Hydrogen Separation Membranes: A Material Perspective
Abstract
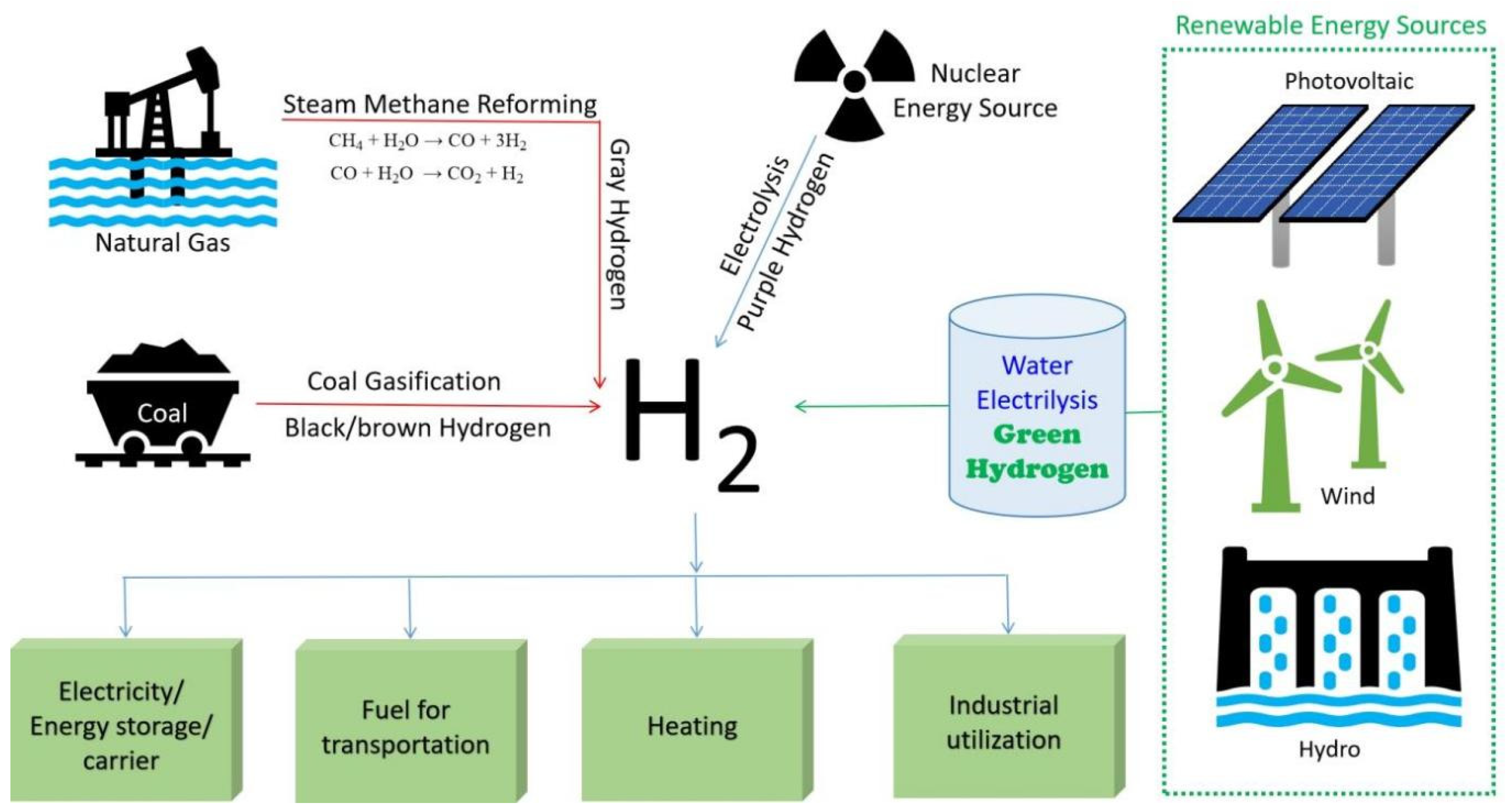
1. Introduction
2. Conventional Membranes
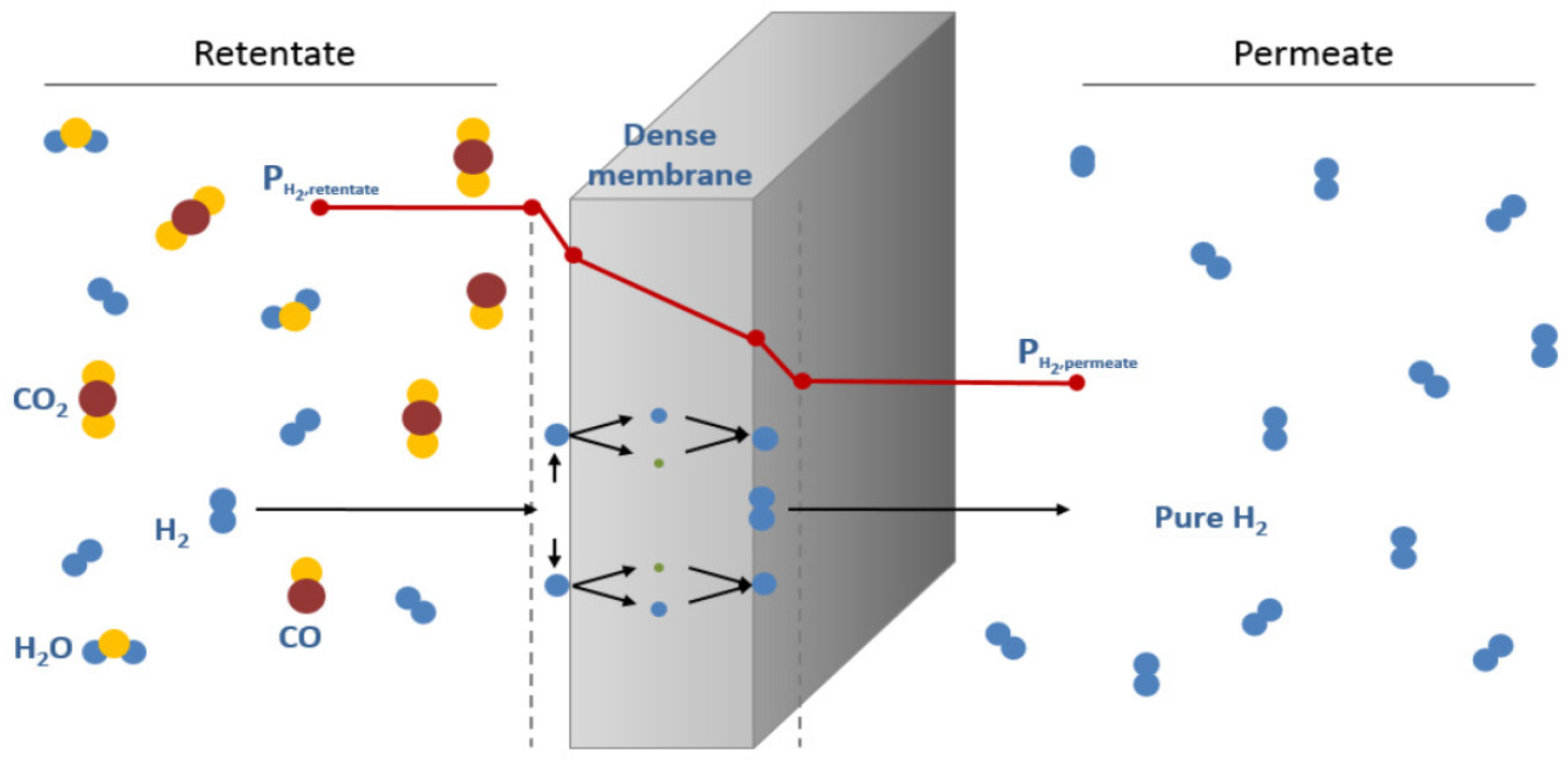
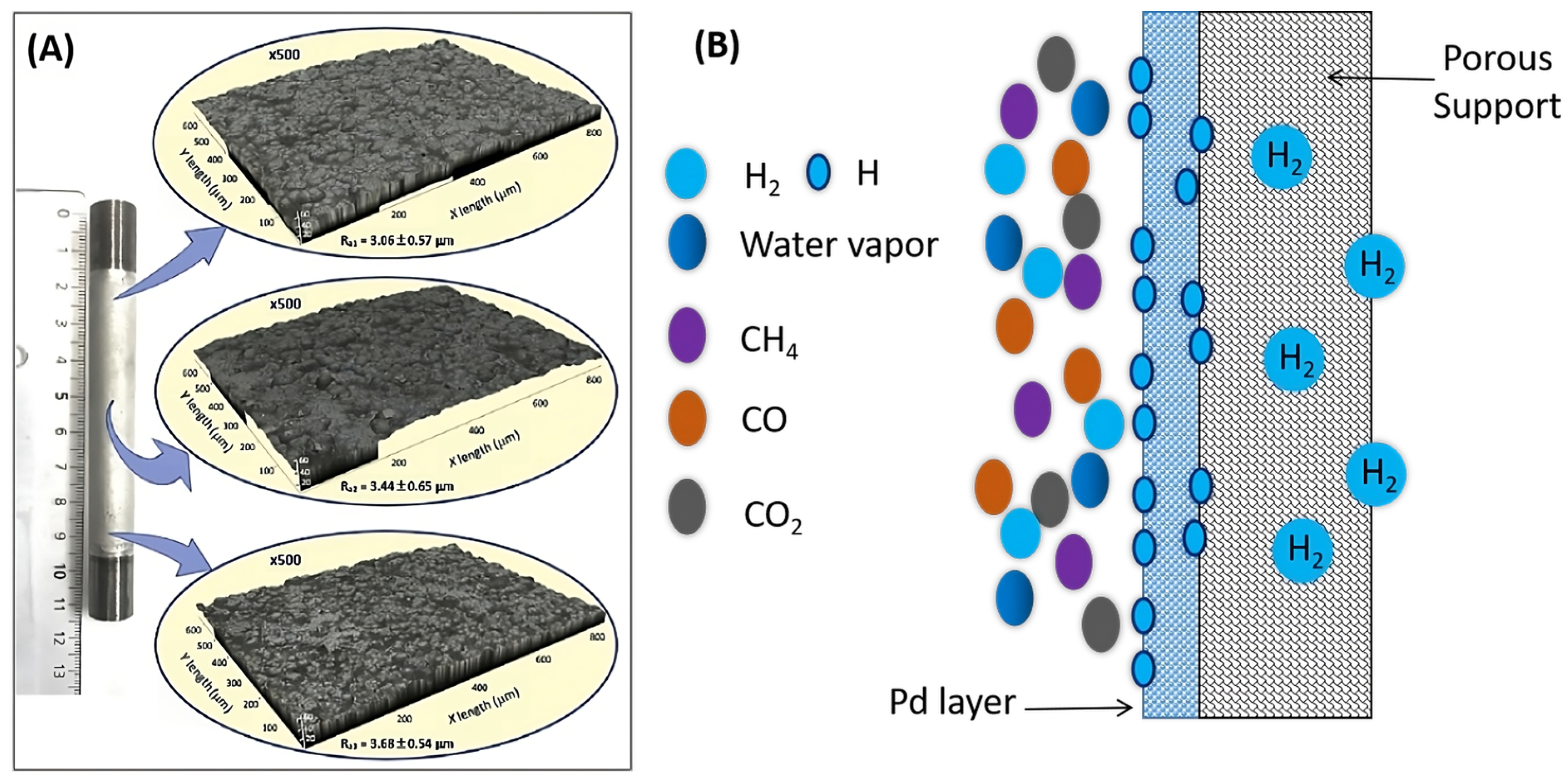
2.1. Metal Membranes
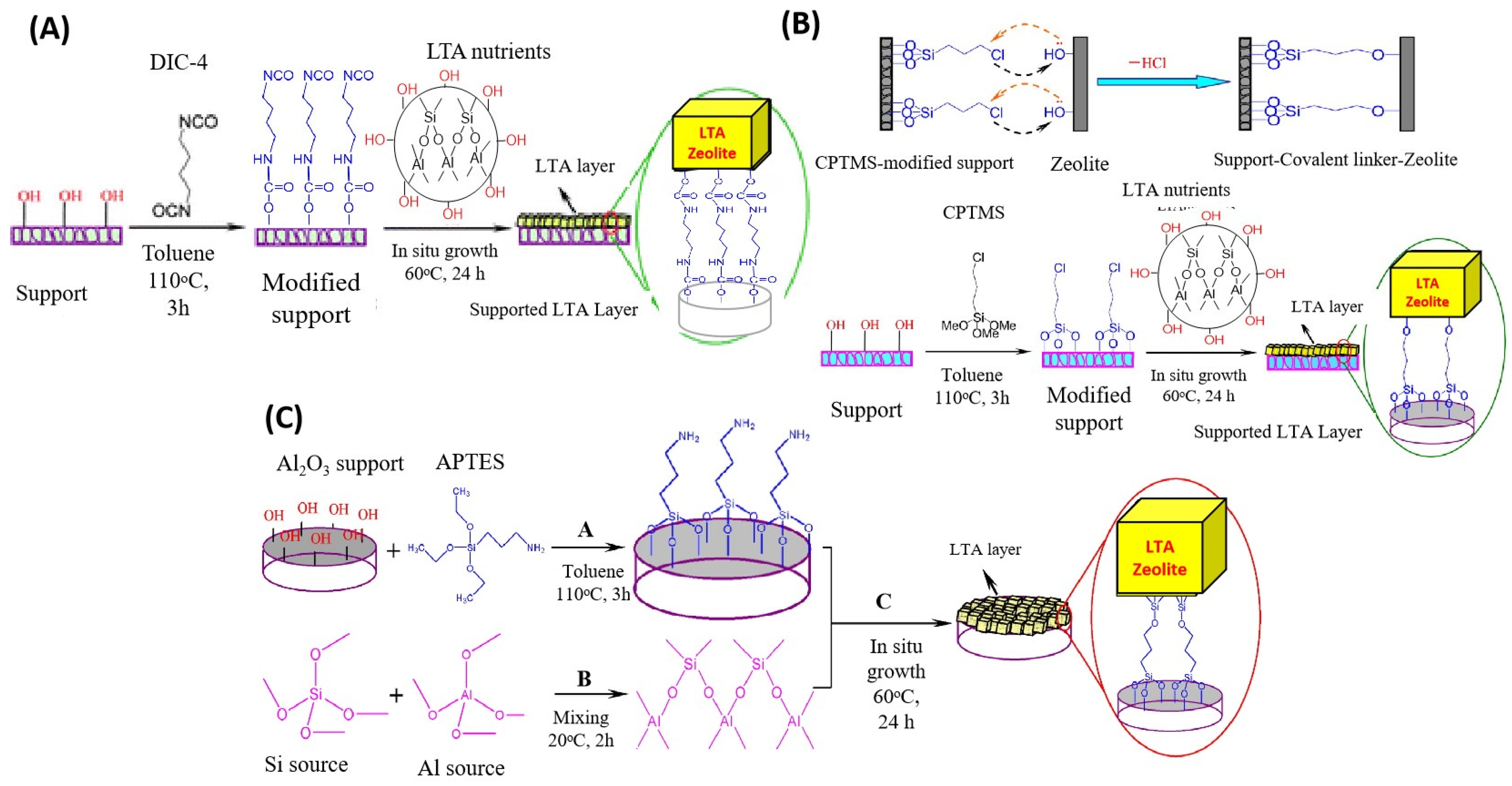
2.2. Zeolite Membranes
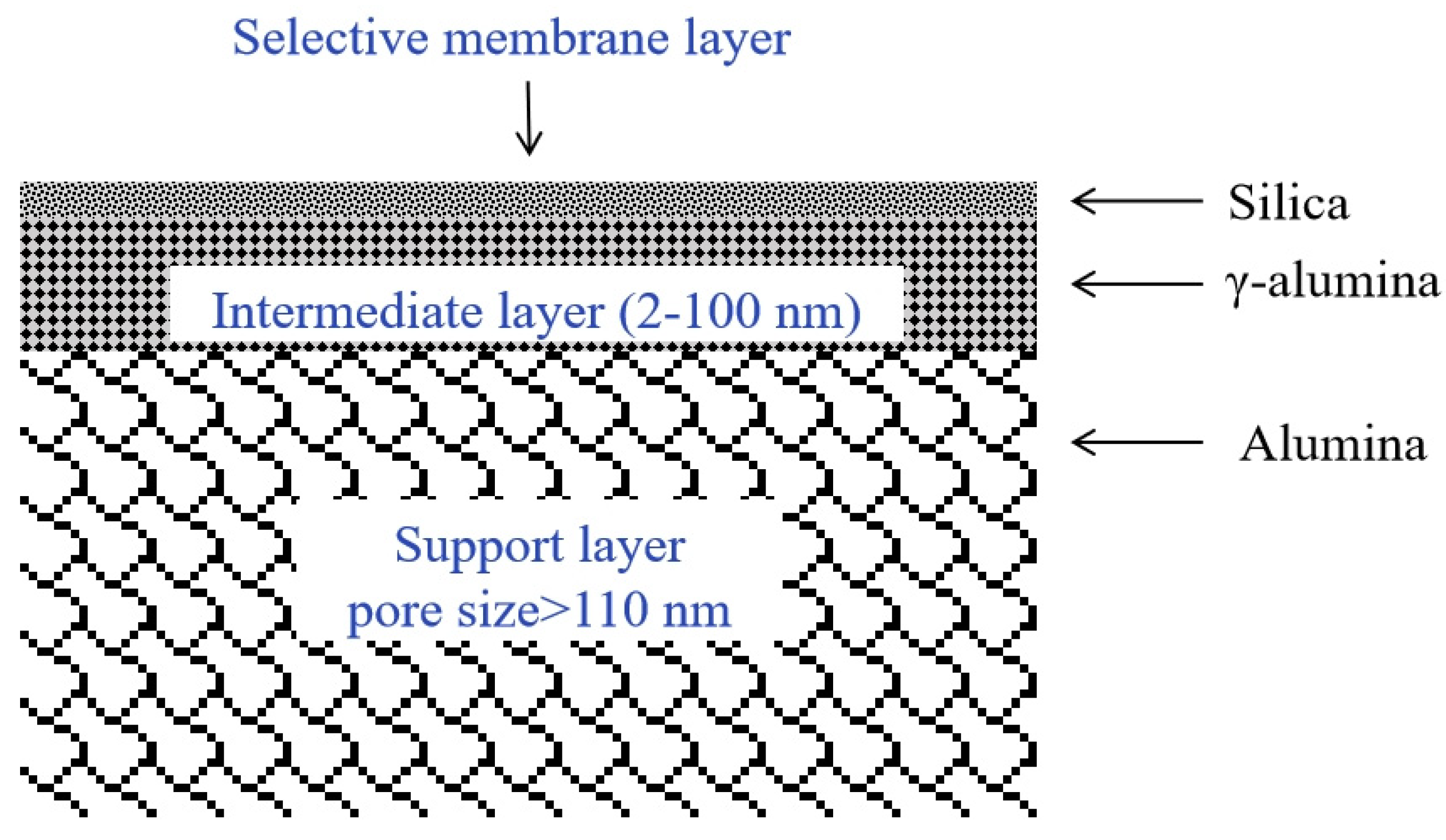
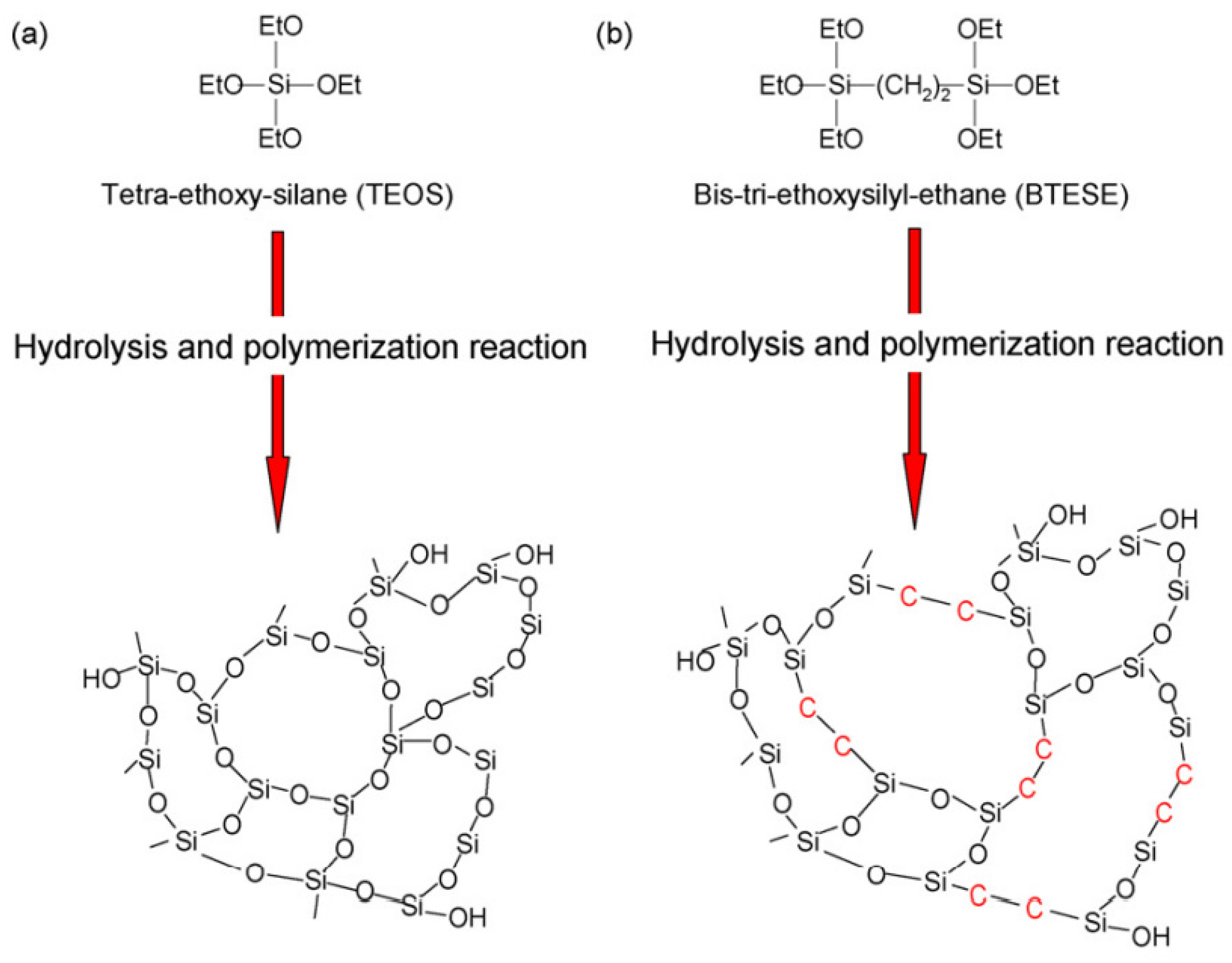
2.3. Silica Membranes
2.4. Carbon Molecular Sieve Membranes
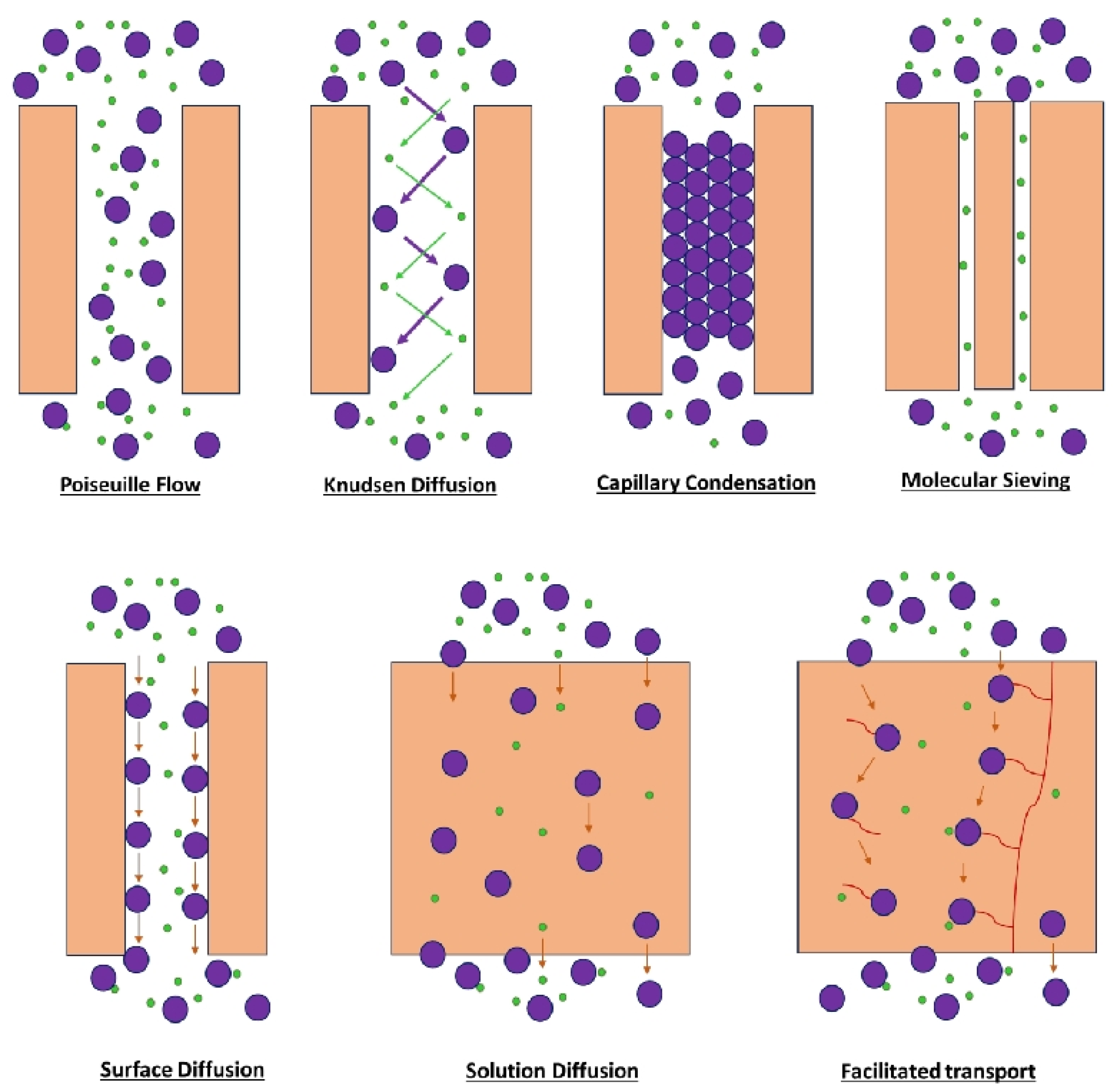
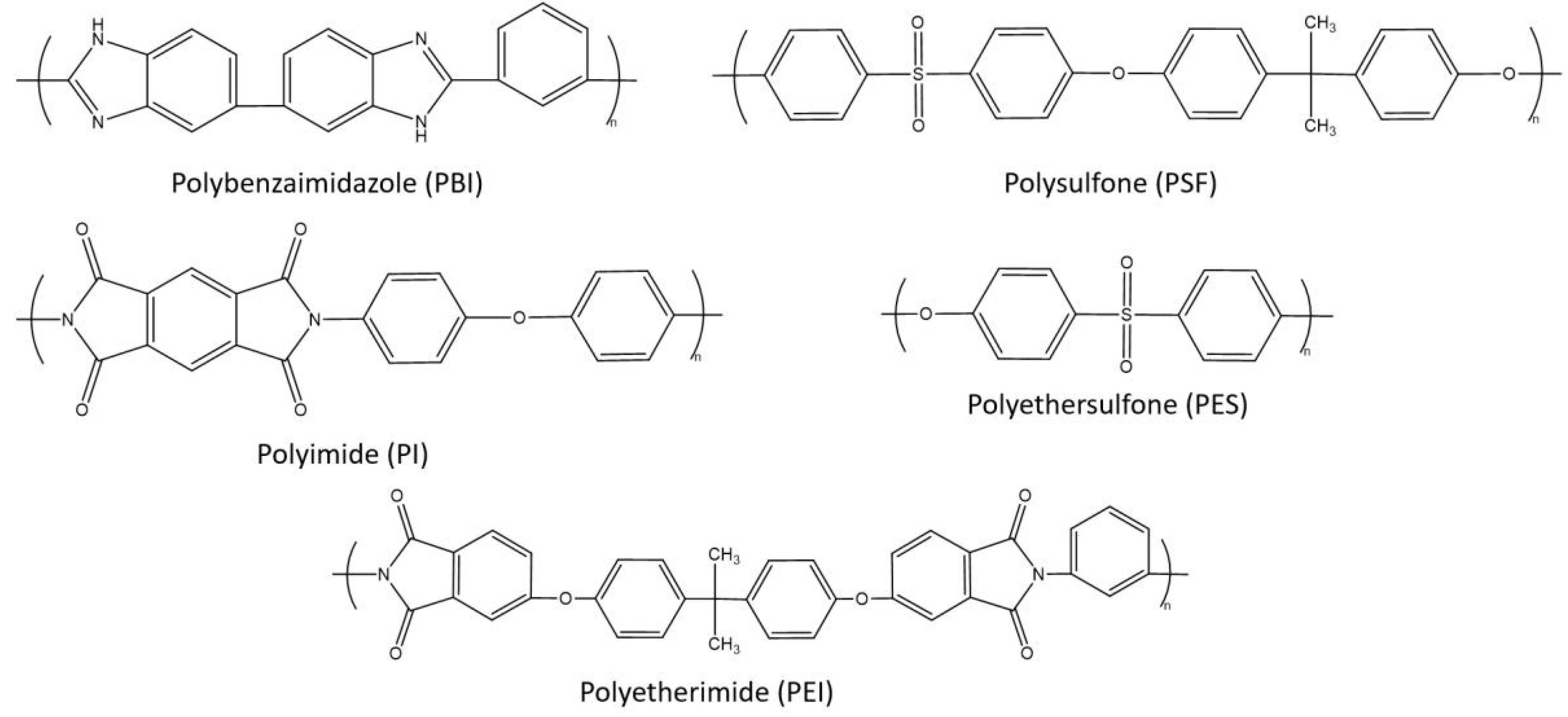
3. Polymeric Membranes for H2 Separation
3.1. H2/CO2-Selective Membrane
3.2. H2/CH4-Selective Membrane
3.3. H2/N2-Selective Membrane
3.4. Limitations of Polymeric Membranes
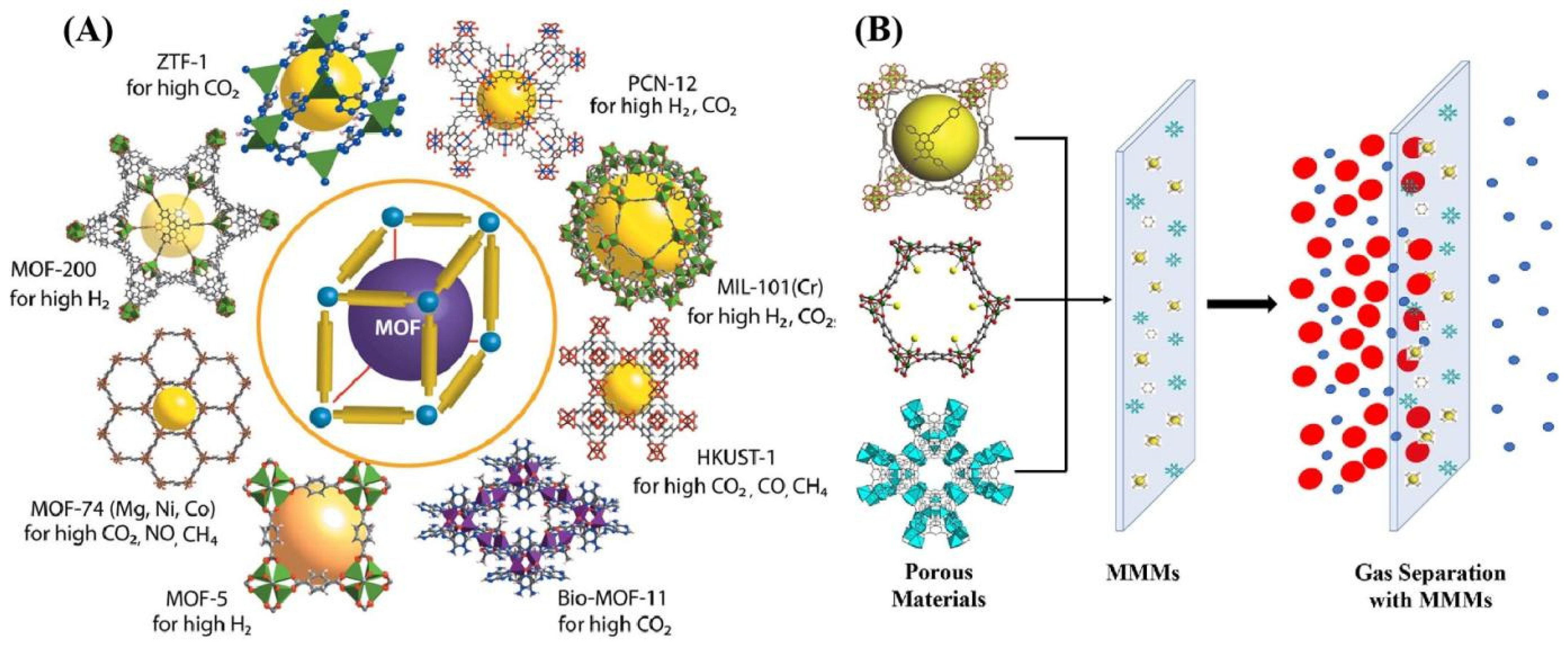
4. Mixed-Matrix Membranes (MMMs)
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect. In Advances in Carbon Capture; Woodhead Publishing: Cambridge, UK, 2020; pp. 3–28. [Google Scholar]
- Dunlop, I. The Role of the Fossil Fuel Industry. In Sustainability and the New Economics: Synthesising Ecological Economics and Modern Monetary Theory; Springer International Publishing: Cham, Germany, 2021; pp. 137–160. [Google Scholar]
- Noll, M. Exponential Life-Threatening Rise of the Global Temperature. EarthArXiv 2023. [Google Scholar]
- Singh, G.J.; Singh, P.K.; Lal, P. Dynamic Approach to Study Relationship Among Carbon Dioxide Emissions, Urbanization, and Economic Growth in BRICS Countries. J. Knowl. Econ. 2024, 1–18. [Google Scholar] [CrossRef]
- Trenberth, K.E. Climate Change Caused by Human Activities Is Happening and It Already Has Major Consequences. J. Energy Nat. Resour. Law 2018, 36, 463–481. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, J.; Li, Y.; Long, X.; Han, J. An Aging Giant at the Center of Global Warming: Population Dynamics and Its Effect on CO2 Emissions in China. J. Environ. Manag. 2023, 327, 116906. [Google Scholar] [CrossRef]
- Capellán-Pérez, I.; Mediavilla, M.; de Castro, C.; Carpintero, Ó.; Miguel, L.J. Fossil Fuel Depletion and Socio-Economic Scenarios: An Integrated Approach. Energy 2014, 77, 641–666. [Google Scholar] [CrossRef]
- Chiari, L.; Zecca, A. Constraints of Fossil Fuels Depletion on Global Warming Projections. Energy Policy 2011, 39, 5026–5034. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of Fossil Fuels and Anthropogenic Climate Change—A Review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Achakulwisut, P.; Erickson, P.; Guivarch, C.; Schaeffer, R.; Brutschin, E.; Pye, S. Global Fossil Fuel Reduction Pathways Under Different Climate Mitigation Strategies and Ambitions. Nat. Commun. 2023, 14, 5425. [Google Scholar] [CrossRef]
- Trout, K.; Muttitt, G.; Lafleur, D.; Van de Graaf, T.; Mendelevitch, R.; Mei, L.; Meinshausen, M. Existing fossil fuel extraction would warm the world beyond 1.5 C. Environ. Res. Lett. 2022, 17, 064010. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.P.; Gallachóir, B.Ó. The role of hydrogen in low carbon energy futures–A review of existing perspectives. Renew. Sustain. Energ. Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.; Sawalhah, M.N.; Valdez, R. A global assessment: Can renewable energy replace fossil fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Baquero, J.E.G.; Monsalve, D.B. From fossil fuel energy to hydrogen energy: Transformation of fossil fuel energy economies into hydrogen economies through social entrepreneurship. Int. J. Hydrogen Energy 2024, 54, 574–585. [Google Scholar] [CrossRef]
- Hosseini, S.E. Hydrogen fuel, a game changer for the world’s energy scenario. Int. J. Green Energy 2024, 21, 1366–1382. [Google Scholar] [CrossRef]
- Hosseini, S.E. An outlook on the global development of renewable and sustainable energy at the time of COVID-19. Energ. Res. Soc. Sci. 2020, 68, 101633. [Google Scholar] [CrossRef]
- Hosseini, S.E. Transition away from fossil fuels toward renewables: Lessons from Russia-Ukraine crisis. Future Energy 2022, 1, 2–5. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green hydrogen: A pathway to a sustainable energy future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energ. Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-enhanced steam methane reforming for combined CO2 capture and hydrogen production: A state-of-the-art review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C. Fuel and hydrogen related problems for conventional steam reforming of natural gas. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–89. [Google Scholar]
