Comparison of Flavor Differences between the Juices and Wines of Four Strawberry Cultivars Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry and Sensory Evaluation
Abstract
1. Introduction
2. Results and Discussion
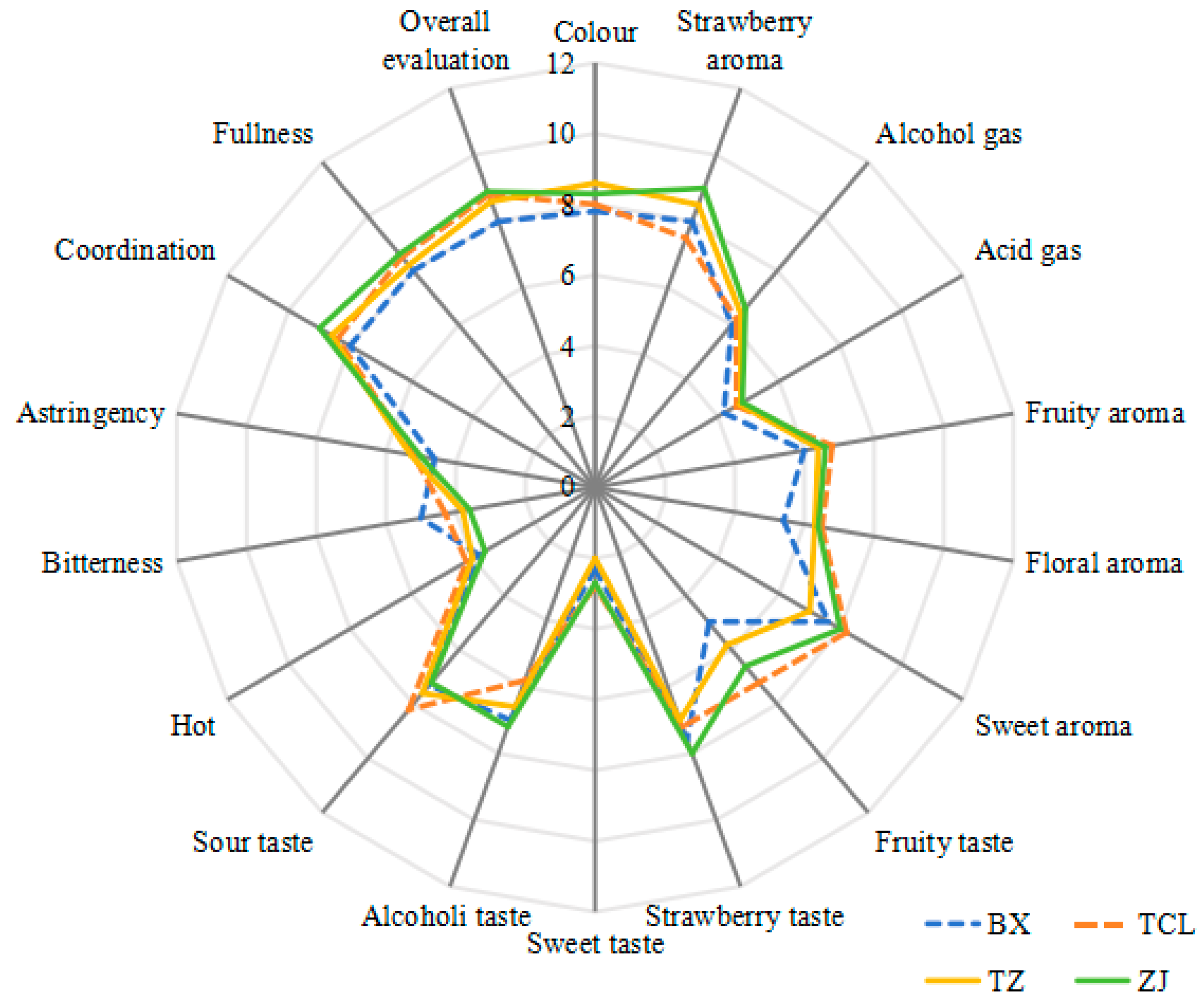
2.1. Sensory Evaluation of Strawberry Wines
2.2. Physicochemical Properties of Juices and Wines
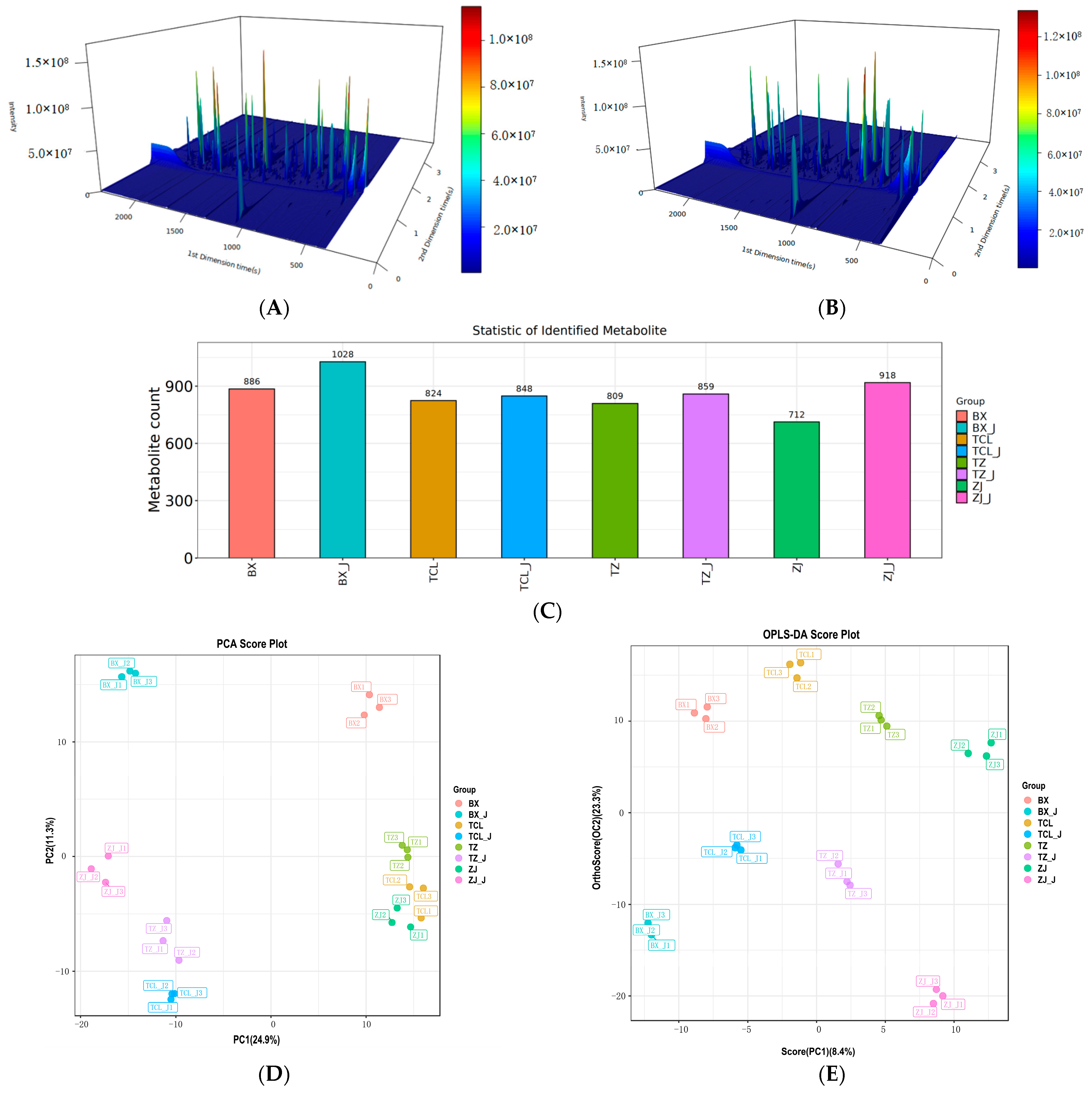
2.3. Identification and Statistical Analysis of Volatile Organic Compounds
2.3.1. Identification of Volatile Organic Compounds
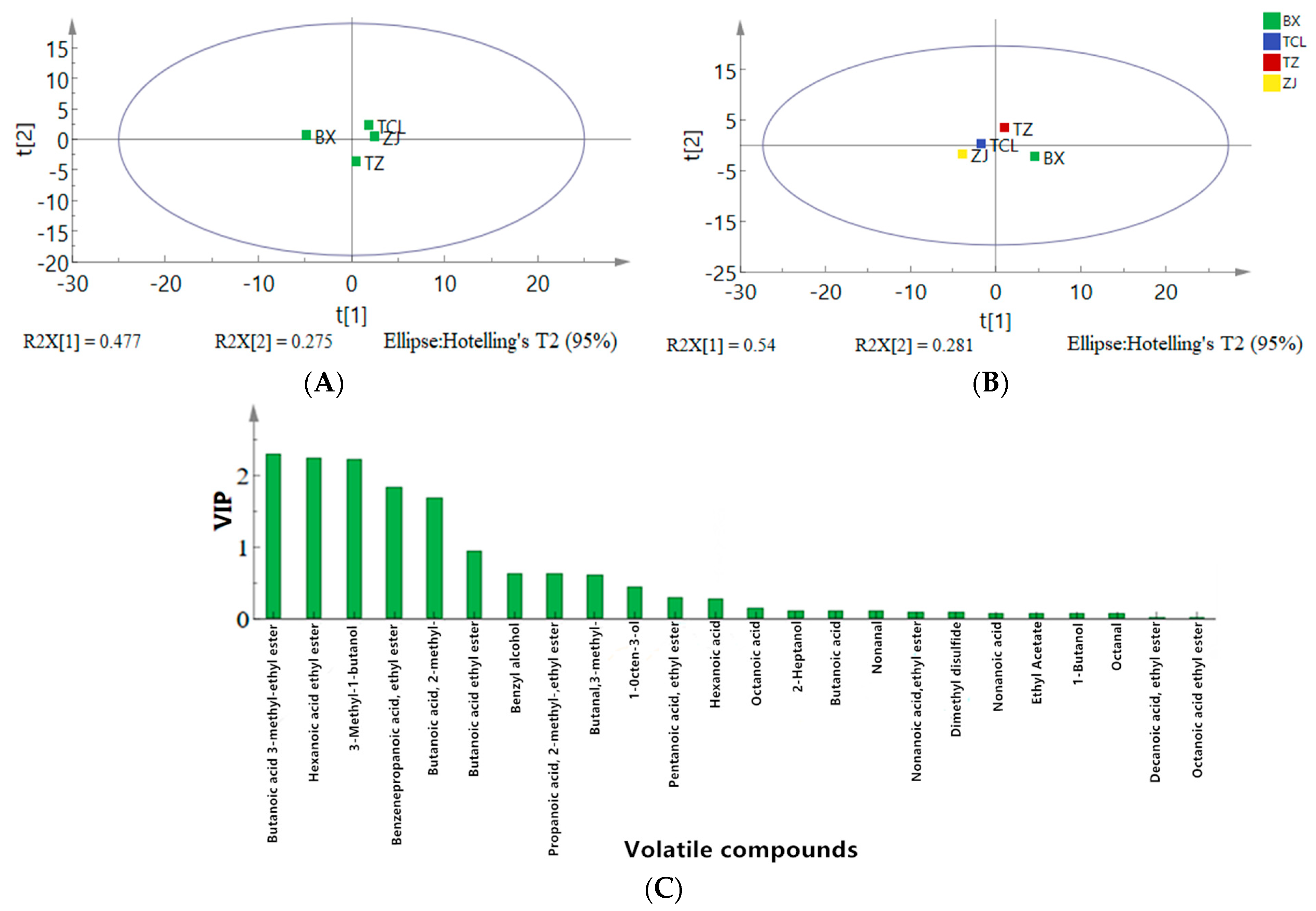
2.3.2. Principal Component Analysis and Orthogonal Partial Least Square-Discriminant Analysis of the Volatile Organic Compounds
2.4. Comparison of Key Differential Volatile Organic Compounds and Their Relative Odor Activity Values in the Strawberry Juices and Wines
2.4.1. Comparison of the Quantities and Relative Amounts of Key Differential Volatile Organic Compounds in Strawberry Juices
2.4.2. Comparison of Relative Odor Activity Values of Key Differential Volatile Organic Compounds in Strawberry Wines
2.4.3. Network Diagram of the Relationships between Various Volatile Organic Compounds for Imparting Unique Sensory Flavor Characteristics in Strawberry Wines
3. Materials and Methods
3.1. Samples and Reagents
3.1.1. Strawberry Cultivars and Yeast Strain for Brewing
3.1.2. Strawberry Juice Preparation and Wine Fermentation
3.1.3. Chemicals and Reagents
3.2. Descriptive Sensory Analysis
3.3. Physicochemical Parameter Detection
3.4. GC × GC–TOF-MS Analysis of Volatile Organic Compounds
3.4.1. Preparation of Internal Standard Solution
3.4.2. HS-SPME Method
3.4.3. GC × GC–TOF-MS Method
3.5. Statistical Analysis
3.5.1. Data Processing
3.5.2. Chemometric Analysis of the GC × GC–TOF-MS Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Cai, G.; Lu, J.; Gómez Plaza, E. The production and application of enzymes related to the quality of fruit wine. Crit. Rev. Food Sci. 2021, 61, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Saranraj, P.; Sivasakthivelan, P.; Naveen, M. Fermentation of fruit wine and its quality analysis: A review. Aust. J. Sci. Technol. 2017, 1, 85–97. Available online: https://aujst.com/vol-1-2/07_AJST.pdf (accessed on 10 August 2024).
