 3 | 3 | 3 |
| 21 | 3-Ethyl-2,5-dimethylpyrazine | 1092 | 1093 | [14] | 1455 | 1449 | [28] | Hazelnut toast | 1 | 3 | -- | -- |
| 22 | Linalool | 1120 | 1102 | [38] | 1550 | 1552 | [28] | Sweet | 2 | 2 | 2 | 3 |
| 23 | Maltol | 1112 | 1112 | [39] | 1996 | 1981 | [40] | Caramel (sweet) | 2 | 2 | 2 | 3 |
| 24 | 1-Furfuryl-2-methylpyrrole | 1142 | 1148 | [26] | 1832 | 1834 | [41] | Crushed plant | 1 | -- | 1 | -- |
| 25 | 2-Furfuryl-5-methylfuran | 1159 | 1157 | [26] | 1687 | 1682 | [10] | Green plant leaves | 2 | 2 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obando, A.M.; Figueroa, J.G. Effect of Roasting Level on the Development of Key Aroma-Active Compounds in Coffee. Molecules 2024, 29, 4723. https://doi.org/10.3390/molecules29194723
Obando AM, Figueroa JG. Effect of Roasting Level on the Development of Key Aroma-Active Compounds in Coffee. Molecules. 2024; 29(19):4723. https://doi.org/10.3390/molecules29194723
Chicago/Turabian StyleObando, Andrea M., and Jorge G. Figueroa. 2024. "Effect of Roasting Level on the Development of Key Aroma-Active Compounds in Coffee" Molecules 29, no. 19: 4723. https://doi.org/10.3390/molecules29194723
APA StyleObando, A. M., & Figueroa, J. G. (2024). Effect of Roasting Level on the Development of Key Aroma-Active Compounds in Coffee. Molecules, 29(19), 4723. https://doi.org/10.3390/molecules29194723






