Research Progress on the Mechanisms of Protocatechuic Acid in the Treatment of Cognitive Impairment
Abstract
1. Introduction
2. Chemical Properties of Protocatechuic Acid
3. Sources of Protocatechuic Acid
3.1. Direct Sources
3.2. Indirect Sources
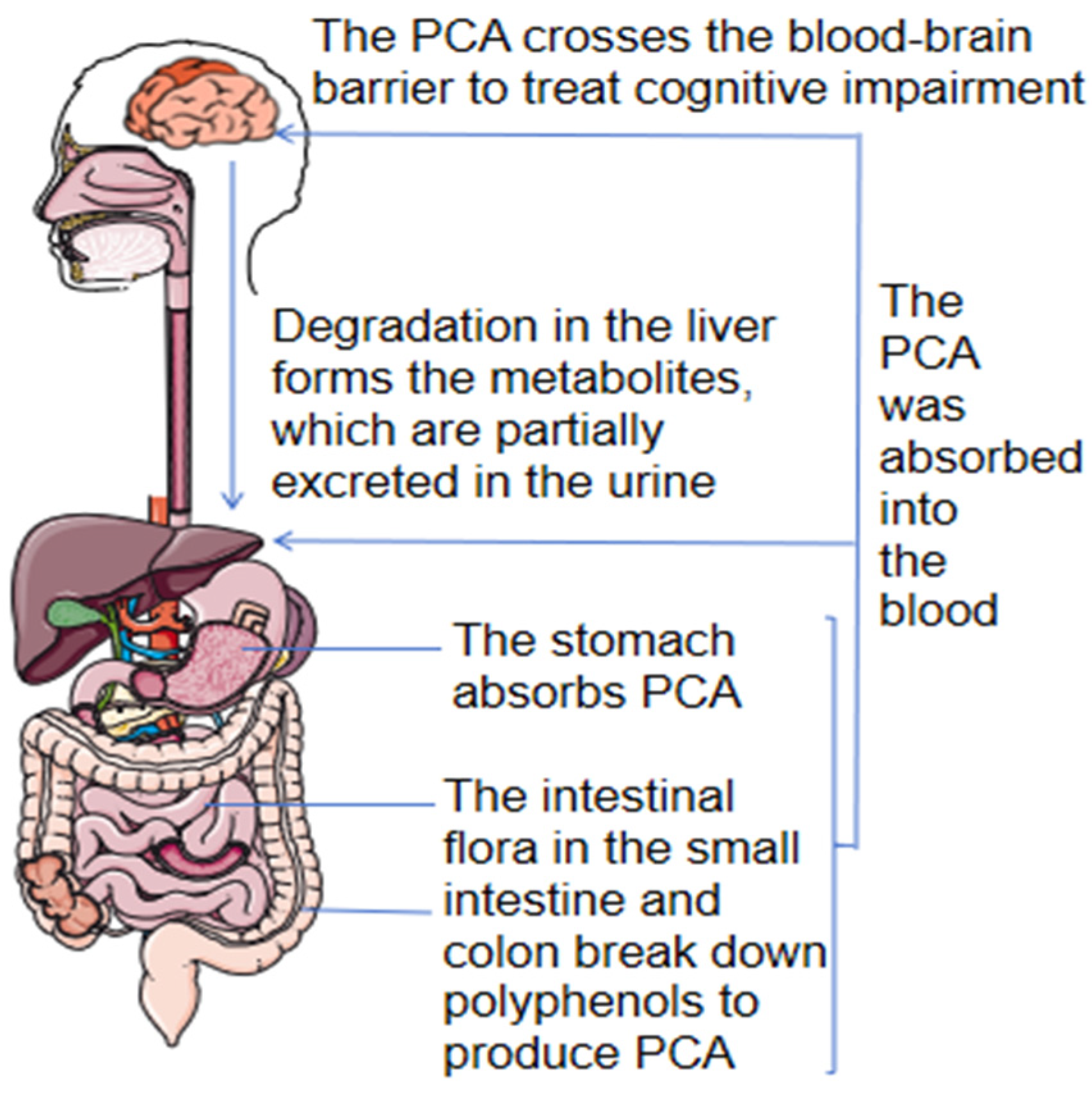
3.3. Pharmacokinetics
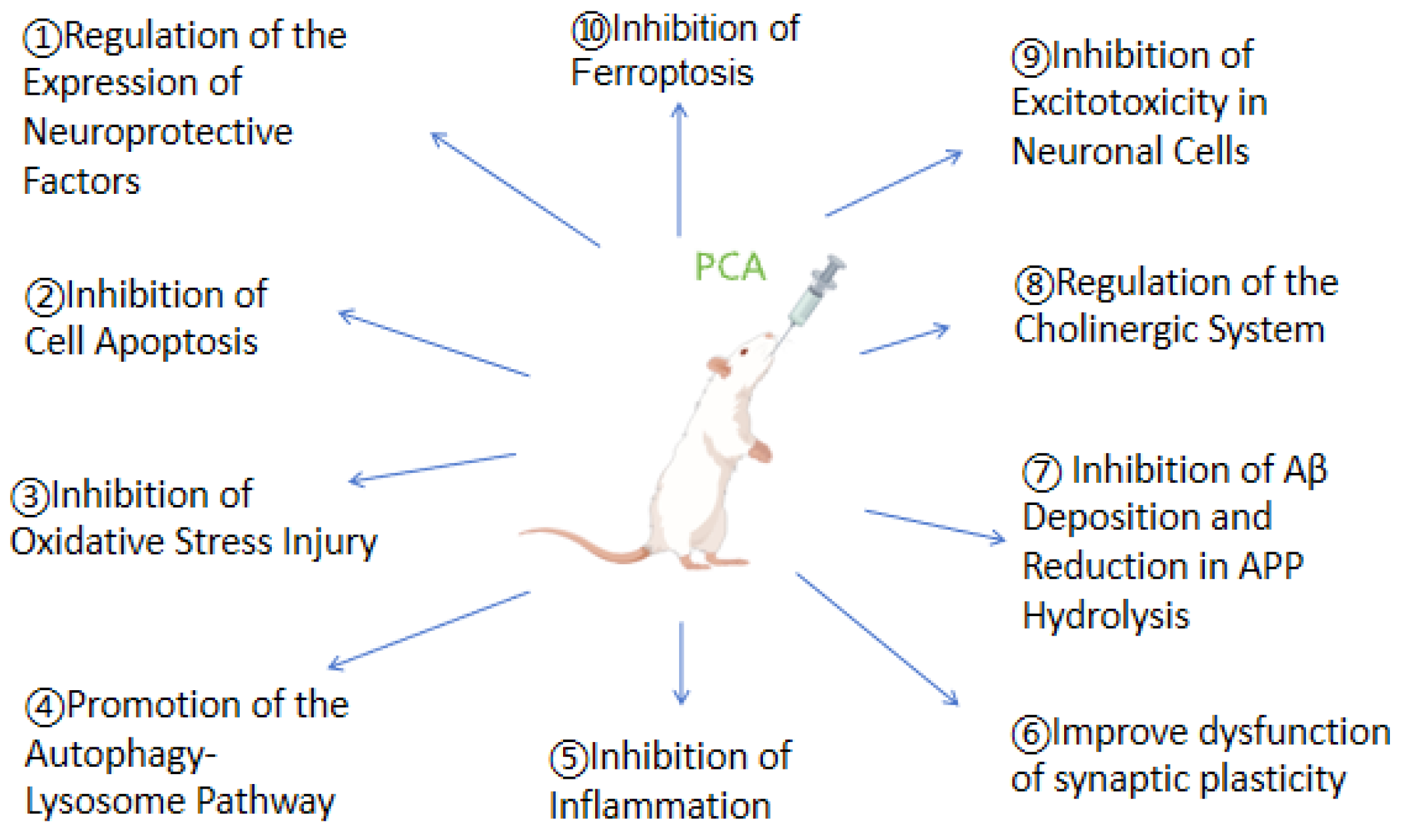
4. The Mechanisms by Which Protocatechuic Acid Treats Cognitive Impairment
4.1. Regulation of the Expression of Neuroprotective Factors
4.2. Inhibition of Cell Apoptosis
4.3. Inhibition of Oxidative Stress Injury
4.4. Promotion of the Autophagy-Lysosome Pathway
4.5. Inhibition of Inflammation
4.6. Improve Dysfunction of Synaptic Plasticity
4.7. Inhibition of Aβ Deposition and Reduction in APP Hydrolysis
4.8. Regulation of the Cholinergic System
4.9. Inhibition of Excitotoxicity in Neuronal Cells
4.10. Inhibition of Ferroptosis
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation List
| Abbreviation | Full title | Abbreviation | Full title |
| CI | Cognitive impairment | PCA | Protocatechuic acid |
| AGEs | Advanced glycation end products | DA | Dopamine |
| 5-HT | 5-hydroxytryptamine | BDNF | Brain-derived neurotrophic factor |
| CREB | cAMP-response element binding protein | NF-κB | Nuclear factor κB |
| PKA | Protein kinase A system | XOD | Xanthine oxidase |
| LC3 | Microtubule-associated protein 1 light chain 3 | MMP-9 | Matrix metalloproteinase 9 |
| HNE | 4-hydroxynonenal | ChAT | Choline acetyltransferase |
| AChE | Acetylcholinesterase | GR | Glutathione reductase |
| GPX | Glutathione peroxidase | GSH | Glutathione |
| LPO | Lipid hydroperoxide | MDA | Malonic dialdehyde |
| LIP | Labile iron pool |
References
- Moritz, S.; Silverstein, S.M.; Dietrichkeit, M.; Gallinat, J. Neurocognitive deficits in schizophrenia are likely to be less severe and less related to the disorder than previously thought. World Psychiatry 2020, 19, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Berron, D.; Vogel, J.W.; Insel, P.S. Early stages of tau pathology and its associations with functional connectivity, atrophy and memory. Brain 2021, 144, 2771–2783. [Google Scholar] [CrossRef] [PubMed]
- Skovgård, K.; Agerskov, C.; Kohlmeier, K.A.; Herrik, K.F. The 5-HT3 receptor antagonist ondansetron potentiates the effects of the acetylcholinesterase inhibitor donepezil on neuronal network oscillations in the rat dorsal hippocampus. Neuropharmacology 2018, 143, 130–142. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R. Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, J.; Chou, F.; Wang, C. Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br. J. Pharmacol. 2011, 162, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Huang, C.; Huang, C.; Yin, M. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J. Agric. Food Chem. 2009, 57, 6661–6667. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Trinha, L.T.P.; Choic, Y.S.; Baea, H.J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crop. Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill.using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, P.; Chen, X.; Wang, Y.; Xu, Y. An ultra high performance liquid chromatography with tandem mass spectrometry method for simultaneous determination of thirteen components extracted from Radix Puerariae in rat plasma and tissues: Application to pharmacokinetic and tissue distribution study. J. Sep. Sci. 2020, 43, 418–437. [Google Scholar] [PubMed]
- Yang, Y.; Wei, M.; Huang, T.; Lee, S. Extraction of protocatechuic acid from Scutellaria barbata D. Don using supercritical carbon dioxide. J. Supercrit. Fluids 2013, 81, 55–66. [Google Scholar] [CrossRef]
- Hou, P.; Wang, Q.; Qi, W.; Zhang, Y.; Xie, J. Comprehensive determination of seven polyphenols in Eucommia ulmoides and its anti oxidative stress activity in C. elegans. J. Food Meas. Charact. 2019, 13, 2903–2909. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Y.; Xu, L. Simultaneous determination of protocatechuic acid, syringin, chlorogenic acid, caffeic acid, liriodendrin and isofraxidin in Acanthopanax senticosus Harms by HPLC-DAD. Biol. Pharm. Bull. 2006, 29, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tang, Q.; Liu, J. Preparative isolation and purification of phenolic acids from Smilax china by high-speed counter-current chromatography. Sep. Purif. Technol. 2008, 61, 477–478. [Google Scholar] [CrossRef]
- Mîrza, C.M.; Mîrza, T.V.; Odagiu, A.C.M. Phytochemical Analysis and Antioxidant Effects of Prunella vulgaris in Experimental Acute Inflammation. Int. J. Mol. Sci. 2024, 25, 4843. [Google Scholar] [CrossRef]
- Lion, Q.; Pichette, A.; Mihoub, M.; Mshvildadze, V.; Legault, J. Phenolic Extract from Aralia nudicaulis L. Rhizomes Inhibits Cellular Oxidative Stresses. Molecules 2021, 26, 4458. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, P.; Liu, B.; Wei, L.; Xu, Y. Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC-MS/MS method: Application to pharmacokinetics and tissue distribution study. J. Pharm. Biomed. Anal. 2018, 159, 490–512. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Bi, S. Simultaneous Determination of Four Ingredients in Plantago Depressa by Single Marker. J. Chem. 2021, 2021, 4040239. [Google Scholar] [CrossRef]
- Shi, J.; Peng, L.; Chen, W. Evaluation of chemical components and quality in Xinhui Chenpi (Citrus reticulata ‘Chachi’) with two different storage times by GC–MS and UPLC. Food Sci. Nutr. 2024, 12, 5036–5051. [Google Scholar] [CrossRef]
- Yener, İ.; Ertaş, A.; Yilmaz, M.A. Characterization of the Chemical Profile of Euphorbia Species from Turkey by Gas Chromatography–Mass Spectrometry (GC-MS), Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS), and Liquid Chromatography–Ion Trap–Time-of-Flight–Mass Spectrometry (LC-IT-TOF-MS) and Chemometric Analysis. Anal. Lett. 2019, 52, 1031–1049. [Google Scholar]
