Microwave Irradiation as a Powerful Tool for Isolating Isoflavones from Soybean Flour
Abstract
1. Introduction
2. Results
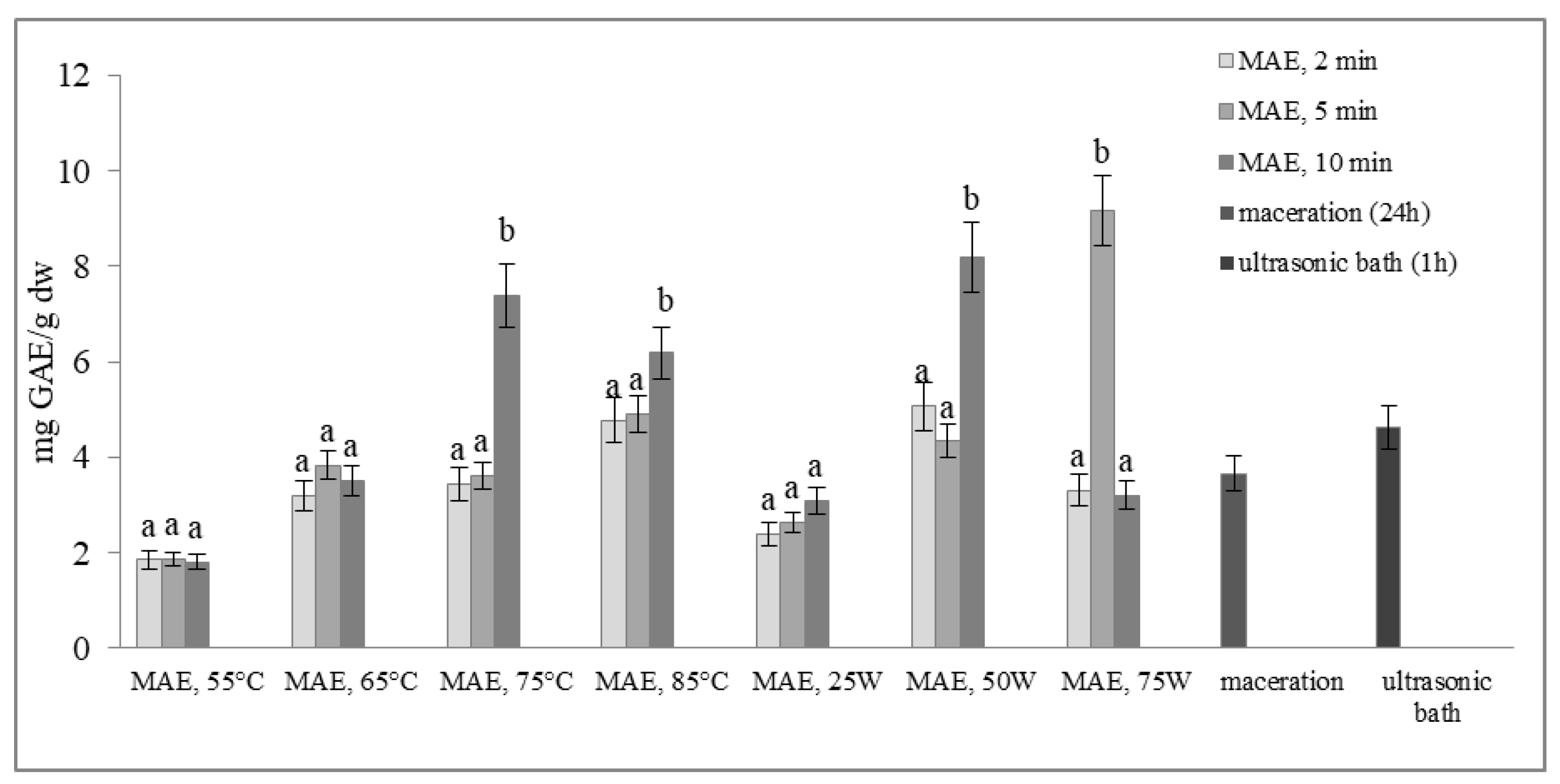
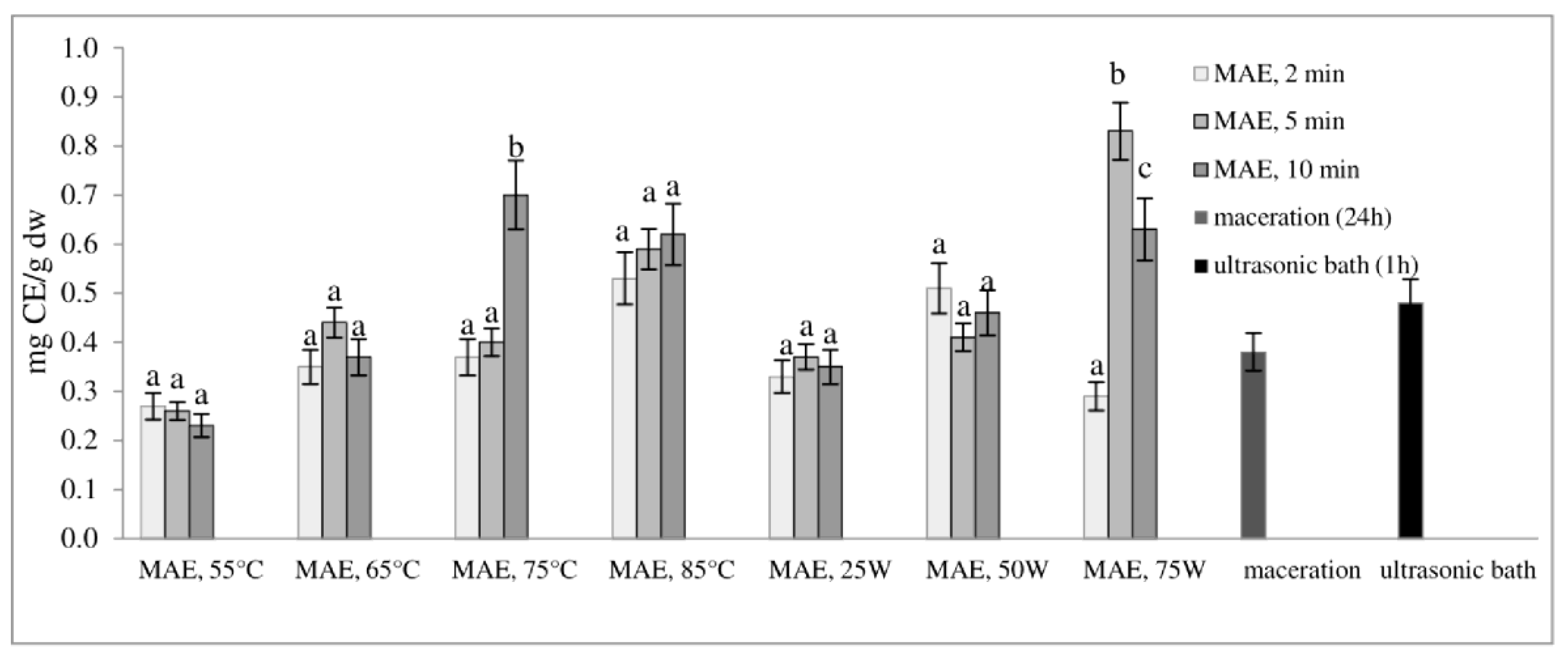
2.1. Total Phenol Content and Total Flavonoid Content
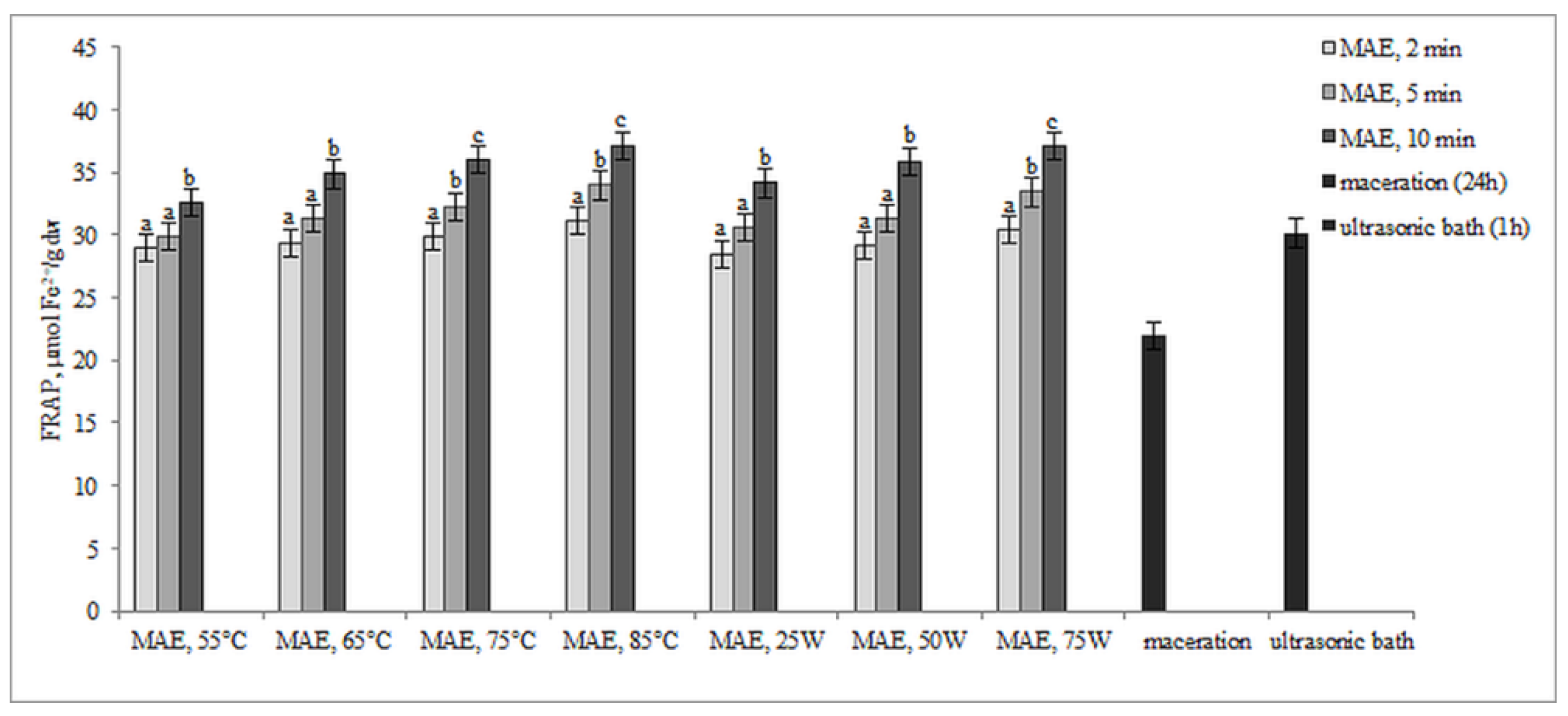
2.2. Antioxidant Activity of Extracts
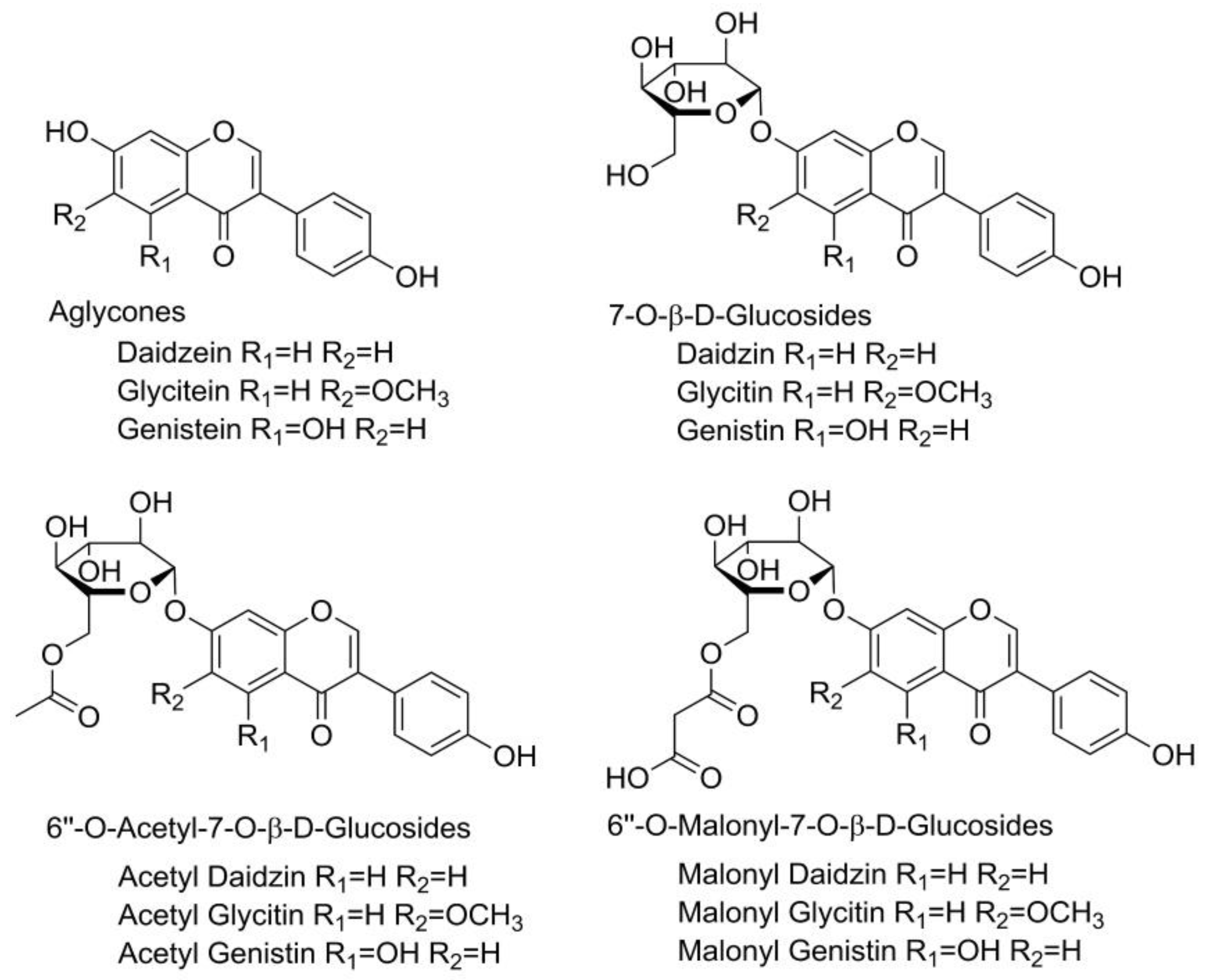
2.3. Individual Isoflavone Content
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Methods
4.3.1. Extraction of Plant Material
4.3.2. Total Phenol Content (TPC)
4.3.3. Total Flavonoid Content (TFC)
4.3.4. Antioxidant Activity
4.3.5. HPLC Analysis of Isoflavones
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Costa, C.; Teodoro, M.; Giambò, F.; Tsatsakis, A.M.; Fenga, C. Polyphenols: A route from bioavailability to bioactivity addressing potential health benefits to tackle human chronic diseases. Arch. Toxicol. 2023, 97, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Farková, V. Isoflavones. In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer: Cham, Switzerland, 2023; Volume 1, pp. 313–339. [Google Scholar]
- Wang, J.F.; Liu, S.S.; Song, Z.Q.; Xu, T.C.; Liu, C.S.; Hou, Y.G.; Huang, R.; Wu, S.H. Naturally Occurring Flavonoids and Isoflavonoids and Their Microbial Transformation: A Review. Molecules 2020, 25, 5112. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M. Phenolic Compounds in Food: Characterization and Health Benefits. Molecules 2022, 27, 783. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef]
- Hod, R.; Maniam, S.; Mohd Nor, N.H. A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer. Molecules 2021, 26, 1105. [Google Scholar] [CrossRef]