- Ganguli, A.; Bhatt, V. Hydrogen production using advanced reactors by steam methane reforming: A review. Front. Therm. Eng. 2023, 3, 1143987. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Saini, A.K.; Thakur, V.K. Recent update on gasification and pyrolysis processes of lignocellulosic and algal biomass for hydrogen production. Fuel 2023, 332, 126169. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology Control and Electronic Tailoring of CoxAy (A = P, S, Se) Electrocatalysts for Water Splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen Society: From Present to Future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-scale hydrogen production via water electrolysis: A techno-economic and environmental assessment. Energ. Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Simoes, S.G.; Catarino, J.; Picado, A.; Lopes, T.F.; Di Berardino, S.; Amorim, F.; de Leao, T.P. Water availability and water usage solutions for electrolysis in hydrogen production. J. Clean. Prod. 2021, 315, 128124. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.; Byun, M.; Lim, H. Comparative techno-economic analysis for steam methane reforming in a sorption-enhanced membrane reactor: Simultaneous H2 production and CO2 capture. Chem. Eng. Res. Des. 2021, 171, 383–394. [Google Scholar] [CrossRef]
- Navas-Anguita, Z.; García-Gusano, D.; Dufour, J.; Iribarren, D. Revisiting the role of steam methane reforming with CO2 capture and storage for long-term hydrogen production. Sci. Total Environ. 2021, 771, 145432. [Google Scholar] [CrossRef] [PubMed]
- Valdés-López, V.F.; Mason, T.; Shearing, P.R.; Brett, D.J. Carbon monoxide poisoning and mitigation strategies for polymer electrolyte membrane fuel cells–A review. Prog. Energ. Combust. Sci. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Dushina, A.; Schmies, H.; Schonvogel, D.; Dyck, A.; Wagner, P. The influence of hydrogen sulphide contamination on platinum catalyst used in polymer electrolyte membrane fuel cells during potential cycling at 0.05–1.05 V vs RHE: An RRDE study. Int. J. Hydrogen Energy 2020, 45, 35073–35084. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Review of Polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933. [Google Scholar] [CrossRef]
- Liemberger, W.; Groß, M.; Miltner, M.; Harasek, M. Experimental analysis of membrane and pressure swing adsorption (PSA) for the hydrogen separation from natural gas. J. Clean. Prod. 2017, 167, 896–907. [Google Scholar] [CrossRef]
- Speight, J.G. Heavy Oil Recovery and Upgrading; Gulf Professional Publishing: Houston, TX, USA, 2019. [Google Scholar]
- Aasadnia, M.; Mehrpooya, M.; Ghorbani, B. A novel integrated structure for hydrogen purification using the cryogenic method. J. Clean. Prod. 2021, 278, 123872. [Google Scholar] [CrossRef]
- Razi, F.; Dincer, I. Challenges, Opportunities and Future Directions in Hydrogen Sector Development in Canada. Int. J. Hydrogen Energy 2022, 47, 9083–9102. [Google Scholar] [CrossRef]
- Ji, G.; Zhao, M. Membrane separation technology in carbon capture. In Recent Advances in Carbon Capture and Storage; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–90. [Google Scholar]
- Pal, N.; Agarwal, M.; Maheshwari, K.; Solanki, Y.S. A review on types, fabrication and support material of hydrogen separation membrane. Mater. Today Proc. 2020, 28, 1386–1391. [Google Scholar] [CrossRef]
- Karousos, D.S.; Qadir, D.; Sapalidis, A.A.; Ahmad, F.; Favvas, E.P. Polymeric, metallic and carbon membranes for hydrogen separation: A review. Gas Sci. Eng. 2023, 120, 205167. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M. Advances in materials process and separation mechanism of the membrane towards hydrogen separation. Int. J. Hydrogen Energy 2021, 46, 27062–27087. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic membranes for hydrogen separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Liguori, S.; Kian, K.; Buggy, N.; Anzelmo, B.H.; Wilcox, J. Opportunities and challenges of low-carbon hydrogen via metallic membranes. Prog. Energ. Combust. Sci. 2020, 80, 100851. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energ. Rev. 2021, 145, 111062. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Lee, J.; Bae, T.H. Graphene-based membranes for H2 separation: Recent progress and future perspective. Membranes 2020, 10, 336. [Google Scholar] [CrossRef]