- Jin, H.; Tang, G.; Li, J.; Ma, L.; Li, Y.; Chang, Y. Simultaneous Determination of Phenolic Acids, Anthraquinones, Flavonoids, and Triterpenes of Cynomorii Herba in Different Harvest Times by LC-MS/MS. J. Anal. Methods Chem. 2020, 2020, 8861765. [Google Scholar] [CrossRef] [PubMed]
- Jargalsaikhan, G.; Wu, J.; Chen, Y.; Yang, L.; Wu, M. Comparison of the Phytochemical Properties, Antioxidant Activity and Cytotoxic Effect on HepG2 Cells in Mongolian and Taiwanese Rhubarb Species. Molecules 2021, 26, 1217. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, L.; Chen, G. Separation and determination of protocatechuic aldehyde and protocatechuic acid in Salivia miltorrhrza by capillary electrophoresis with amperometric detection. Analyst 2001, 126, 1519–1523. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, S.; Zou, X.; Teng, Y.; Wang, W.; Fu, L. Determination of Phenolic Acids Using Ultra-High-Performance Liquid Chromatography Coupled with Triple Quadrupole (UHPLC-QqQ) in Fruiting Bodies of Sanghuangporus baumii (Pilát) L.W. Zhou and Y.C. Dai. Plants 2023, 12, 3565. [Google Scholar] [CrossRef] [PubMed]
- Osmic, N.; Culum, D.; Ibragic, S. Catechins and other phenolic compounds in herb of eight Ephedra species in comparison to Camellia sinensis. Nat. Prod. Res. 2024, 38, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Lin, S.; Li, H. Green Extraction of Natural Antioxidants from the Sterculia nobilis Fruit Waste and Analysis of Phenolic Profile. Molecules 2018, 23, 1059. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef]
- Wang, X.; Yan, K.; Ma, X. Simultaneous Determination and Pharmacokinetic Study of Protocatechuic Aldehyde and Its Major Active Metabolite Protocatechuic Acid in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. Sci. 2016, 54, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Deng, Q.; Zhou, X. The Tissue Distribution and Urinary Excretion Study of Gallic Acid and Protocatechuic Acid after Oral Administration of Polygonum Capitatum Extract in Rats. Molecules 2016, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Pan, M.; Wang, Y. In vitro bloodbrain barrier permeability study of four main active ingredients from Alpiniae oxyphyllae fructus. J. Pharm. Biomed. Anal. 2023, 235, 115637. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, D.; Wang, L. Pharmacokinetics of protocatechuic acid in mouse and its quantification in human plasma using LC-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 908, 39–44. [Google Scholar] [CrossRef]
- Habib, S.A.; Suddek, G.M.; Abdel, R.M.; Abdelrahman, R.S. The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci. 2021, 277, 119485. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Piechal, A.; Blecharz-Klin, K. Administration of protocatechuic acid affects memory and restores hippocampal and cortical serotonin turnover in rat model of oral D-galactose-induced memory impairment. Behav. Brain Res. 2019, 368, 111896. [Google Scholar] [CrossRef]