- Ohishi, T.; Miyoshi, N.; Mori, M.; Sagara, M.; Yamori, Y. Health Effects of Soy Isoflavones and Green Tea Catechins on Cancer and Cardiovascular Diseases Based on Urinary Biomarker Levels. Molecules 2022, 27, 8899. [Google Scholar] [CrossRef]
- Farhan, M.; El Oirdi, M.; Aatif, M.; Nahvi, I.; Muteeb, G.; Alam, M.W. Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties. Molecules 2023, 28, 2925. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef]
- Juma, S.S.; Ezzat-Zadeh, Z.; Khalil, D.A.; Hooshmand, S.; Akhter, M.; Arjmandi, B.H. Soy protein with or without isoflavones failed to preserve bone density in gonadal hormone-deficient male rat model of osteoporosis. Nutr. Res. 2012, 32, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Ostatnikova, D.; Celec, P.; Putz, Z.; Lapcík, O.; Matucha, P. Short-term effect of soy consumption on thyroid hormone levels and correlation with phytoestrogen level in healthy subjects. Endocr. Regul. 2008, 42, 53–61. [Google Scholar] [PubMed]
- Pejčić, T.; Zeković, M.; Bumbaširević, U.; Kalaba, M.; Vovk, I.; Bensa, M.; Popović, L.; Tešić, Ž. The Role of Isoflavones in the Prevention of Breast Cancer and Prostate Cancer. Antioxidants 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Frankel, F.; Takahashr, H.; Vance, N.; Stiegerwald, C.; Edelstein, S. Collected literature on isoflavones and chronic diseases. Cogent Food Agric. 2016, 2, 1135861. [Google Scholar] [CrossRef]
- Portman, M.A. Kawasaki disease and soy: Potential role for isoflavone interaction with Fcγ receptors. Pediatr. Res. 2013, 73, 130–134. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Chen, X.R.; Luo, Y.J.; Qi, B.; Wan, Y.H. Simultaneous extraction of oil and soy isoflavones from soy sauce residue using ultrasonic-assisted two-phase solvent extraction technology. Sep. Purif. Technol. 2014, 128, 72–79. [Google Scholar] [CrossRef]