- Tan, J.; Ji, M.; Gong, J.; Chitrakar, B. The formation of volatiles in fruit wine process and its impact on wine quality. Appl. Microbiol. Biotechnol. 2024, 108, 420. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Zhang, M.; Xie, X.; Li, R.; Cheng, W.; Ma, T.; Zhou, Y. Effects of cultivar factors on fermentation characteristics and volatile organic components of strawberry wine. Foods 2024, 13, 2874. [Google Scholar] [CrossRef] [PubMed]
- Cordenunsi, B.R.; Genovese, M.I.; do Nascimento, J.R.O.; Hassimotto, N.M.A.; dos Santos, R.J.; Lajolo, F.M. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005, 91, 113–121. [Google Scholar] [CrossRef]
- Yang, B.; Liu, S.; Zang, H.; Dai, Y.; Zhang, S.; Lin, X.; Liang, H.; Chen, Y. Flavor profile and quality of strawberry wine are improved through sequential fermentation with indigenous non-Saccharomyces yeasts and Saccharomyces cerevisiae. Food Biosci. 2024, 59, 104021. [Google Scholar] [CrossRef]
- Sturm, K.; Koron, D.; Stampar, F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003, 83, 417–422. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Qiu, J.; Qian, Y.; Wang, M. Comparative metabolomic analysis of the nutritional aspects from ten cultivars of the strawberry fruit. Foods 2023, 12, 1153. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, X.; Chen, H.; Liu, Y.; Xao, Y.; Chen, H.; Tang, Z.; Li, Q.; Yao, H. Evaluation of a strawberry fermented beverage with potential health benefits. Peer J. 2021, 9, e11974. [Google Scholar] [CrossRef]
- Bezerra, M.; Ribeiro, M.; Cosme, F.; Nunes, F.M. Overview of the distinctive characteristics of strawberry, raspberry, and blueberry in berries, berry wines, and berry spirits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13354. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, S.; Marsol-Vall, A.; Tähti, R.; Laaksonen, O.; Karhu, S.; Yang, B.; Ma, X. Chemical composition, sensory profile and antioxidant capacity of low-alcohol strawberry beverages fermented with Saccharomyces cerevisiae and Torulaspora delbrueckii. LWT 2021, 149, 111910. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Xu, X.; Zhang, X.; Lan, W.; Wang, Y.; Gao, X. Volatile compositions and sensorial properties of strawberry fruit wines fermented with Torulaspora delbrueckii and Saccharomyces cerevisiae in sequential and simultaneous inoculations. Eur. Food Res. Technol. 