- Luo, L.; Li, R.; Wang, G. Age-dependent effects of a high-fat diet combined with dietary advanced glycation end products on cognitive function and protection with voluntary exercise. Food Funct. 2022, 13, 4445–4458. [Google Scholar] [CrossRef]
- Krzysztoforska, K.; Piechal, A.; Blecharz-Klin, K. Effect of protocatechuic acid on cognitive processes and central nervous system neuromodulators in the hippocampus, prefrontal cortex, and striatum of healthy rats. Nutr. Neurosci. 2022, 25, 1362–1373. [Google Scholar] [CrossRef]
- Li, S.; Sheng, Z. Energy matters: Presynaptic metabolism and the maintenance of synaptic transmission. Nat. Rev. Neurosci. 2022, 23, 4–22. [Google Scholar] [CrossRef]
- Jurič, D.M.; Kržan, M.; Lipnik-Stangelj, M. Histamine and astrocyte function. Pharmacol. Res. 2016, 111, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cui, T.; Xie, N.; Zhang, X.; Qian, Z.; Liu, J. Protocatechuic acid improves cognitive deficits and attenuates amyloid deposits, inflammatory response in aged AβPP/PS1 double transgenic mice. Int. Immunopharmacol. 2014, 20, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, B.J.; Mohammad, A.; Finch, M.S. Exercise training and BDNF injections alter amyloid precursor protein (APP) processing enzymes and improve cognition. J. Appl. Physiol. 2023, 135, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Krzysztoforska, K.; Piechal, A.; Wojnar, E. Protocatechuic Acid Prevents Some of the Memory-Related Behavioural and Neurotransmitter Changes in a Pyrithiamine-Induced Thiamine Deficiency Model of Wernicke-Korsakoff Syndrome in Rats. Nutrients 2023, 15, 625. [Google Scholar] [CrossRef]
- Wang, M.; Zong, H.; Chang, K. 5-HT1AR alleviates Aβ-induced cognitive decline and neuroinflammation through crosstalk with NF-κB pathway in mice. Int. Immunopharmacol. 2020, 2020, 106354. [Google Scholar] [CrossRef]
- Kale, S.; Sarode, L.P.; Kharat, A. Protocatechuic Acid Prevents Early Hour Ischemic Reperfusion Brain Damage by Restoring Imbalance of Neuronal Cell Death and Survival Proteins. J. Stroke Cerebrovasc. Dis. 2021, 30, 105507. [Google Scholar] [CrossRef]
- Xing, J.; Han, D.; Xu, D.; Li, X.; Sun, L. CREB Protects against Temporal Lobe Epilepsy Associated with Cognitive Impairment by Controlling Oxidative Neuronal Damage. Neurodegener. Dis. 2019, 19, 225–237. [Google Scholar] [CrossRef]
- Xi, Z.; Hu, X.; Chen, X. Protocatechuic acid exerts protective effects via suppression of the P38/JNK- NF-κB signalling pathway in an experimental mouse model of intracerebral haemorrhage. Eur. J. Pharmacol. 2019, 854, 128–138. [Google Scholar] [CrossRef]
- Guan, S.; Ge, D.; Liu, T.; Ma, X.; Cui, Z. Protocatechuic acid promotes cell proliferation and reduces basal apoptosis in cultured neural stem cells. Toxicol. Vitr. 2009, 23, 201–208. [Google Scholar] [CrossRef]
- Kho, A.R.; Choi, B.Y.; Lee, S. Effects of Protocatechuic Acid (PCA) on Global Cerebral Ischemia-Induced Hippocampal Neuronal Death. Int. J. Mol. Sci. 2018, 19, 1420. [Google Scholar] [CrossRef]