- Patel, A.K.; Acharya, N.K. Thermally rearranged (TR) HAB-6FDA nanocomposite membranes for hydrogen separation. Int. J. Hydrogen Energy 2020, 45, 18685–18692. [Google Scholar] [CrossRef]
- Ward, T.L.; Dao, T. Model of hydrogen permeation behavior in palladium membranes. J. Membr. Sci. 1999, 153, 211–231. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H. A review for consistent analysis of hydrogen permeability through dense metallic membranes. Membranes 2020, 10, 120. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of supported Pd-based membranes preparation by electroless plating for ultra-pure hydrogen production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef]
- Hatlevik, Ø.; Gade, S.K.; Keeling, M.K.; Thoen, P.M.; Davidson, A.P.; Way, J.D. Palladium and palladium alloy membranes for hydrogen separation and production: History, fabrication strategies, and current performance. Sep. Purif. Technol. 2010, 73, 59–64. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process. Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Gallucci, F.; Medrano, J.A.; Fernandez, E.; Melendez, J.; Van Sint Annaland, M.; Pacheco-Tanaka, D.A. Advances on high temperature Pd-based membranes and membrane reactors for hydrogen purification and production. J. Membr. Sci. Res. 2017, 3, 142–156. [Google Scholar]
- Tosto, E.; Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A.; Caravella, A.; Gallucci, F. Stability of pore-plated membranes for hydrogen production in fluidized-bed membrane reactors. Int. J. Hydrogen Energy 2020, 45, 7374–7385. [Google Scholar] [CrossRef]
- Vermaak, L.; Neomagus, H.W.; Bessarabov, D.G. Recent advances in membrane-based electrochemical hydrogen separation: A review. Membranes 2021, 11, 127. [Google Scholar] [CrossRef]
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen production technologies-Membrane based separation, storage and challenges. J. Environ. Manag. 2022, 302, 113963. [Google Scholar] [CrossRef]
- Dolan, M.D. Non-Pd BCC alloy membranes for industrial hydrogen separation. J. Membr. Sci. 2010, 362, 12–28. [Google Scholar] [CrossRef]
- Braun, F.; Tarditi, A.M.; Miller, J.B.; Cornaglia, L.M. Pd-based binary and ternary alloy membranes: Morphological and perm-selective characterization in the presence of H2S. J. Membr. Sci. 2014, 450, 299–307. [Google Scholar] [CrossRef]
- Morreale, B.D.; Howard, B.H.; Iyoha, O.; Enick, R.M.; Ling, C.; Sholl, D.S. Experimental and computational prediction of the hydrogen transport properties of Pd4S. Ind. Eng. Chem. Res. 2007, 46, 6313–6319. [Google Scholar] [CrossRef]
- Li, A.; Boyd, T.; Lim, J.C.; Grace, J.R. Development of palladium-alloy membranes for hydrogen separation and purification. J. Membr. Sci. Res. 2020, 6, 99–106. [Google Scholar]
- Park, Y.; Kwak, Y.; Yu, S.; Badakhsh, A.; Lee, Y.J.; Jeong, H.; Jo, Y.S. Degradation mechanism of a Pd/Ta composite membrane: Catalytic surface fouling with inter-diffusion. J. Alloys Compd. 2021, 854, 157196. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-based membranes for hydrogen separation: Review of alloying elements and their influence on membrane properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Cooney, D.A.; Way, J.D.; Wolden, C.A. A comparison of the performance and stability of Pd/BCC metal composite membranes for hydrogen purification. Int. J. Hydrogen Energy 2014, 39, 19009–19017. [Google Scholar] [CrossRef]
- Weber, M.; Drobek, M.; Rebiere, B.; Charmette, C.; Cartier, J.; Julbe, A.; Bechelany, M. Hydrogen selective palladium-alumina composite membranes prepared by Atomic Layer Deposition. J. Membr. Sci. 2020, 596, 117701. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Gapontsev, A.V.; Kondrat’ev, V.V. Hydrogen diffusion in disordered metals and alloys. Phys. Uspekhi 2003, 46, 1077. [Google Scholar] [CrossRef]
- Hara, S.; Hatakeyama, N.; Itoh, N.; Kimura, H.M.; Inoue, A. Hydrogen permeation through amorphous-Zr36−xHfxNi64-alloy membranes. J. Membr. Sci. 2003, 211, 149–156. [Google Scholar] [CrossRef]
- Hara, S.; Sakaki, K.; Itoh, N.; Kimura, H.M.; Asami, K.; Inoue, A. An amorphous alloy membrane without noble metals for gaseous hydrogen separation. J. Membr. Sci. 2000, 164, 289–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Ozaki, T.; Komaki, M.; Nishimura, C. Hydrogen permeation of Pd–Ag alloy coated V–15Ni composite membrane: Effects of overlayer composition. J. Membr. Sci. 2003, 224, 81–91. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Zhai, J.; Li, X.; Guo, X.; He, G. Research Progress and progress and prospects on hydrogen separation membranes. Clean Energy 2023, 7, 217–241. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardo, P.; Barbieri, G.; Drioli, E. A novel seeding procedure for preparing tubular NaY zeolite membranes. Microporous Mesoporous Mater. 2009, 119, 129–136. [Google Scholar] [CrossRef]