- Cong-Cong, X.; Bing, W.; Yi-Qiong, P.; Jian-Sheng, T.; Tong, Z. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules 2010, 15, 6365–6374. [Google Scholar] [CrossRef]
- Chiremba, C.; Rooney, L.W.; Beta, T. Microwave-assisted extraction of bound phenolic acids in bran and flour fractions from sorghum and maize cultivars varying in hardness. J. Agric. Food Chem. 2012, 60, 4735–4742. [Google Scholar] [CrossRef]
- Lovrić, V.; Putnik, P.; Kovačević, D.B.; Jukić, M.; Dragović-Uzelac, V. Effect of Microwave-Assisted Extraction on the Phenolic Compounds and Antioxidant Capacity of Blackthorn Flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Álvarez, A.; Poejo, J.; Matias, A.A.; Duarte, C.M.M.; Cocero, M.J.; Mato, R.B. Microwave pretreatment to improve extraction efficiency and polyphenol extract richness from grape pomace. Effect on antioxidant bioactivity. Food Bioprod. Process. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariépy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Li, Y.; Lin, S.-J.; Li, H.-B. Green Extraction of Natural Antioxidants from the Sterculia nobilis Fruit Waste and Analysis of Phenolic Profile. Molecules 2018, 23, 1059. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Microwave-assisted extraction (MAE) conditions using polynomial design for improving antioxidant phytochemicals in Berberis asiatica Roxb. Ex DC. Leaves. Ind. Crops Prod. 2017, 95, 393–403. [Google Scholar] [CrossRef]