- Liu, H.; Gale, J.; Reynolds, I.; Weiss, J.; Aizenman, E. The Multifaceted Roles of Zinc in Neuronal Mitochondrial Dysfunction. Biomedicines 2021, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, B.; Bao, Y.; An, L. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol. Vitr. 2008, 22, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Li, S.; Xu, B. p38α MAPK is a MTOC-associated protein regulating spindle assembly, spindle length and accurate chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle 2010, 9, 4130–4143. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, X.; Lv, C. Protocatechuic acid ameliorates neurocognitive functions impairment induced by chronic intermittent hypoxia. Sci. Rep. 2015, 5, 14507. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic Acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Han, l.; Yang, Q.; Ma, W. Protocatechuic acid ame-liorated palmitic-acid-induced oxidative damage in en-dothelial cells through activating endogenous antioxi-dant enzymes via an adenosine-monophosphat e-activated-protein-kinase-dependent pathway. J. Agric. Food chem. 2018, 66, 10400–10409. [Google Scholar] [CrossRef]
- Choi, J.R.; Kim, J.H.; Lee, S.; Cho, E.J.; Kim, H.Y. Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer’s disease mouse model. Food Chem. Toxicol. 2020, 144, 111571. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wang, F. Neuroprotective effects of protocatechuic acid on sodium arsenate induced toxicity in mice: Role of oxidative stress, inflammation, and apoptosis. Chem. Biol. Interact. 2021, 337, 109392. [Google Scholar] [CrossRef]
- Tobón-Velasco, J.C.; Carmona-Aparicio, L.; Ali, S.F. Biomarkersof cell damage induced by oxidative stress in Parkinson’s disease andrelated models. Cent. Nerv. Syst. Agents. Med. Chem. 2010, 10, 278–286. [Google Scholar] [CrossRef]
- Husain, K.; Mejia, J.; Lalla, J.; Kazin, S. Dose response of alcohol-in-duced changes in BP, nitric oxide and antioxidants in rat plasma. Pharmacol. Res. 2005, 51, 337–343. [Google Scholar] [CrossRef]
- Adedara, I.A.; Fasina, O.B.; Ayeni, M.F.; Ajayi, O.M.; Farombi, E.O. Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem. Toxicol. 2019, 125, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Adeyanju, A.A.; Oso, B.J.; Molehin, O.R. Treatment with protocatechuic acid attenuates cisplatin-induced toxic-ity in the brain and liver of male wistar rats. Adv. Tradit. Med. 2023, 23, 121–131. [Google Scholar] [CrossRef]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tao, W.; Wang, J. Progress of researches on acupuncture treatment of cerebral ischemia-reperfusion injury by regulating autophagy in the ischemic cerebral tissue. Zhen Ci Yan Jiu 2019, 44, 459–464. [Google Scholar]
- Old, E.A.; Clark, A.K.; Malcangio, M. The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb. Exp. Pharmacol. 2015, 227, 145–170. [Google Scholar]
- Cheng, X.; Xie, Y.; Zhou, B.; Huang, N.; Farfel-Becker, T.; Sheng, Z. Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons. J. Cell Biol. 2018, 217, 3127–3139. [Google Scholar] [CrossRef]
