Breath Analysis: Identification of Potential Volatile Biomarkers for Non-Invasive Diagnosis of Chronic Kidney Disease (CKD)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Study Design
4.2. Breath Sampling and Analysis
4.3. Data Analysis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golshayan, D.; Pascual, M. Burden of end-stage renal disease and evolving challenges in kidney transplantation. Transpl. Int. 2019, 32, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Improving Global Outcomes (KDIGO). KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105 (Suppl. S4), S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Polanowska, B.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Śliwka, I. The application of chromatographic breath analysis in the search of volatile biomarkers of chronic kidney disease and coexisting type 2 diabetes mellitus. J. Chromatogr. B 2017, 1060, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Goerl, T.; Kischkel, S.; Sawacki, A.; Fuchs, P.; Miekisch, W.; Schubert, J.K. Volatile breath biomarkers for patient monitoring during haemodialysis. J. Breath Res. 2013, 7, 017116. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, S.; Paschke, K.M.; Wang, X.; Grove, D.; Heyka, R.J.; Dweik, R.A. Molecular breath analysis identifies the breathprint of renal failure. J. Breath Res. 2017, 11, 026009. [Google Scholar] [CrossRef]
- Endre, Z.H.; Pickering, J.W.; Storer, M.K.; Hu, W.P.; Moorhead, K.T.; Allardyce, R.; McGregor, D.O.; Scotter, J.M. Breath ammonia and trimethylamine allow real-time monitoring of haemodialysis efficacy. Physiol. Meas. 2011, 32, 115–130. [Google Scholar] [CrossRef]
- Lamote, K.; Hiddinga, B.; Van Cleemput, J.; Nackaerts, K.; Thas, O.; Van Meerbeeck, J.P. A breath test for diagnosing malignant pleural mesothelioma. Ann. Oncol. 2014, 25 (Suppl. S4), 542–545. [Google Scholar] [CrossRef][Green Version]
- Brusselmans, L.; Arnouts, L.; Millevert, C.; Vandersnickt, J.; Van Meerbeeck, J.P.; Lamote, K. Breath analysis as a diagnostic and screening tool for malignant pleural mesothelioma: A systematic review (Review). Transl. Lung Cancer Res. 2018, 7, 520–536. [Google Scholar] [CrossRef]