- Shahid, T.; Khan, A.A.; Khalil, A.A.; Batool, M.; Khan, S.; Aslam, A. Effect of Microwave Power and Time on Total Phenolic Contents and Antioxidant Characteristics of Microwave Assisted Extracts of Watermelon Rind Powder: Microwave Assisted Extracts of Watermelon Rind Powder. Pakistan BioMed. J. 2021, 4, pbmj.v4i1.52. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Lai, Q.; Li, T.; Fu, X.; Guo, X.; Liu, R.H. The dynamic changes of ascorbic acid, tocopherols and antioxidant activity during germination of soya bean (Glycine max). Int. J. Food Sci. Technol. 2015, 50, 2367–2374. [Google Scholar] [CrossRef]
- Gebregziabher, B.S.; Gebremeskel, H.; Debesa, B.; Ayalneh, D.; Mitiku, T.; Wendwessen, T.; Habtemariam, E.; Nur, S.; Getachew, T. Carotenoids: Dietary sources, health functions, biofortification, marketing trend and affecting factors—A review. J. Agric. Food Res. 2023, 14, 100834. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Pop, O.L.; Suharoschi, R.; Socaci, S.A.; Berger Ceresino, E.; Weber, A.; Gruber-Traub, C.; Vodnar, D.C.; Fărcas, A.C.; Johansson, E. Polyphenols—Ensured Accessibility from Food to the Human Metabolism by Chemical and Biotechnological Treatments. Antioxidants 2023, 12, 865. [Google Scholar] [CrossRef]
- López-Hortas, L.; Torres, M.D.; Domínguez, H. Chapter 15—Equipment and recent advances in microwave processing. In Innovative and Emerging Technologies in the Bio-marine Food Sector: Applications, Regulations, and Prospects; Garcia-Vaquero, M., Rajauria, G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 333–360. [Google Scholar]
- Azam, M.; Zhang, S.; Abdelghany, A.M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Seed isoflavone profiling of 1168 soybean accessions from major growing ecoregions in China. Food Res. Int. 2020, 130, 108957. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Rodríguez-Gonzalo, E.; Domínguez-Álvarez, J. Analysis of Isoflavones in Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Jung, Y.S.; Nam, T.G.; Rha, C.S.; Ko, M.J.; Jang, D.; Kim, H.S.; Kim, D.O. pH-adjusted solvent extraction and reversed-phase HPLC quantification of isoflavones from soybean (Glycine max (L.) Merr.). J. Food Sci. 2020, 85, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Terigar, B.G.; Balasubramanian, S.; Boldor, D.; Xu, Z.; Lima, M.; Sabliov, C.M. Continuous microwave-assisted isoflavone extraction system: Design and performance evaluation. Bioresour. Technol. 2010, 101, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Soyata, A.; Hasanah, A.N.; Rusdiana, T. Isoflavones in Soybean as a Daily Nutrient: The Mechanisms of Action and How They Alter the Pharmacokinetics of Drugs. Turk. J. Pharm. Sci. 2021, 18, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Miljin, D.; Knežević-Jugović, Z. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds. Ind. Crops Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Woumbo, C.Y.; Kuate, D.; Klang, M.J.; Womeni, H.M. Valorization of Glycine max (Soybean) Seed Waste: Optimization of the Microwave-Assisted Extraction (MAE) and Characterization of Polyphenols from Soybean Meal Using Response Surface Methodology (RSM). J. Chem. 2021, 4869909. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Payum, T.; Shankar, R.; Chandan, T.; Moushumi, H. Antioxidant Potential of Solanum spirale Shoot and Berry: A Medicinal Food Plant Used in Arunachal Pradesh. Int. J. Pharmtech. Res. 2015, 5, 307–314. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]

| n = 3 (µg/g dw) | Time | Temperature | Level of Significance | |||||
|---|---|---|---|---|---|---|---|---|
| Isoflavones | 55 °C | 65 °C | 75 °C | 85 °C | Te | Ti | TexTi | |