2024, 1–13. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, P.; Du, G.; Zhai, J.; Guo, Y.; Wang, X. Advancements and challenges for brewing aroma-enhancement fruit wines: Microbial metabolizing and brewing techniques. Food Chem. 2024, 456, 139981. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.J.; Liu, N.; Ye, D.Q.; Gong, X.; Qin, Y.; Liu, Y.L. Volatile compounds in wild strawberry and their odorants of wild strawberry wines: Effects of different stages of fermentation. Int. J. Food Prop. 2017, 20, S399–S415. [Google Scholar] [CrossRef]
- Ulrich, D.; Kecke, S.; Olbricht, K. What do we know about the chemistry of strawberry aroma? J. Agr. Food Chem. 2018, 66, 3291–3301. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, K.; He, Z.; Zhao, D.; Zheng, J.; Qian, M.C. Comparison of two data processing approaches for aroma marker identification in different distilled liquors using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry dataset. J. Food Sci. 2023, 88, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Lu, J.; Gao, M.; Li, C.; Chen, S. Optimization and validation of a headspace solid-phase microextraction with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometric detection for quantification of trace aroma compounds in Chinese liquor (Baijiu). Molecules 2021, 26, 6910. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A.; et al. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, X.; Zhu, F.; Liu, G.; Chu, L.; Yan, X.; Ma, Y.; He, F.; Li, G.; Zhang, Y.; et al. Effects of different winemaking yeasts on the composition of aroma-active compounds and flavor of the fermented jujube wine. Processes 2021, 9, 970. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Peña, Á.; Robert, P.; Bartolomé, B.; Gómez-Cordovés, C. Influence of the genotype on the anthocyanin composition, antioxidant capacity and color of Chilean pomegranate (Punica granatum L.) juices. Chil. J. Agric. Res. 2010, 70, 50–57. Available online: https://digital.csic.es/bitstream/10261/44992/1/Sepulveda_Elena.pdf (accessed on 10 August 2024). [CrossRef]