- Di Gilio, A.; Catino, A.; Lombardi, A.; Palmisani, J.; Facchini, L.; Mongelli, T.; Varesano, N.; Bellotti, R.; Galetta, D.; de Gennaro, G.; et al. Breath Analysis for early detection of malignant pleural mesothelioma: Volatile Organic Compounds (VOCs) determination and possible biochemical pathways. Cancers 2020, 12, 1262. [Google Scholar] [CrossRef]
- Davies, S.J.; Španěl, P.; Smith, D. Breath analysis of ammonia, volatile organiccompounds and deuterated water vapor in chronic kidney disease and duringdialysis. Bioanalysis 2014, 6, 843–857. [Google Scholar] [CrossRef]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pagonas, N.; Vautz, W.; Seifert, L.; Slodzinski, R.; Jankowski, J.; Zidek, W.; Westhoff, T.H. Volatile Organic Compounds in Uremia. PLoS ONE 2012, 7, e46258. [Google Scholar] [CrossRef] [PubMed]
- Schönermarck, U.; Dengler, C.; Gmeinwieser, A.; Praun, S.; Schelling, G.; Fischereder, M.; Boulesteix, A.-L.; Dolch, M.E. Exhaled breath volatile organic and inorganic compound composition in end-stage renal disease. Clin. Nephrol. 2016, 86, 132–140. [Google Scholar] [CrossRef]
- Mochalski, P.; King, J.; Haas, M.; Unterkofler, K.; Amann, A.; Mayer, G. Blood and breath profiles of volatile organic compounds in patients with end-stage renal disease. BMC Nephrol. 2014, 15, 43. [Google Scholar] [CrossRef]
- Simenhoff, M.L.; Burke, J.F.; Saukkonen, J.J.; Ordinario, A.T.; Doty, R. Biochemical profile or uremic breath. N. Engl. J. Med. 1977, 297, 132–135. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Lu, P.C.; Tain, Y.L. Association of Trimethylamine, Trimethylamine N-oxide, and Dimethylamine with Cardiovascular Risk in Children with Chronic Kidney Disease. J. Clin. Med. 2020, 9, 336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romani, A.; Marrone, G.; Celotto, R.; Campo, M.; Vita, C.; Chiaramonte, C.; Carretta, A.; Di Daniele, N.; Noce, A. Utility of SIFT MS to evaluate volatile organic compounds in nephropathic patients’ breath. Sci. Rep. 2022, 12, 10413. [Google Scholar] [CrossRef]