| Daidzin | 2 min | 30.68 ± 0.56 a | 80.65 ± 0.79 bA | 84.28 ± 1.20 bA | 116.62 ± 2.63 cA | *** | *** | *** |
| 5 min | 28.29 ± 0.24 a | 89.82 ± 0.07 bB | 93.02 ± 0.66 bB | 121.52 ± 2.12 cA | ||||
| 10 min | 30.57 ± 0.94 a | 105.51 ± 1.66 bC | 107.09 ± 2.91 bC | 156.28 ± 3.03 cB | ||||
| Glycitin | 2 min | 0.00 ± 0.00 aA | 0.81 ± 0.05 bA | 2.60 ± 0.47 cB | 3.30 ± 0.14 d | *** | *** | *** |
| 5 min | 0.00 ± 0.00 aA | 1.55 ± 0.08 bB | 1.76 ± 0.28 bA | 3.37 ± 0.37 c | ||||
| 10 min | 0.71 ± 0.22 aB | 3.04 ± 1.05 bC | 2.87 ± 0.05 bB | 3.71 ± 0.07 c | ||||
| Genistin | 2 min | 30.71 ± 1.52 a | 91.59 ± 1.00 bA | 88.26 ± 2.64 bA | 122.38 ± 4.04 cA | *** | *** | *** |
| 5 min | 29.90 ± 0.91 a | 99.73 ± 1.69 bA | 103.34 ± 1.73 bB | 126.38 ± 3.08 cA | ||||
| 10 min | 32.90 ± 0.70 a | 117.95 ± 2.88 bB | 124.01 ± 0.41 bC | 157.51 ± 7.10 cB | ||||
| Daidzein | 2 min | 5.43 ± 0.05 cC | 5.25 ± 0.46 bcB | 4.50 ± 0.28 bB | 1.90 ± 0.09 aA | *** | *** | *** |
| 5 min | 4.35 ± 0.10 bB | 4.97 ± 0.13 bB | 2.67 ± 0.25 aA | 2.40 ± 0.57 aA | ||||
| 10 min | 1.34 ± 0.17 aA | 2.84 ± 0.18 bA | 5.08 ± 0.36 cB | 5.34 ± 0.11 cB | ||||
| Glycitein | 2 min | 141.20 ± 6.34 a | 449.90 ±3.01 bcAB | 438.19 ± 4.09 bA | 495.90 ± 15.77 c | *** | NS | *** |
| 5 min | 146.03 ± 4.25 a | 477.97 ± 5.46 bB | 443.73 ± 8.94 bA | 487.59 ± 55.32 bc | ||||
| 10 min | 110.98 ± 1.79 a | 418.67 ± 21.25 bA | 559.99 ± 5.44 cB | 452.15 ± 8.17 b | ||||
| Genistein | 2 min | 2.77 ± 0.05 a | 3.28 ± 0.05 aA | 3.13 ± 0.09 aA | 6.02 ± 0.35 bA | *** | *** | *** |
| 5 min | 2.62 ± 0.11 a | 3.37 ± 0.07 abA | 4.06 ± 0.24 bB | 6.48 ± 0.48 cA | ||||
| 10 min | 3.43 ± 0.26 a | 5.71 ± 0.26 bB | 4.11 ± 0.17 aB | 8.41 ± 0.66 cB | ||||