- Li, H.; Zheng, T.; Lian, F.; Xu, T.; Yin, W.; Jiang, Y. Anthocyanin-rich blueberry extracts and anthocyanin metabolite protocatechuic acid promote autophagy-lysosomal pathway and alleviate neurons damage in in vivo and in vitro models of Alzheimer’s disease. Nutrition 2022, 93, 111473. [Google Scholar] [CrossRef]
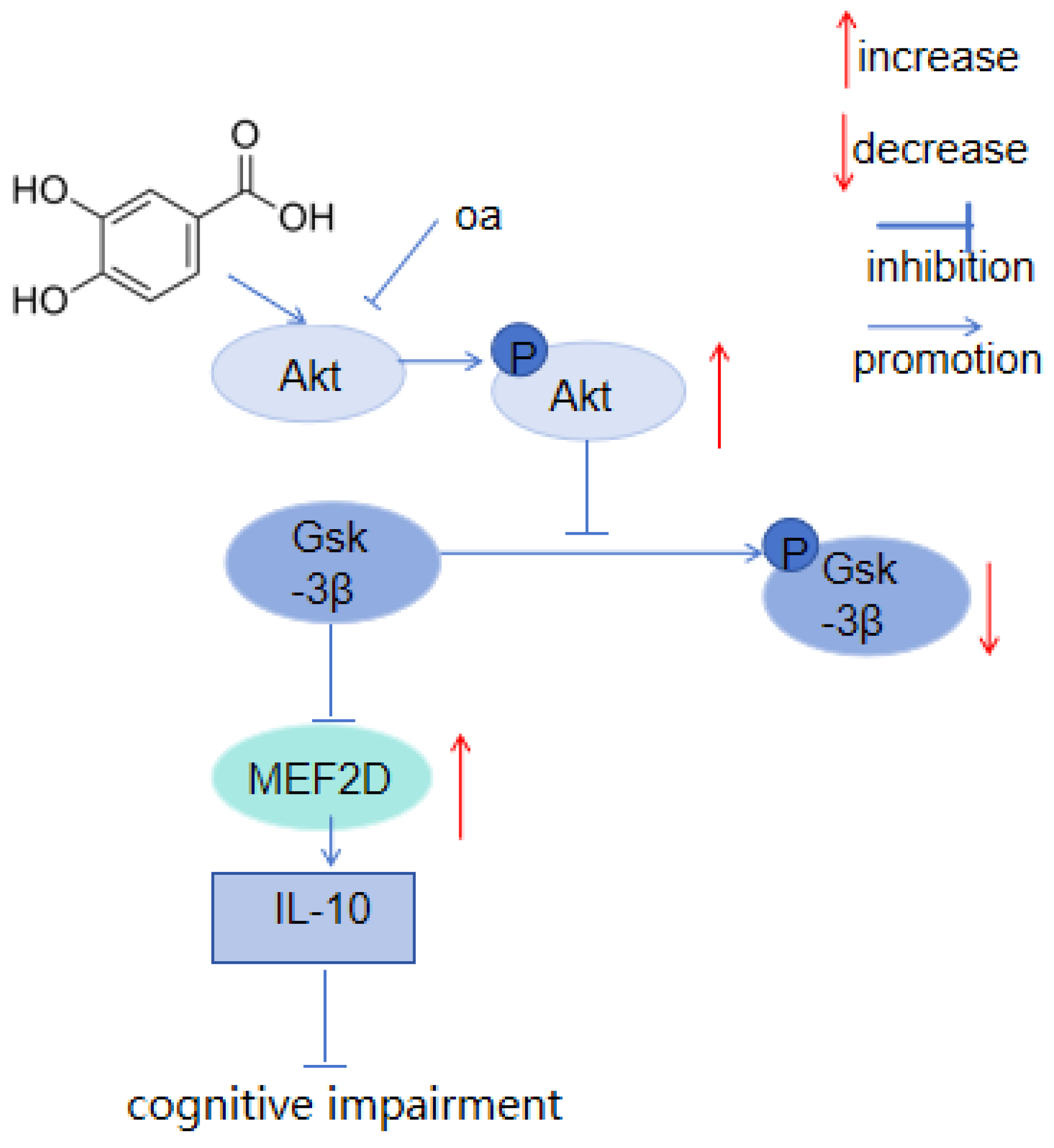
- Huang, L.; Zhong, X.; Qin, S.; Deng, M. Protocatechuic acid attenuates β secretase activity and okadaic acid induced autophagy via the Akt/GSK 3β/MEF2D pathway in PC12 cells. Mol. Med. Rep. 2020, 21, 1328–1335. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, X.; Zhou, Q.; Li, H. Inhibition of excitatory amino acid efflux contributes to protective effects of puerarin against cerebral ischemia in rats. Biomed. Environ. Sci. 2007, 20, 336–342. [Google Scholar] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Kaewmool, C.; Kongtawelert, P.; Phitak, T.; Pothacharoen, P.; Udomruk, S. Protocatechuic acid inhibits inflammatory responses in LPS-activated BV2 microglia via regulating SIRT1/NF-κB pathway contributed to the suppression of microglial activation-induced PC12 cell apoptosis. J. Neuroimmunol. 2020, 341, 577164. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.N.; Harris, K.M. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 2008, 31, 47–67. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.; Lee, S. Administration of Protocatechuic Acid Reduces Traumatic Brain Injury-Induced Neuronal Death. Int. J. Mol. Sci. 2017, 18, 2510. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, X.; Ge, D.; Liu, T.; Ma, X.; Cui, Z. Protocatechuic acid promotes the neuronal differentiation and facilitates survival of phenotypes differentiated from cultured neural stem and progenitor cells. Eur. J. Pharmacol. 2011, 670, 471–478. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Duan, S.; Sawyer, T.W.; Sontz, R.A.; Wieland, B.A.; Diaz, A.F.; Merchant, J.L. GFAP-directed Inactivation of Men1 Exploits Glial Cell Plasticity in Favor of Neuroendocrine Reprogramming. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 1025–1051. [Google Scholar] [CrossRef]
- Albrecht, P.J.; Dahi, J.P.; Stoltfus, O.K. Ciliary neurophicfactor activates spinal cord astrocytes stimulating their produc-tion and release of firoblast growth factor-2 to increase motorneutron surbvival. J. Exp. Nerrol. 2002, 173, 46–62. [Google Scholar] [CrossRef]
- Li, S.; Jin, M.; Koeglsperger, T. Soluble abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011, 31, 6627–6638. [Google Scholar] [CrossRef]
- Tolar, M.; Hey, J.; Power, A.; Abushakra, S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef]
- Rummel, N.G.; Butterfield, D.A. Altered metabolism in Alzheimer disease brain: Role of oxidative stress. Antioxid. Redox Signal. 2022, 36, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.A.; Garcia-Parrilla, M.A.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, L.; Zheng, J. Polygoni Multiflori Radix Praeparata and Acori Tatarinowii Rhizoma ameliorate scopolamine-induced cognitive impairment by regulating the cholinergic and synaptic associated proteins. J. Ethnopharmacol. 2023, 311, 116400. [Google Scholar] [CrossRef]