- Severance, M.; Wang, B.; Ramasubramanian, K.; Zhao, L.; Ho, W.W.; Dutta, P.K. Rapid crystallization of faujasitic zeolites: Mechanism and application to zeolite membrane growth on polymer supports. Langmuir 2014, 30, 6929–6937. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, S.G.; Singh, P.S. In situ hydrothermal growth of Zeolite-A membrane on polysulfone hollow fibers. Microporous Mesoporous Mater. 2024, 366, 112952. [Google Scholar] [CrossRef]
- Lin, Y.S.; Duke, M.C. Recent progress in polycrystalline zeolite membrane research. Curr. Opin. Chem. Eng. 2013, 2, 209–216. [Google Scholar] [CrossRef]
- Nazir, L.S.M.; Yeong, Y.F.; Chew, T.L. Methods and synthesis parameters affecting the formation of FAU type zeolite membrane and its separation performance: A review. J. Asian Ceram. Soc. 2020, 8, 553–571. [Google Scholar] [CrossRef]
- Kong, C.; Lu, J.; Yang, J.; Wang, J. Preparation of silicalite-1 membranes on stainless steel supports by a two-stage varying-temperature in situ synthesis. J. Membr. Sci. 2006, 285, 258–264. [Google Scholar] [CrossRef]
- Simplício, M.; Afonso, M.D.; Borisevich, O.; Lefebvre, X.; Demange, D. Permeation of single gases and binary mixtures of hydrogen and helium through a MFI zeolite hollow fibers membrane for application in nuclear fusion. Sep. Purif. Technol. 2014, 122, 199–205. [Google Scholar] [CrossRef]
- Zhou, R.; Pan, Y.; Xing, W.; Xu, N. Advanced microporous membranes for H2/CH4 separation: Challenges and perspectives. Adv. Membr. 2021, 1, 100011. [Google Scholar] [CrossRef]
- Li, K.; Tian, Z.; Li, X.; Xu, R.; Xu, Y.; Wang, L.; Lin, L. Ionothermal synthesis of aluminophosphate molecular sieve membranes through substrate surface conversion. Angew. Chem. Int. Ed. 2012, 18, 4397–4400. [Google Scholar] [CrossRef]
- Sen, M.; Dana, K.; Das, N. Development of LTA zeolite membrane from clay by sonication assisted method at room temperature for H2-CO2 and CO2-CH4 separation. Ultrason. Sonochem. 2018, 48, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kanezashi, M.; O’Brien-Abraham, J.; Lin, Y.S.; Suzuki, K. Gas Permeation through DDR-Type Zeolite Membranes at High Temperatures. AIChE J. 2008, 54, 1478–1486. [Google Scholar] [CrossRef]
- Huang, A.; Caro, J. Facile Synthesis of LTA Molecular Sieve Membranes on Covalently Functionalized Supports by Using Diisocyanates as Molecular Linkers. J. Mater. Chem. 2011, 21, 11424–11429. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Q.; Wang, N.; Tong, X.; Huang, B.; Wang, M.; Caro, J. Covalent Synthesis of Dense Zeolite LTA Membranes on Various 3-Chloropropyltrimethoxysilane Functionalized Supports. J. Membr. Sci. 2013, 437, 57–64. [Google Scholar] [CrossRef]
- Huang, A.; Liang, F.; Steinbach, F.; Caro, J. Preparation and Separation Properties of LTA Membranes by Using 3-Aminopropyltriethoxysilane as Covalent Linker. J. Membr. Sci. 2010, 350, 5–9. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Tilebon, S.M.S.; Basile, A. Silica Membranes Application for Hydrogen Separation. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 243–264. [Google Scholar]
- Wei, Q.; Wang, F.; Nie, Z.R.; Song, C.L.; Wang, Y.L.; Li, Q.Y. Highly Hydrothermally Stable Microporous Silica Membranes for Hydrogen Separation. J. Phys. Chem. B 2008, 112, 9354–9359. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Silica Membranes for Hydrogen Separation Prepared by Chemical Vapor Deposition (CVD). Sep. Purif. Technol. 2013, 111, 20–42. [Google Scholar] [CrossRef]
- Smart, S.; Beltramini, J.; da Costa, J.D.; Katikaneni, S.P.; Pham, T. Microporous Silica Membranes: Fundamentals and Applications in Membrane Reactors for Hydrogen Separation. In Handbook of Membrane Reactors; Woodhead Publishing: Cambridge, UK, 2013; pp. 337–369. [Google Scholar]
- Lee, D.; Oyama, S.T. Gas Permeation Characteristics of a Hydrogen Selective Supported Silica Membrane. J. Membr. Sci. 2002, 210, 291–306. [Google Scholar] [CrossRef]
- Verweij, H.; Lin, Y.S.; Dong, J. Microporous Silica and Zeolite Membranes for Hydrogen Purification. MRS Bull. 2006, 31, 756–764. [Google Scholar] [CrossRef]