- Lirk, P.; Bodrogi, F.; Raifer, H.; Greiner, K.; Ulmer, H.; Rieder, J. Elective haemodialysis increases exhaled isoprene. Nephrol. Dial. Transpl. Transplant. 2003, 18, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Davies, S. A new ‘online’ method to measure increased exhaled isoprene in end-stage renal failure. Nephrol. Dial. Transpl. Transplant. 2001, 16, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar] [CrossRef]
- Das, S.; Pal, S.; Mitra, M. Significance of exhaled breath test in clinical diagnosis: A special focus on the detection of diabetes mellitus. J. Med. Biol. Eng. 2016, 36, 605–624. [Google Scholar] [CrossRef]
- Lehman-McKeeman, L.D. Absorption, distribution, and excretion of toxicants. In Casarett and Dowll’s Toxicology: The Basic Science of Poisons; Klaassen, C.D., Ed.; McGraw-Hill Education: New York, NY, USA, 2013; pp. 153–183. [Google Scholar]
- Catino, A.; de Gennaro, G.; Di Gilio, A.; Facchini, L.; Galetta, D.; Palmisani, J.; Porcelli, F.; Varesano, N. Breath Analysis: A Systematic Review of Volatile Organic Compounds (VOCs) in Diagnostic and Therapeutic Management of Pleural Mesothelioma. Cancers 2019, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Varadwaj, P. Smelling the Disease: Diagnostic Potential of Breath Analysis. Mol. Diagn. Ther. 2023, 27, 321–347. [Google Scholar] [CrossRef]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Ferrannini, M.; Fabrini, R.; Bocedi, A.; Dessì, M.; Galli, F.; Federici, G.; Palumbo, R.; Di Daniele, N.; Ricci, G. Erythrocyte glutathione transferase: A new biomarker for hemodialysis adequacy, overcoming the Kt/V(urea) dogma? Cell Death Dis. 2012, 3, e377. [Google Scholar] [CrossRef][Green Version]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska-Bender, M.; Dykowska, G.; Zuk, W.; Milewska, M.; Staniszewska, A. The impact on quality of life of dialysis patients with renal insufficiency. Patient Prefer. Adherence 2018, 12, 577–583. [Google Scholar] [CrossRef]
- Colussi, G.; Brunati, C.C.M.; Gervasi, F.; Montoli, A.; Vergani, D.; Curci, F.; Minetti, E. A simple method for the calculation of dialysis Kt factor as a quantitative measure of removal efficiency of uremic retention solutes: Applicability to high-dialysate vs low-dialysate volume technologies. PLoS ONE 2020, 15, e0233331. [Google Scholar] [CrossRef]