| Total | 2 min | 210.78 ± 8.35 a | 631.48 ± 4.47 b | 620.96 ± 7.02 bA | 746.11 ± 22.06 c | *** | *** | *** |
| 5 min | 211.19 ± 5.22 a | 677.41 ± 6.22 b | 648.58 ±10.65 bA | 747.73 ± 60.03 c | ||||
| 10 min | 179.94 ± 1.27 a | 653.72 ± 25.95 b | 803.15 ± 8.24 cB | 783.40 ± 17.58 c | ||||
| n = 3 (µg/g dw) | Time | Power | Level of Significance | ||||
|---|---|---|---|---|---|---|---|
| Isoflavones | 25 W | 50 W | 75 W | P | Ti | PxTi | |
| Daidzin | 2 min | 59.35 ± 2.97 aA | 117.05 ± 4.11 cA | 90.54 ± 0.29 bA | *** | *** | *** |
| 5 min | 71.17 ± 2.42 aB | 122.50 ± 3.27 bA | 184.24 ± 3.84 cC | ||||
| 10 min | 81.42 ± 7.26 aB | 147.24 ± 4.45 cB | 131.94 ± 1.86 bB | ||||
| Glycitin | 2 min | 0.00 ± 0.00 aA | 2.92 ± 0.10 cC | 1.94 ± 0.15 bB | *** | *** | *** |
| 5 min | 0.70 ± 0.21 aB | 2.32 ± 0.17 bB | 7.31 ± 0.23 cC | ||||
| 10 min | 1.22 ± 0.15 C | 1.11 ± 0.08 A | 0.85 ± 0.02 A | ||||
| Genistin | 2 min | 61.77 ± 4.29 aA | 127.30 ± 2.18 cA | 103.84 ± 1.12 bA | *** | *** | *** |
| 5 min | 81.91 ± 1.03 aB | 130.51 ± 4.09 bA | 211.10 ± 3.37 cC | ||||
| 10 min | 95.01 ± 4.53 aC | 145.09 ± 4.74 cB | 132.26 ± 2.69 bB | ||||
| Daidzein | 2 min | 0.00 ± 0.00 a | 0.00 ± 0.00 aA | 1.35 ± 0.07 bA | *** | *** | *** |
| 5 min | 0.00 ± 0.00 a | 0.50 ± 0.08 bB | 1.88 ± 0.11 cB | ||||
| 10 min | 0.00 ± 0.00 a | 0.96 ± 0.05 bC | 1.32 ± 0.10 cA | ||||
| Glycitein | 2 min | 196.73 ± 10.94 aA | 514.26 ± 13.47 cB | 434.37 ± 1.92 bB | *** | *** | *** |
| 5 min | 323.01 ± 6.56 aB | 463.80 ± 15.12 bA | 551.84 ± 9.78 cC | ||||
| 10 min | 349.40 ± 15.60 aB | 534.12 ± 13.80 bB | 366.74 ± 10.35 aA | ||||
| Genistein | 2 min | 3.69 ± 0.10 | 4.18 ± 0.16 A | 3.43 ± 0.14 A | *** | *** | *** |
| 5 min | 3.42 ± 0.08 a | 5.16 ± 0.83 bA | 7.13 ± 0.32 cB | ||||