- Moon, J.H.; Bae, J.H.; Bae, Y.S.; Chung, J.T.; Lee, C.H. Hydrogen Separation from Reforming Gas Using Organic Templating Silica/Alumina Composite Membrane. J. Membr. Sci. 2008, 318, 45–55. [Google Scholar] [CrossRef]
- Koutsonikolas, D.E.; Pantoleontos, G.; Karagiannakis, G.; Konstandopoulos, A.G. Development of H2 Selective Silica Membranes: Performance Evaluation through Single Gas Permeation and Gas Separation Tests. Sep. Purif. Technol. 2021, 264, 118432. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y.S.; Kanezashi, M.; Tang, Z. Microporous Inorganic Membranes for High Temperature Hydrogen Purification. J. Appl. Phys. 2008, 104, 121301. [Google Scholar] [CrossRef]
- Lu, G.Q.; Da Costa, J.D.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic Membranes for Hydrogen Production and Purification: A Critical Review and Perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, T.; Hauler, F.; Bram, M.; Meulenberg, W.A.; Buchkremer, H.P. Synthesis and Characterization of Hydrogen-Selective Sol–Gel SiO2 Membranes Supported on Ceramic and Stainless Steel Supports. Sep. Purif. Technol. 2014, 121, 20–29. [Google Scholar] [CrossRef]
- Lin, Y.S.; Kumakiri, I.; Nair, B.N.; Alsyouri, H. Microporous Inorganic Membranes. Sep. Purif. Methods 2002, 31, 229–379. [Google Scholar] [CrossRef]
- Comite, A. Preparation of Silica Membranes by Sol-Gel Method. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–23. [Google Scholar]
- Khatib, S.J.; Oyama, S.T.; de Souza, K.R.; Noronha, F.B. Review of Silica Membranes for Hydrogen Separation Prepared by Chemical Vapor Deposition. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 14, pp. 25–60. [Google Scholar]
- Ohta, Y.; Akamatsu, K.; Sugawara, T.; Nakao, A.; Miyoshi, A.; Nakao, S.I. Development of Pore Size-Controlled Silica Membranes for Gas Separation by Chemical Vapor Deposition. J. Membr. Sci. 2008, 315, 93–99. [Google Scholar] [CrossRef]
- Ayral, A.; Julbe, A.; Rouessac, V.; Roualdes, S.; Durand, J. Microporous Silica Membrane: Basic Principles and Recent Advances. Membr. Sci. Technol. 2008, 13, 33–79. [Google Scholar]
- Duke, M.C.; Da Costa, J.D.; Do, D.D.; Gray, P.G.; Lu, G.Q. Hydrothermally Robust Molecular Sieve Silica for Wet Gas Separation. Adv. Funct. Mater. 2006, 16, 1215–1220. [Google Scholar] [CrossRef]
- Gu, Y.; Hacarlioglu, P.; Oyama, S.T. Hydrothermally Stable Silica–Alumina Composite Membranes for Hydrogen Separation. J. Membr. Sci. 2008, 310, 28–37. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J. Zirconia-Doped Methylated Silica Membranes via Sol-Gel Process: Microstructure and Hydrogen Permselectivity. Nanomaterials 2022, 12, 2159. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Organic–Inorganic Hybrid Silica Membranes with Controlled Silica Network Size: Preparation and Gas Permeation Characteristics. J. Membr. Sci. 2010, 348, 310–318. [Google Scholar] [CrossRef]
- De Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic Silica Membranes for Gas Separation. J. Membr. Sci. 1999, 158, 277–288. [Google Scholar] [CrossRef]
- Yan, M.; Yang, J.; Mu, R.; Guo, Y.; Cui, X.; Song, J. Fabrication, Characteristics and Hydrothermal Stability of Methyl-Modified Ni-Co/SiO2 Membranes for H2/CO2 Separation. J. CO2 Util. 2023, 68, 102393. [Google Scholar] [CrossRef]
- Igi, R.; Yoshioka, T.; Ikuhara, Y.H.; Iwamoto, Y.; Tsuru, T. Characterization of Co-Doped Silica for Improved Hydrothermal Stability and Application to Hydrogen Separation Membranes at High Temperatures. J. Am. Ceram. Soc. 2008, 91, 2975–2981. [Google Scholar] [CrossRef]
- Genduso, G.; Ogieglo, W.; Wang, Y.; Pinnau, I. Carbon Molecular Sieve Gas Separation Materials and Membranes: A Comprehensive Review. J. Membr. Sci. 2024, 699, 122533. [Google Scholar] [CrossRef]
- Ismail, A.F.; David, L.I.B. A Review on the Latest Development of Carbon Membranes for Gas Separation. J. Membr. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Rao, M.; Sircar, S. Nanoporous Carbon Membranes for Separation of Gas Mixtures by Selective Surface Flow. J. Membr. Sci. 1993, 85, 253–264. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Ismail, N.H.; Kumaran, K.; Othman, M.H.D.; Harun, Z. Advanced in Carbon Membrane by Relating to Coating-Carbonization-Cycles for Oxygen Performance. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 736, No. 2; p. 022008. [Google Scholar]