- Chen, W.; Metsala, M.; Vaittinen, O.; Halonen, L. The origin of mouth-exhaled ammonia. J. Breath Res. 2014, 8, 036003. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J.K.; Staude, H.; Fischer, D.-C.; Miekisch, W. Exhaled volatile substances mirror clinical conditions in pediatric chronic kidney disease. PLoS ONE 2017, 12, e0178745. [Google Scholar] [CrossRef]
- Saidi, T.; Zaim, O.; Moufid, M.; El Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Seong, S.-H.; Kim, H.S.; Lee, Y.-M.; Kim, J.-S.; Park, S.; Oh, J. Exploration of Potential Breath Biomarkers of Chronic Kidney Disease through Thermal Desorption–Gas Chromatography/Mass Spectrometry. Metabolites 2023, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.A.; Walker, V. Headspace solid-phase microextraction profiling of volatile compounds in urine: Application to metabolic investigations. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Wahl, H.G.; Hoffmann, A.; Luft, D.; Liebich, H.M. Analysis of volatile organic compounds in human urine by headspace gas chromatography-mass spectrometry with a multipurpose sampler. J. Chromatogr. A. 1999, 847, 117–125. [Google Scholar] [CrossRef]
- Hensley, C.T.; Faubert, B.; Yuan, Q. Metabolic heterogeneity in human lung tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef]
- Otsuka, M.; Mine, T.; Ohuchi, K.; Ohmori, S. A detoxication route for acetaldehyde: Metabolism of diacetyl, acetoin, and 2,3-butanediol in liver homogenate and perfused liver of rats. J. Biochem. 1996, 119, 246–251. [Google Scholar] [CrossRef]
- Jepson, C.; Hsu, J.Y.; Fischer, M.J.; Kusek, J.W.; Lash, J.P.; Ricardo, A.C.; Schelling, J.R.; Feldman, H.I.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Incident Type 2 Diabetes Among Individuals With CKD: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2019, 73, 72–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mochalski, P.; Krapf, K.; Ager, C.; Wiesenhofer, H.; Agapiou, A.; Statheropoulos, M.; Fuchs, D.; Ellmerer, E.; Buszewski, B.; Amann, A. Temporal profiling of human urine VOCs and its potential role under the ruins of collapsed buildings. Toxicol. Mech. Methods. 2012, 22, 502–511. [Google Scholar] [CrossRef]