- Zarei, M.; Azizi, M.; Bashir-Sadr, Z. Evaluation of physicochemical characteristics of pomegranate (Punica granatum L.) fruit during ripening. Fruits 2011, 66, 121–129. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Teliban, I.V.; Zamfir, C.I.; Luchian, C.E.; Colibaba, L.C.; Niculaua, M.; Cotea, V.V. Effect of different winemaking conditions on organic acids compounds of white wines. Foods 2021, 10, 2569. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Liu, H.; Yang, W.; Zhang, Q.; Chen, D.; Jiao, Z.; Liu, J. Comprehensive analysis of physicochemical properties and volatile compounds in different strawberry wines under various pre-treatments. Molecules 2024, 29, 2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Wu, Y.; Zhao, D. Uncover the flavor code of strong-aroma Baijiu: Research progress on the revelation of aroma compounds in strong-aroma Baijiu by means of modern separation technology and molecular sensory evaluation. J. Food Compos. Anal. 2022, 109, 104499. [Google Scholar] [CrossRef]
- Jia, W.; Fan, Z.; Du, A.; Li, Y.; Zhang, R.; Shi, Q.; Shi, L.; Chu, X. Recent advances in Baijiu analysis by chromatography based technology–A review. Food Chem. 2020, 324, 126899. [Google Scholar] [CrossRef]
- Song, C.; Hong, X.; Zhao, S.; Liu, J.; Schulenburg, K.; Huang, F.C.; Franz-Oberdorf, K.; Schwab, W. Glucosylation of 4-hydroxy-2,5-dimethyl-3(2H)-furanone, the key strawberry flavor compound in strawberry fruit. Plant Physiol. 2016, 171, 139–151. [Google Scholar] [CrossRef]
- Massera, A.; Assof, M.; Sari, S.; Ciklic, I.; Mercado, L.; Jofré, V.; Combina, M. Effect of low temperature fermentation on the yeast-derived volatile aroma composition and sensory profile in Merlot wines. LWT 2021, 142, 111069. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhao, H.; Huang, M.; Sun, Y.; Zhang, J.; Sun, B. Recent advances in the understanding of off-flavors in alcoholic beverages: Generation, regulation, and challenges. J. Food Compos. Anal. 2021, 103, 104117. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, X.; Guo, Y.; Zhou, D.; Zeng, H.; Fu, H. The microbial diversity and flavour metabolism of Chinese strong flavour Baijiu: A review. J. Inst. Brew. 2023, 129, 15–38. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Ren, Y.; Wang, X.; Li, H.; Liu, Z.; Yue, T.; Gao, Z. Effect of inoculation method on the quality and nutritional characteristics of low-alcohol kiwi wine. LWT 2022, 156, 113049. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.-J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties, sugar and organic acid profiles, and antioxidant compositions of strawberries. Food Sci. Biotechnol. 2019, 28, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, X.; Xue, X.; Lan, W.; Zeng, H.; Li, R.; Pan, T.; Li, N.; Gong, Z.; Yang, H. Comparison of the correlations of microbial community and volatile compounds between pit-mud and fermented grains of compound-flavor Baijiu. Foods 2024, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.; Goel, M.; Batra, D.; Garg, N.; Tuwani, R.; Sethupathy, A.; Bagler, G. Flavor DB2: An updated database of flavor molecules. arXiv 2022, arXiv:2205.05451. [Google Scholar]
- Kamatou, G.P.; Viljoen, A.M. Linalool–A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef]
- Amiri, P.; Shahpiri, A.; Asadollahi, M.A.; Momenbeik, F.; Partow, S. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol. Lett. 2016, 38, 503–508. [Google Scholar] [CrossRef]
- Chen, D.; Chia, J.Y.; Liu, S.-Q. Impact of addition of aromatic amino acids on non-volatile and volatile compounds in lychee wine fermented with Saccharomyces cerevisiae MERIT.ferm. Int. J. Food Microbiol. 2014, 170, 12–20. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the key aroma volatile compounds in nine different grape varieties wine by headspace gas chromatography–ion mobility spectrometry (HS-GC-IMS), odor activity values (OAV) and sensory analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Ma, T.; Liu, X.; Huang, W.; Zhan, J. Profiles of phenolic acids and flavan-3-ols for select Chinese red wines: A comparison and differentiation according to geographic origin and grape variety. J. Food Sci. 2015, 80, C2170–C2179. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Development of a sensitive non-targeted method for characterizing the wine volatile profile using headspace solid-phase microextraction comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 504–517. [Google Scholar] [CrossRef]