- Nan, Z.; Dan, L.; Yi, W. Screening and identification of anti-acetylcholinesterase ingredients from tianzhi granule based on ultrafiltration combined with ultra-performance liquid chromatography-mass spectrometry and in silico analysis. J. Ethnopharmacol. 2022, 298, 115641. [Google Scholar]
- Yamini, P.; Ray, R.S.; Yadav, S. α7nAChR activation protects against oxidative stress, neuroinflammation and central insulin resistance in ICV-STZ induced sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2022, 217, 173402. [Google Scholar] [CrossRef]
- Kang, T.; Yang, Q.; Bais, S. Neuroprotective effect of procate-chuic acid through MaO-B inhibition in aluminium chloride induced dementia of alzheimer’s type in rats. Int. J. Pharmacol. 2018, 14, 879–888. [Google Scholar]
- Ferdous, R.; Islam, M.B.; Al-Amin, M.Y. Anticholinesterase and antioxidant activity of Drynaria quercifolia and its ameliorative effect in scopolamine-induced memory impairment in mice. J. Ethnopharmacol. 2024, 319, 117095. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Mai, D. Molecular mechanisms of glutamate toxicity in parkinson’s disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef]
- Sultana, R.; Perluigi, M.; Newman, S.F. Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer’s disease. Antioxid. Redox Signal. 2010, 12, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Cho, S.; Jeon, S.; Bae, K.; Song, K.; Seong, Y. 3,4-dihydroxybenzoic acid from Smilacis chinae rhizome protects amyloid beta protein (25–35)-induced neurotoxicity in cultured rat cortical neurons. Neurosci. Lett. 2007, 420, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.M.; Teng, K.M.; Vogt, N.M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J. Comp. Neurol. 2013, 521, 3508–3523. [Google Scholar] [CrossRef]
- Mert, H.; Kerem, Ö.; Mıs, L.; Yıldırım, S.; Mert, N. Effects of protocatechuic acid against cisplatin-induced neurotoxicity in rat brains: An experimental study. Int. J. Neurosci. 2024, 134, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Omojokun, O.S.; Adefegha, O.M. Alterations of Na+/K+-ATPase, cholinergic and antioxidant enzymes activity by protocatechuic acid in cadmium-induced neurotoxicity and oxidative stress in Wistar rats. Biomed. Pharmacother. 2016, 83, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xiao, X.; Bi, M. ATP-sensitive potassium channels:a double-edged sword in neurodegenerative diseases. Ageing Res. Rev. 2022, 80, 101676. [Google Scholar] [CrossRef]