- De Vietro, N.; Aresta, A.M.; Picciariello, A.; Altomare, D.; Lucarelli, G.; Di Gilio, A.; Palmisani, J.; De Gennaro, G.; Zambonin, C. Optimization of a Breath Analysis Methodology to Potentially Diagnose Transplanted Kidney Rejection: A Preclinic Study. Appl. Sci. 2023, 13, 2852. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath analysis: Comparison among methodological approaches for breath sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clin. Chim. Acta 2004, 47, 25–39. [Google Scholar] [CrossRef]
- Phillips, M.; Basa-Dalay, V.; Bothamley, G.; Cataneo, R.N.; Lam, P.K.; Natividad, M.P.; Schmitt, P.; Wai, J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosi 2010, 90, 145–151. [Google Scholar] [CrossRef]
- Pabst, F.; Miekisch, W.; Fuchs, P.; Kischkel, S.; Schubert, J.K. Monitoring of oxidative and metabolic stress during cardiac surgery by means of breath biomarkers: An observational study. J. Cardiothorac. Surg. 2007, 9, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Halliwel, B. Free radicals, antioxidants, and human disease: Where are we now? J. Lab. Clin. Med. 1992, 119, 598. [Google Scholar]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Weinstein, T. Haemolysis in haemodialysis patients: Evidence for impaired defence mechanisms against oxidative stress. Nephrol. Dial. Transpl. Transplant. 2000, 15, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Mamatha, S.; Yoav, Y.B.; Abraham, M.; Hossam, H. Profiling Single Cancer Cells with Volatolomics Approach. IScience 2019, 11, 178–188. [Google Scholar]
- Di Gilio, A.; Palmisani, J.; Picciariello, A.; Altomare, D.F.; de Gennaro, G. Identification of a characteristic VOCs pattern in the exhaled breath of post-COVID subjects: Are metabolic alterations induced by the infection still detectable? J. Breath. Res. 2023, 17, 047101. [Google Scholar] [CrossRef]
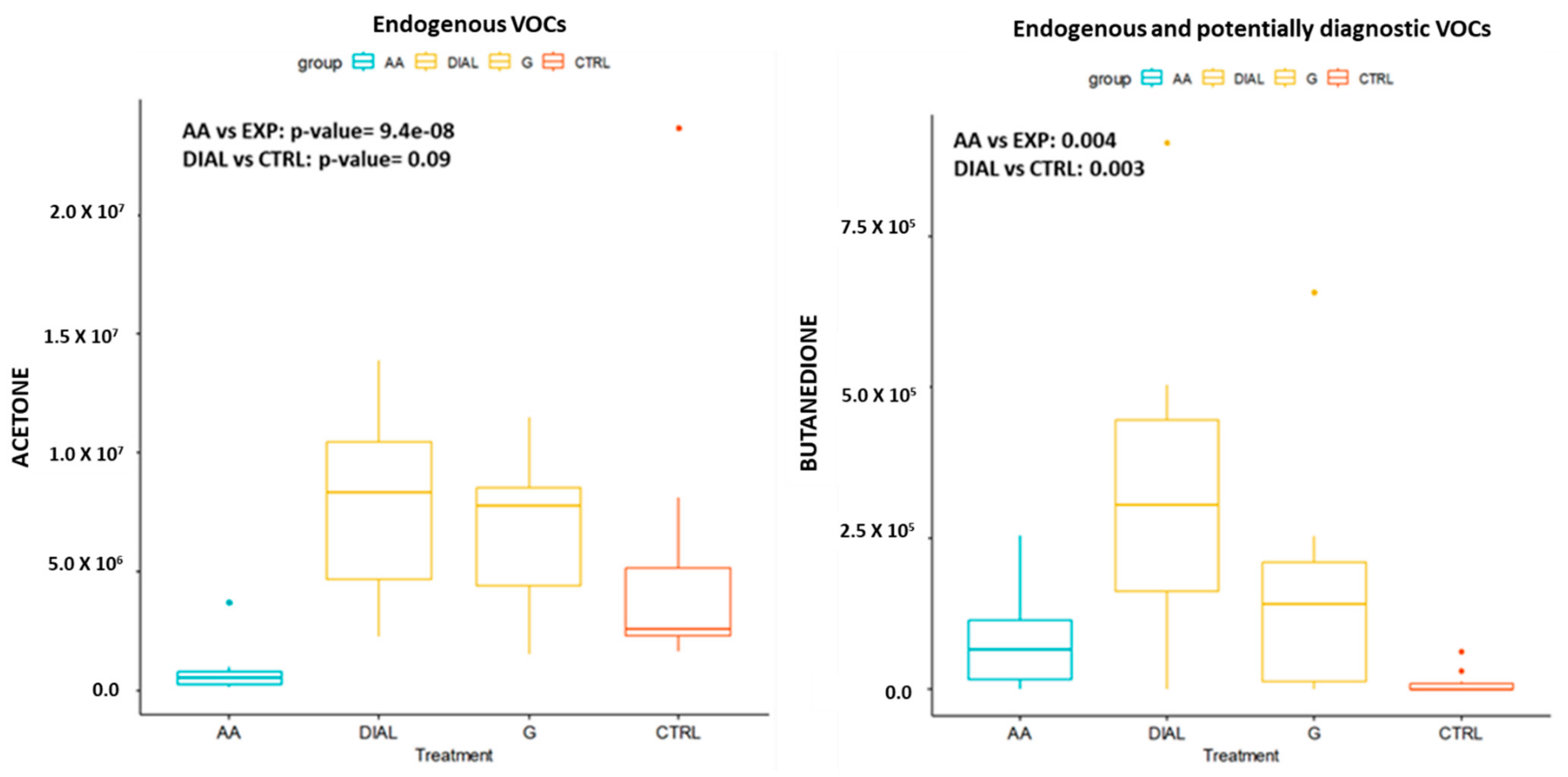

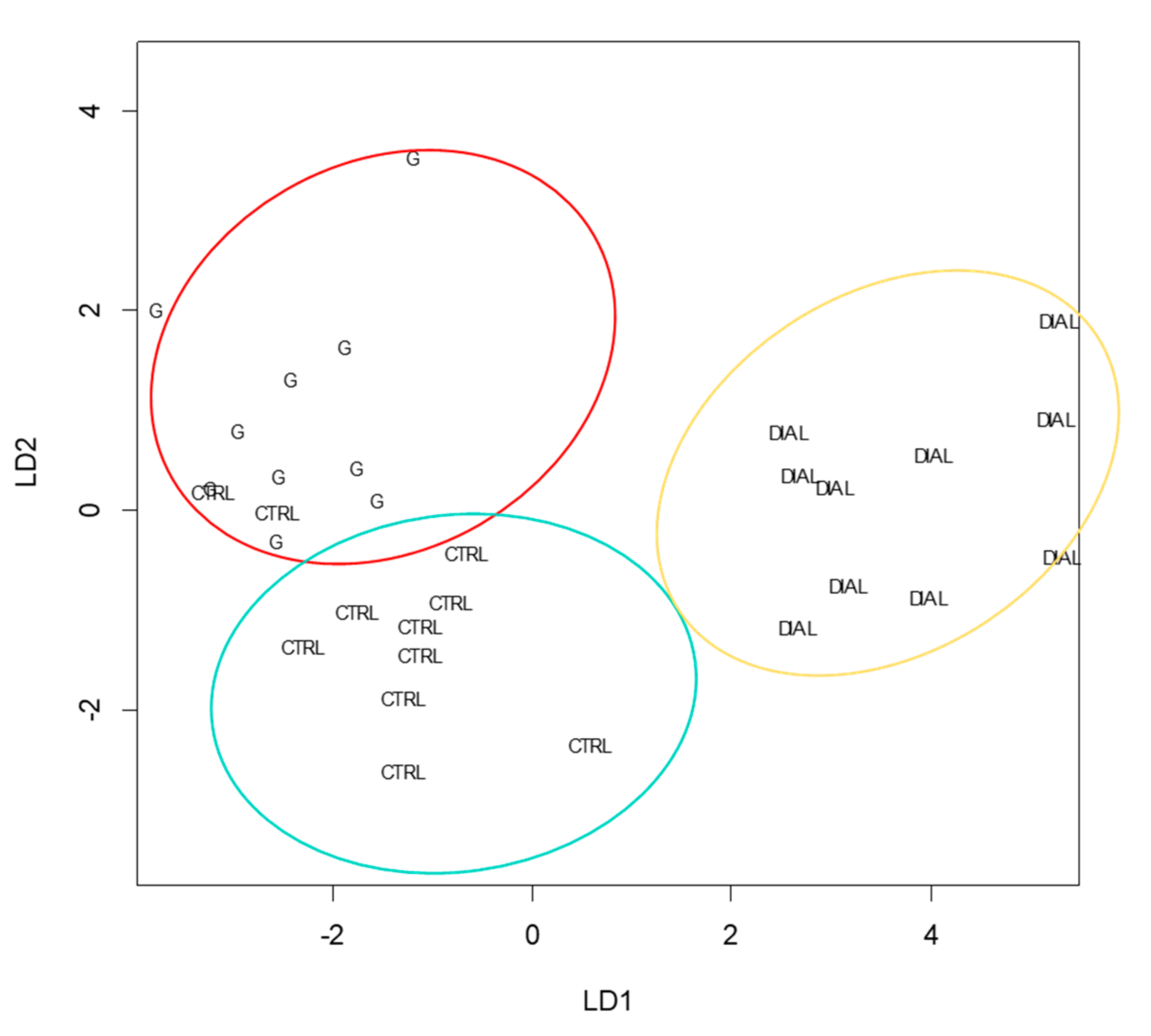
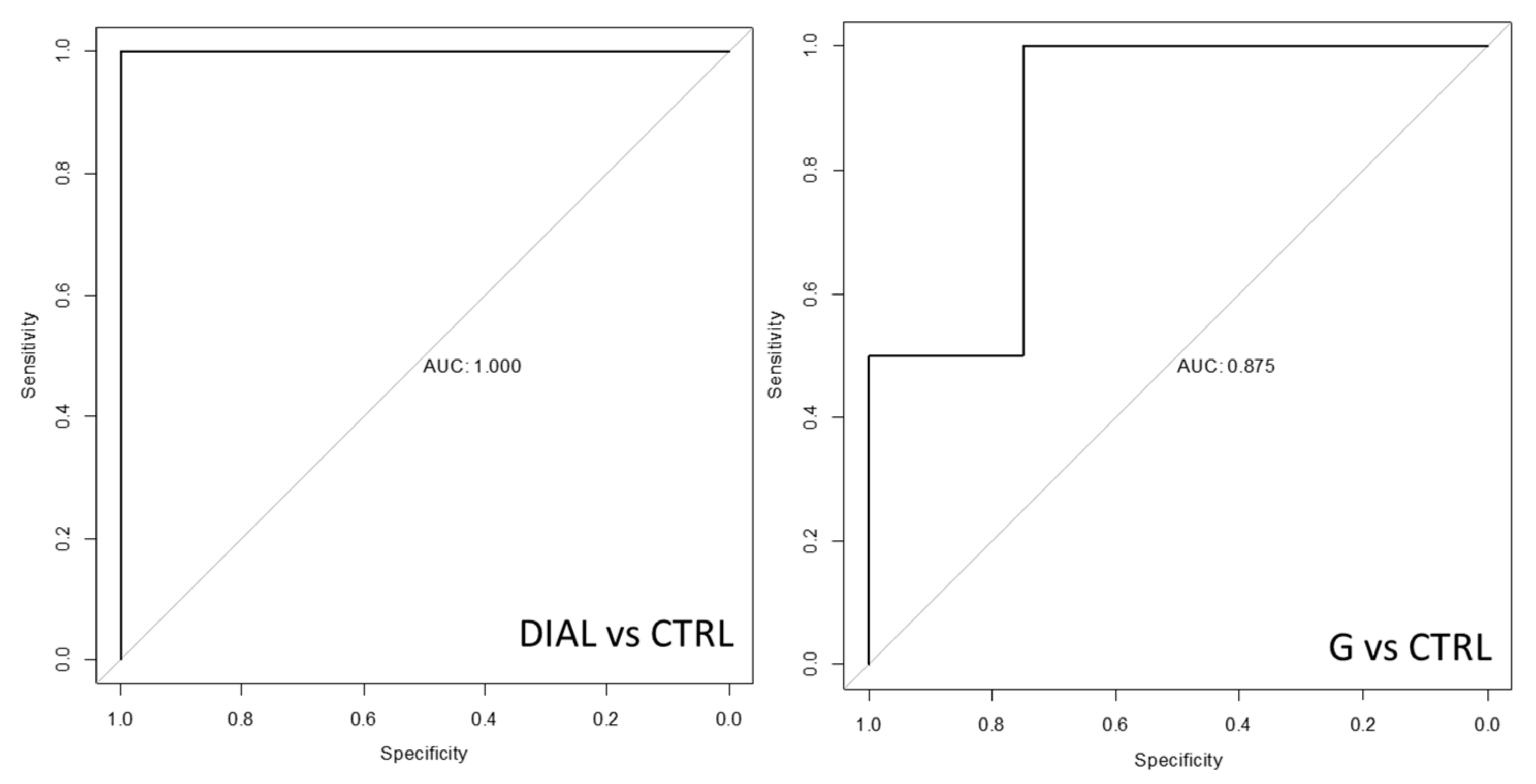
| Endogenous VOCs | CTRL vs. G | CTRL vs. DIAL |
|---|---|---|
| Pentane | 0.21 | 0.03 |
| Octane | 0.32 | 0.02 |
| Eptene | 0.72 | 0.005 |
| Undecane | 0.03 * | 0.01 |