- He, X.; Jeleń, H.H. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC × GC-TOFMS) in conventional and reversed column configuration for the investigation of Baijiu aroma types and regional origin. J. Chromatogr. A 2021, 1636, 461774. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Petronilho, S.; Camara, J.S.; Rocha, S.M. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction as a powerful tool for quantification of ethyl carbamate in fortified wines. The case study of Madeira wine. J. Chromatogr. A 2010, 1217, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, S.; Nie, Y.; Xu, Y. Characterization of volatile sulfur compounds in soy sauce aroma type Baijiu and changes during fermentation by GC×GC-TOFMS, organoleptic impact evaluation, and multivariate data analysis. Food Res. Int. 2020, 131, 109043. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Zhou, Z.L.; Yu, H.B.; Mao, L.G.; Pan, X.X.; Ji, Z.W.; Mao, J. Characterization of volatile aging markers in traditional semi-dry Huangjiu by comprehensive two-dimensional gas chromatography-time of flight mass spectrometry and chemometrics. Food Sci. 2023, 44, 249–258. [Google Scholar] [CrossRef]
- Liang, H.; Gao, D.; Wang, C.; Gao, H.; Guo, Y.; Zhao, Z.; Shi, H. Effect of fermentation strategy on the quality and aroma characteristics of yellow peach wines. Fermentation 2022, 8, 604. [Google Scholar] [CrossRef]


| Cultivar/Sample | Ethanol (%, v/v) | Residual Sugar (g/L) |
|---|---|---|
| BX | 13.25 ± 0.59 | 3.47 ± 0.65 a |
| TCL | 13.02 ± 0.6 | 3.44 ± 0.17 a |
| TZ | 13.12 ± 0.08 | 1.86 ± 0.02 b |
| ZJ | 13.69 ± 0.1 | 3.22 ± 0.04 a |
| Cultivar/ Sample | pH | Total Acid (g/L) | ||
|---|---|---|---|---|
| Juice | Wine | Juice | Wine | |
| BX | 4.06 ± 0.05 a | 4.03 ± 0.05 a | 4.03 ± 0.05 d | 5.46 ± 0.37 b |
| TCL | 3.36 ± 0.05 d | 3.53 ± 0.05 d | 6.63 ± 0.15 a | 7.7 ± 0.1 a |
| TZ | 3.7 ± 0 b | 3.83 ± 0.05 b | 6.26 ± 0.05 b | 7.53 ± 0.23 a |
| ZJ | 3.6 ± 0 c | 3.73 ± 0.05 c | 5.46 ± 0.32 c | 6.56 ± 0.11 b |
| Group | Ketones | Hydrocarbons | Heterocyclic Compounds | Aldehydes | Esters | Alcohols | Carboxylic Acids | Others | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Wine | BX | 48 | 88 | 16 | 9 | 130 | 82 | 35 | 478 | 886 |
| TCL | 47 | 74 | 15 | 6 | 125 | 86 | 30 | 441 | 824 | |
| TZ | 43 | 73 | 20 | 9 | 121 | 79 | 30 | 434 | 809 | |
| ZJ | 40 | 58 | 23 | 9 | 100 | 73 | 27 | 382 | 712 | |