- Sazali, N. A Review of the Application of Carbon-Based Membranes to Hydrogen Separation. J. Mater. Sci. 2020, 55, 11052–11070. [Google Scholar] [CrossRef]
- Hägg, M.B.; He, X. Carbon Molecular Sieve Membranes for Gas Separation; Royal Society of Chemistry: Tokyo, Japan, 2011. [Google Scholar]
- Saufi, S.M.; Ismail, A.F. Fabrication of Carbon Membranes for Gas Separation––A Review. Carbon 2004, 42, 241–259. [Google Scholar] [CrossRef]
- Ismail, A.F.; Li, K. From Polymeric Precursors to Hollow Fiber Carbon and Ceramic Membranes. Membr. Sci. Technol. 2008, 13, 81–119. [Google Scholar]
- Fu, Y.J.; Hu, C.C.; Lin, D.W.; Tsai, H.A.; Huang, S.H.; Hung, W.S.; Lai, J.Y. Adjustable Microstructure Carbon Molecular Sieve Membranes Derived from Thermally Stable Polyetherimide/Polyimide Blends for Gas Separation. Carbon 2017, 113, 10–17. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Bae, T.H. Polyimide-Derived Carbon Molecular Sieve Membranes for Advanced Gas Separation: From Membrane Development to Pilot-Scale Operations. Sep. Purif. Technol. 2023, 320, 124114. [Google Scholar] [CrossRef]
- Bartocci, P.; Russo, G.; Yang, H.; Hu, S.; Skreiberg, Ø.; Wang, L.; Fantozzi, F. Carbon Nanotubes for Hydrogen Purification and Storage. In Green Synthesis of Nanomaterials for Bioenergy Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 211–238. [Google Scholar]
- Song, C.; Wang, T.; Jiang, H.; Wang, X.; Cao, Y.; Qiu, J. Gas Separation Performance of C/CMS Membranes Derived from Poly (Furfuryl Alcohol)(PFA) with Different Chemical Structure. J. Membr. Sci. 2010, 361, 22–27. [Google Scholar] [CrossRef]
- Araújo, T.; Bernardo, G.; Mendes, A. Cellulose-Based Carbon Molecular Sieve Membranes for Gas Separation: A Review. Molecules 2020, 25, 3532. [Google Scholar] [CrossRef]
- Hou, M.; Li, L.; He, Z.; Xu, R.; Lu, Y.; Wang, T. High Hydrogen Permselective Carbon Molecular Sieve Membrane and Its Structural Formation Mechanism. Carbon 2023, 205, 194–206. [Google Scholar] [CrossRef]
- Lagorsse, S.; Magalhães, F.D.; Mendes, A. Carbon Molecular Sieve Membranes: Sorption, Kinetic and Structural Characterization. J. Membr. Sci. 2004, 241, 275–287. [Google Scholar] [CrossRef]
- Lagorsse, S.; Leite, A.; Magalhães, F.D.; Bischofberger, N.; Rathenow, J.; Mendes, A. Novel Carbon Molecular Sieve Honeycomb Membrane Module: Configuration and Membrane Characterization. Carbon 2005, 43, 809–819. [Google Scholar] [CrossRef]
- Ismail, A.F.; Rana, D.; Matsuura, T.; Foley, H.C. Carbon-Based Membranes for Separation Processes; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Lagorsse, S.; Campo, M.C.; Magalhães, F.D.; Mendes, A. Water Adsorption on Carbon Molecular Sieve Membranes: Experimental Data and Isotherm Model. Carbon 2005, 43, 2769–2779. [Google Scholar] [CrossRef]
- Campo, M.C.; Magalhães, F.D.; Mendes, A. Carbon Molecular Sieve Membranes from Cellophane Paper. J. Membr. Sci. 2010, 350, 180–188. [Google Scholar] [CrossRef]
- Sazali, N. A Comprehensive Review of Carbon Molecular Sieve Membranes for Hydrogen Production and Purification. Int. J. Adv. Manuf. Technol. 2021, 107, 2465–2483. [Google Scholar] [CrossRef]
- Ge, L.; Du, A.; Hou, M.; Rudolph, V.; Zhu, Z. Enhanced Hydrogen Separation by Vertically-Aligned Carbon Nanotube Membranes with Zeolite Imidazolate Frameworks as a Selective Layer. RSC Adv. 2012, 2, 11793–11800. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Robeson, L.M.; Liu, Q.; Freeman, B.D.; Paul, D.R. Comparison of Transport Properties of Rubbery and Glassy Polymers and the Relevance to the Upper Bound Relationship. J. Membr. Sci. 2015, 476, 421–431. [Google Scholar] [CrossRef]
- Yáñez, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Comparative Performance of Commercial Polymeric Membranes in the Recovery of Industrial Hydrogen Waste Gas Streams. Int. J. Hydrogen Energy 2021, 46, 17507–17521. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qiao, Z.; Liu, Y.; Cao, X.; Li, W.; Wang, S. Recent Developments in Membranes for Efficient Hydrogen Purification. J. Membr. Sci. 2015, 495, 130–168. [Google Scholar] [CrossRef]