| Total | 10 min | 3.87 ± 0.26 a | 10.46 ± 0.58 cB | 8.03 ± 0.20 bB | |||
| 2 min | 321.55 ± 18.00 aA | 765.72 ± 19.81 cA | 635.47 ± 1.64 bA | *** | *** | *** | |
| 5 min | 480.21 ± 8.91 aB | 724.30 ± 21.55 bA | 963.52± 17.35 cB | ||||
| 10 min | 530.92 ± 27.55 aB | 838.02 ± 23.19 cB | 641.16± 15.09 bA | ||||
| Isoflavones | Maceration | Ultrasound Bath |
|---|---|---|
| daidzin | 87.442 ± 0.74 a | 135.58 ± 1.62 b |
| glycitin | 0.62 ± 0.05 a | 3.21 ± 0.13 b |
| genistin | 99.13 ± 2.03 a | 140.95 ± 1.89 b |
| daidzein | 3.2 ± 0.08 b | 1.53 ± 0.13 a |
| glycitein | 468.43 ± 14.59 a | 454.31 ± 2.45 a |
| genistein | 2.99 ± 0.08 a | 6.71 ± 0.20 b |
| Total | 661.81 | 742.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurović, S.; Nikolić, B.; Pisinov, B.; Mijin, D.; Knežević-Jugović, Z. Microwave Irradiation as a Powerful Tool for Isolating Isoflavones from Soybean Flour. Molecules 2024, 29, 4685. https://doi.org/10.3390/molecules29194685
Đurović S, Nikolić B, Pisinov B, Mijin D, Knežević-Jugović Z. Microwave Irradiation as a Powerful Tool for Isolating Isoflavones from Soybean Flour. Molecules. 2024; 29(19):4685. https://doi.org/10.3390/molecules29194685
Chicago/Turabian StyleĐurović, Sanja, Bogdan Nikolić, Boris Pisinov, Dušan Mijin, and Zorica Knežević-Jugović. 2024. "Microwave Irradiation as a Powerful Tool for Isolating Isoflavones from Soybean Flour" Molecules 29, no. 19: 4685. https://doi.org/10.3390/molecules29194685
APA StyleĐurović, S., Nikolić, B., Pisinov, B., Mijin, D., & Knežević-Jugović, Z. (2024). Microwave Irradiation as a Powerful Tool for Isolating Isoflavones from Soybean Flour. Molecules, 29(19), 4685. https://doi.org/10.3390/molecules29194685