| Butanedione | 0.03 * | 0.004 |
| Pentanone | 0.33 | 0.002 |
| Trioxane | 0.86 | 0.01 |
| 2-Ethylhexanol | 0.02 * | 0.0003 |
| Acetophenone | 0.85 | 0.01 |
| Benzaldehyde | 0.97 | 0.02 |
| Nonanal | 0.66 | 0.01 |
| Benzene | 0.62 | 0.02 |
| Toluene | 0.97 | 0.01 |
| Ethylbenzene | 0.79 | 0.0004 |
| Xylene | 0.48 | 0.0006 |
| Total Samples n: 30 | CKD (DIAL+G) | |||
|---|---|---|---|---|
| CTRL (n: 10) | DIAL (n: 10) | CKD Patients (Stage G3) (n: 7) | CKD Patients Stage G2 (n: 3) | |
| Age | 63 (49–81) | 67 (54–82) | 75 (60–83) | 66 (65–68) |
| M:F | 6:4 (60% vs. 40%) | 6:4 (60% vs. 40%) | 6:1 (86% vs. 14%) | 6:4 (67% vs. 33%) |
| BMI mean | 25 | 25 | 25 | 27 |
| diabetes | 0 | 2 | 2 | 1 |
| hypertension | 4 | 3 | 3 | 2 |
| hypercholesterolemia | 3 | 1 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gilio, A.; Palmisani, J.; Nisi, M.; Pizzillo, V.; Fiorentino, M.; Rotella, S.; Mastrofilippo, N.; Gesualdo, L.; de Gennaro, G. Breath Analysis: Identification of Potential Volatile Biomarkers for Non-Invasive Diagnosis of Chronic Kidney Disease (CKD). Molecules 2024, 29, 4686. https://doi.org/10.3390/molecules29194686
Di Gilio A, Palmisani J, Nisi M, Pizzillo V, Fiorentino M, Rotella S, Mastrofilippo N, Gesualdo L, de Gennaro G. Breath Analysis: Identification of Potential Volatile Biomarkers for Non-Invasive Diagnosis of Chronic Kidney Disease (CKD). Molecules. 2024; 29(19):4686. https://doi.org/10.3390/molecules29194686
Chicago/Turabian StyleDi Gilio, Alessia, Jolanda Palmisani, Marirosa Nisi, Valentina Pizzillo, Marco Fiorentino, Stefania Rotella, Nicola Mastrofilippo, Loreto Gesualdo, and Gianluigi de Gennaro. 2024. "Breath Analysis: Identification of Potential Volatile Biomarkers for Non-Invasive Diagnosis of Chronic Kidney Disease (CKD)" Molecules 29, no. 19: 4686. https://doi.org/10.3390/molecules29194686
APA StyleDi Gilio, A., Palmisani, J., Nisi, M., Pizzillo, V., Fiorentino, M., Rotella, S., Mastrofilippo, N., Gesualdo, L., & de Gennaro, G. (2024). Breath Analysis: Identification of Potential Volatile Biomarkers for Non-Invasive Diagnosis of Chronic Kidney Disease (CKD). Molecules, 29(19), 4686. https://doi.org/10.3390/molecules29194686