| Juice | BX_J | 63 | 109 | 26 | 20 | 123 | 96 | 39 | 552 | 1028 |
| TCL_J | 61 | 90 | 22 | 10 | 103 | 90 | 16 | 456 | 848 | |
| TZ_J | 53 | 88 | 16 | 10 | 132 | 84 | 20 | 456 | 859 | |
| ZJ_J | 63 | 90 | 11 | 17 | 112 | 102 | 24 | 499 | 918 | |
| Group | Alcohols | Aldehydes | Carboxylic Acids | Esters | Heterocyclic Compounds | Hydrocarbons | Ketones | Others | |
|---|---|---|---|---|---|---|---|---|---|
| Wine | BX | 29.77 | 0.09 | 5.82 | 27.92 | 5.78 | 1.14 | 0.81 | 28.62 |
| TCL | 21.80 | 0.14 | 5.51 | 37.77 | 0.25 | 1.58 | 1.40 | 31.50 | |
| TZ | 29.75 | 0.10 | 3.78 | 28.38 | 1.56 | 2.30 | 1.01 | 33.09 | |
| ZJ | 38.81 | 0.16 | 5.09 | 19.27 | 6.30 | 0.51 | 0.84 | 29.00 | |
| Juice | BX_J | 18.82 | 0.69 | 7.16 | 31.36 | 0.53 | 1.18 | 3.12 | 37.11 |
| TCL_J | 22.19 | 0.20 | 8.98 | 18.13 | 3.37 | 1.47 | 2.23 | 43.40 | |
| TZ_J | 26.37 | 0.08 | 1.79 | 21.57 | 0.28 | 0.63 | 4.26 | 44.98 | |
| ZJ_J | 17.44 | 0.64 | 3.72 | 21.99 | 0.07 | 2.58 | 6.45 | 47.07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, W.; Cheng, W.; Li, R.; Zhang, M.; Li, M.; Zhang, Y.; Zhou, Y. Comparison of Flavor Differences between the Juices and Wines of Four Strawberry Cultivars Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry and Sensory Evaluation. Molecules 2024, 29, 4691. https://doi.org/10.3390/molecules29194691
Lan W, Cheng W, Li R, Zhang M, Li M, Zhang Y, Zhou Y. Comparison of Flavor Differences between the Juices and Wines of Four Strawberry Cultivars Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry and Sensory Evaluation. Molecules. 2024; 29(19):4691. https://doi.org/10.3390/molecules29194691
Chicago/Turabian StyleLan, Wei, Wei Cheng, Ruilong Li, Mei Zhang, Mengmeng Li, Yuan Zhang, and Yibin Zhou. 2024. "Comparison of Flavor Differences between the Juices and Wines of Four Strawberry Cultivars Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry and Sensory Evaluation" Molecules 29, no. 19: 4691. https://doi.org/10.3390/molecules29194691
APA StyleLan, W., Cheng, W., Li, R., Zhang, M., Li, M., Zhang, Y., & Zhou, Y. (2024). Comparison of Flavor Differences between the Juices and Wines of Four Strawberry Cultivars Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry and Sensory Evaluation. Molecules, 29(19), 4691. https://doi.org/10.3390/molecules29194691