- Vaezi, M.J.; Kojabad, M.E.; Beiragh, M.M.; Babaluo, A.A. Transport Mechanism and Modeling of Microporous Zeolite Membranes. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–203. [Google Scholar]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Gas Separation Membranes; Switzerland Springer: Cham, Switzerland, 2015; Volume 10, pp. 973–978. [Google Scholar]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A Review of the Latest Development of Polyimide-Based Membranes for CO2 Separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Basile, A.; Gugliuzza, A.; Iulianelli, A.D.O.L.F.O.; Morrone, P. Membrane Technology for Carbon Dioxide (CO2) Capture in Power Plants. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2011; pp. 113–159. [Google Scholar]
- Yin, H.; Yip, A.C. A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies. Catalysts 2017, 7, 297. [Google Scholar] [CrossRef]
- Abetz, V.; Brinkmann, T.; Dijkstra, M.; Ebert, K.; Fritsch, D.; Ohlrogge, K.; Schossig, M. Developments in Membrane Research: From Material via Process Design to Industrial Application. Adv. Eng. Mater. 2006, 8, 328–358. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.S. Highly Selective Sulfonated Polyethersulfone (SPES)-Based Membranes with Transition Metal Counterions for Hydrogen Recovery and Natural Gas Separation. J. Membr. Sci. 2008, 308, 128–135. [Google Scholar] [CrossRef]
- Orme, C.J.; Stone, M.L.; Benson, M.T.; Peterson, E.S. Testing of Polymer Membranes for the Selective Permeability of Hydrogen. Sep. Sci. Technol. 2003, 38, 3225–3238. [Google Scholar] [CrossRef]
- Girma, H.G.; Park, K.H.; Ji, D.; Kim, Y.; Lee, H.M.; Jeon, S.; Jung, S.H.; Kim, J.Y.; Noh, Y.Y.; Lim, B. Room-Temperature Hydrogen Sensor with High Sensitivity and Selectivity Using Chemically Immobilized Monolayer Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2023, 33, 2213381. [Google Scholar] [CrossRef]
- Girma, H.G.; Lee, H.M.; Kim, Y.; Ryu, G.S.; Jeon, S.; Kim, J.Y.; Jung, S.H.; Kim, S.H.; Noh, Y.Y.; Lim, B. Highly Sensitive and Wrappable Room Temperature Wireless Gasochromic and Chemiresistive Dual-Response H2 Sensors Using Spray Coating. Nano Energy 2023, 113, 108551. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Wang, J.; Wang, Z.; Liu, X. Network Polyimide Membranes Prepared by Interfacial Polymerization for Hot H2 Purification. AIChE J. 2023, 69, e17983. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Izwanne, M.N.; Harun, Z.; Kadirgama, K. Precursor Selection for Carbon Membrane Fabrication: A Review. J. Appl. Membr. Sci. Technol. 2018, 22. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kapantaidakis, G.C.; Nolan, J.W.; Mitropoulos, A.C.; Kanellopoulos, N.K. Preparation, Characterization and Gas Permeation Properties of Carbon Hollow Fiber Membranes Based on Matrimid® 5218 Precursor. J. Mater. Process. Technol. 2007, 186, 102–110. [Google Scholar] [CrossRef]
- Klaehn, J.R.; Orme, C.J.; Peterson, E.S. Blended Polybenzimidazole and Melamine-Co-Formaldehyde Thermosets. J. Membr. Sci. 2016, 515, 1–6. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Teoh, M.M.; Chung, T.S. Hydrogen Separation and Purification in Membranes of Miscible Polymer Blends with Interpenetration Networks. Polymer 2008, 49, 1594–1603. [Google Scholar] [CrossRef]
- Zhu, L.; Swihart, M.T.; Lin, H. Unprecedented Size-Sieving Ability in Polybenzimidazole Doped with Polyprotic Acids for Membrane H2/CO2 Separation. Energy Environ. Sci. 2018, 11, 94–100. [Google Scholar] [CrossRef]
- Arabi Shamsabadi, A.; Kargari, A.; Bahrami Babaheidari, M. Preparation, Characterization and Gas Permeation Properties of PDMS/PEI Composite Asymmetric Membrane for Effective Separation of Hydrogen from H2/CH4 Mixed Gas. Int. J. Hydrogen Energy 2014, 39, 1410–1419. [Google Scholar] [CrossRef]
- Kargari, A.; Arabi Shamsabadi, A.; Bahrami Babaheidari, M. Influence of Coating Conditions on the H2 Separation Performance from H2/CH4 Gas Mixtures by the PDMS/PEI Composite Membrane. Int. J. Hydrogen Energy 2014, 39, 6588–6597. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Peng, N.; Chung, T.S. Gas Separation Membranes Developed through Integration of Polymer Blending and Dual-Layer Hollow Fiber Spinning Process for Hydrogen and Natural Gas Enrichments. J. Membr. Sci. 2010, 349, 156–166. [Google Scholar] [CrossRef]
- Shishatskiy, B.S.; Nistor, C.; Popa, M.; Pereira Nunes, S.; Peinemann, K.V. Polyimide Asymmetric Membranes for Hydrogen Separation: Influence of Formation Conditions on Gas Transport Properties. Adv. Eng. Mater. 2006, 8, 390–397. [Google Scholar] [CrossRef]