- Varì, R.; D’Archivio, M.; Filesi, C. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Muley, M.M.; Thakare, V.N.; Patil, R.R.; Bafna, P.A.; Naik, S.R. Amelioration of cognitive, motor and endogenous defense functions with silymarin, piracetam and protocatechuic acid in the cerebral global ischemic rat model. Life Sci. 2013, 93, 51–57. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, ferroptosis, and diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X. Glutathione: A key component of the cytoplasmic labile iron pool. Biometals 2011, 24, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Vollmer, M.K.; Kelly, M.G. Reactive Gliosis Contributes to Nrf2-Dependent Neuroprotection by Pretreatment with Dimethyl Fumarate or Korean Red Ginseng Against Hypoxic-Ischemia: Focus on Hippocampal Injury. Mol. Neurobiol. 2020, 57, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zheng, K.; Li, S. Insight into the potential role of ferroptosis in neurodegenerative diseases. Front. Cell. Neurosci. 2022, 16, 1005182. [Google Scholar] [CrossRef] [PubMed]



| Name | Content (mg/g) | Detection Methods | References |
|---|---|---|---|
| rose | 0.476–1.632 | Extraction using an ethanol aqueous solution at 180 °C | [8] |
| wolfberry | 0.1819–0.9114 | HPLC-MS | [9] |
| schizandra | 0.0722–0.3569 | HPLC-DAD | [10] |
| pueraria root | 0.04 | HPLC-/MS | [11] |
| scutellaria barbata | 0.0361–0.066178 | Supercritical CO2 Extraction | [12] |
| eucommia ulmoides | 0–0.20694 | HPLC | [13] |
| acanthopanax senticosus | 0.003165–0.02312 | HPLC-DAD | [14] |
| smilax glabra | 66 | HSCCC | [15] |
| prunella vulgaris | 27 | HPLC-DAD-ESI | [16] |
| solanum nigrum | 4.1–48 | LC-MS | [17] |
| hedyotis diffusa | 0.561 | UHPLC-MS/MS | [18] |
| plantago seed | 0.001881–0.004856 | QAMS | [19] |
| dried tangerine peel | 0.043–0.075 | GC-MS, UPLC | [20] |
| euphorbia pekinensis | 0.02603–0.361 | LC-MS/MS | [21] |
| cynomorium songaricum | 0.012134–0.025287 | LC-MS/MS | [22] |
| rheum officinale | 0.03899 | HPLC | [23] |
| salvia miltiorrhiza | 0.915 | cce-UV | [24] |
| phellinus igniarius | 0.016–0.032 | UHPLC-QqQ | [25] |
| ephedra | 0.05–0.75 | HPLC-DAD | [26] |
| sterculia lychnophora | 0.0219 | UPLC-MS/MS | [27] |
| cinnamon | 0.1931–0.2278 | LC-HRMS | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Zhao, Z.; Liu, L.; Zhang, Y.; Liu, X. Research Progress on the Mechanisms of Protocatechuic Acid in the Treatment of Cognitive Impairment. Molecules 2024, 29, 4724. https://doi.org/10.3390/molecules29194724
Liang S, Zhao Z, Liu L, Zhang Y, Liu X. Research Progress on the Mechanisms of Protocatechuic Acid in the Treatment of Cognitive Impairment. Molecules. 2024; 29(19):4724. https://doi.org/10.3390/molecules29194724
Chicago/Turabian StyleLiang, Shuzhi, Zhongmin Zhao, Leilei Liu, Yan Zhang, and Xijian Liu. 2024. "Research Progress on the Mechanisms of Protocatechuic Acid in the Treatment of Cognitive Impairment" Molecules 29, no. 19: 4724. https://doi.org/10.3390/molecules29194724
APA StyleLiang, S., Zhao, Z., Liu, L., Zhang, Y., & Liu, X. (2024). Research Progress on the Mechanisms of Protocatechuic Acid in the Treatment of Cognitive Impairment. Molecules, 29(19), 4724. https://doi.org/10.3390/molecules29194724