- Macchione, M.; Jansen, J.C.; De Luca, G.; Tocci, E.; Longeri, M.; Drioli, E. Experimental Analysis and Simulation of the Gas Transport in Dense Hyflon® AD60X Membranes: Influence of Residual Solvent. Polymer 2007, 48, 2619–2635. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Gas and Vapor Transport Properties of Amorphous Perfluorinated Copolymer Membranes Based on 2,2-Bistrifluoromethyl-4,5-Difluoro-1,3-Dioxole/Tetrafluoroethylene. J. Membr. Sci. 1996, 109, 125–133. [Google Scholar] [CrossRef]
- Tanaka, K.; Okano, M.; Toshino, H.; Kita, H.; Okamoto, K.-I. Effect of Methyl Substituents on Permeability and Permselectivity of Gases in Polyimides Prepared from Methyl-Substituted Phenylenediamines. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 907–914. [Google Scholar] [CrossRef]
- Tanaka, K.; Osada, Y.; Kita, H.; Okamoto, K.I. Gas permeability and permselectivity of polyimides with large aromatic rings. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1907–1915. [Google Scholar] [CrossRef]
- Tanaka, K.; Islam, M.N.; Kido, M.; Kita, H.; Okamoto, K.-I. Gas Permeation and Separation Properties of Sulfonated Polyimide Membranes. Polymer 2006, 47, 4370–4377. [Google Scholar] [CrossRef]
- Rezac, M.E.; Schöberl, B. Transport and Thermal Properties of Poly(Ether Imide)/Acetylene-Terminated Monomer Blends. J. Membr. Sci. 1999, 156, 211–222. [Google Scholar] [CrossRef]
- Bernardo, P.; Tasselli, F.; Chiappetta, G.; Clarizia, G. Effect of the Post-Spinning Solvent Exchange on the Performance of Asymmetric, Polyimide Hollow Fibers Prepared by Using a Triple-Orifice Spinneret. Materials 2019, 12, 3632. [Google Scholar] [CrossRef]
- Yousef, S.; Tuckute, S.; Tonkonogovas, A.; Stankevičius, A.; Mohamed, A. Ultra-Permeable CNTs/PES Membranes with a Very Low CNTs Content and High H2/N2 and CH4/N2 Selectivity for Clean Energy Extraction Applications. J. Mater. Res. Technol. 2021, 15, 5114–5127. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Fila, V.; Dung, C.T. Mixed Matrix Membranes Based on PIMs for Gas Permeation: Principles, Synthesis, and Current Status. Chem. Eng. Commun. 2017, 204, 295–309. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Khaled, M.; Jamal, A.; Zahid, U. Mixed Matrix Membranes for H2/CO2 Gas Separation—A Critical Review. Fuel 2023, 333, 126285. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent Advances of Inorganic Fillers in Mixed Matrix Membrane for Gas Separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Friebe, S.; Diestel, L.; Knebel, A.; Wollbrink, A.; Caro, J. MOF-Based Mixed-Matrix Membranes in Gas Separation—Mystery and Reality. Chemie Ing. Tech. 2016, 88, 1788–1797. [Google Scholar] [CrossRef]
- Kang, Z.; Peng, Y.; Hu, Z.; Qian, Y.; Chi, C.; Yeo, L.Y.; Zhao, D. Mixed Matrix Membranes Composed of Two-Dimensional Metal–Organic Framework Nanosheets for Pre-Combustion CO2 Capture: A Relationship Study of Filler Morphology versus Membrane Performance. J. Mater. Chem. A 2015, 3, 20801–20810. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chaudhari, H.D.; Banerjee, R.; Kharul, U.K. Chemically Stable Covalent Organic Framework (COF)-Polybenzimidazole Hybrid Membranes: Enhanced Gas Separation through Pore Modulation. Chem. A Eur. J. 2016, 22, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Park, S.; Cho, K.Y.; Jeong, H.K. Polyimide/ZIF-7 Mixed-Matrix Membranes: Understanding the in Situ Confined Formation of the ZIF-7 Phases Inside a Polymer and Their Effects on Gas Separations. J. Mater. Chem. A 2020, 8, 11210–11217. [Google Scholar] [CrossRef]
- Ma, X.; Wu, X.; Caro, J.; Huang, A. Polymer Composite Membrane with Penetrating ZIF-7 Sheets Displays High Hydrogen Permselectivity. Angew. Chem. Int. Ed. 2019, 58, 16156–16160. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, B.; Bian, C.; Meng, X.; Meng, B.; Wong, S.I.; Yang, N.; Sunarso, J.; Tan, X.; Liu, S. Elevated-Temperature H2 Separation Using a Dense Electron and Proton Mixed Conducting Polybenzimidazole-Based Membrane with 2D Sulfonated Graphene. Green Chem. 2021, 23, 3374–3385. [Google Scholar] [CrossRef]
- Zornoza, B.; Gorgojo, P.; Casado, C.; Téllez, C.; Coronas, J. Mixed Matrix Membranes for Gas Separation with Special Nanoporous Fillers. Desalin. Water Treat. 2011, 27, 42–47. [Google Scholar] [CrossRef]
- Safak Boroglu, M.; Yumru, A.B. Gas Separation Performance of 6FDA-DAM-ZIF-11 Mixed-Matrix Membranes for H2/CH4 and CO2/CH4 Separation. Sep. Purif. Technol. 2017, 173, 269–279. [Google Scholar] [CrossRef]
- Augustus, E.N.; Nimibofa, A.; Kesiye, I.A.; Donbebe, W. Metal-Organic Frameworks as Novel Adsorbents: A Preview. Am. J. Environ. Prot. 2017, 5, 61–67. [Google Scholar]
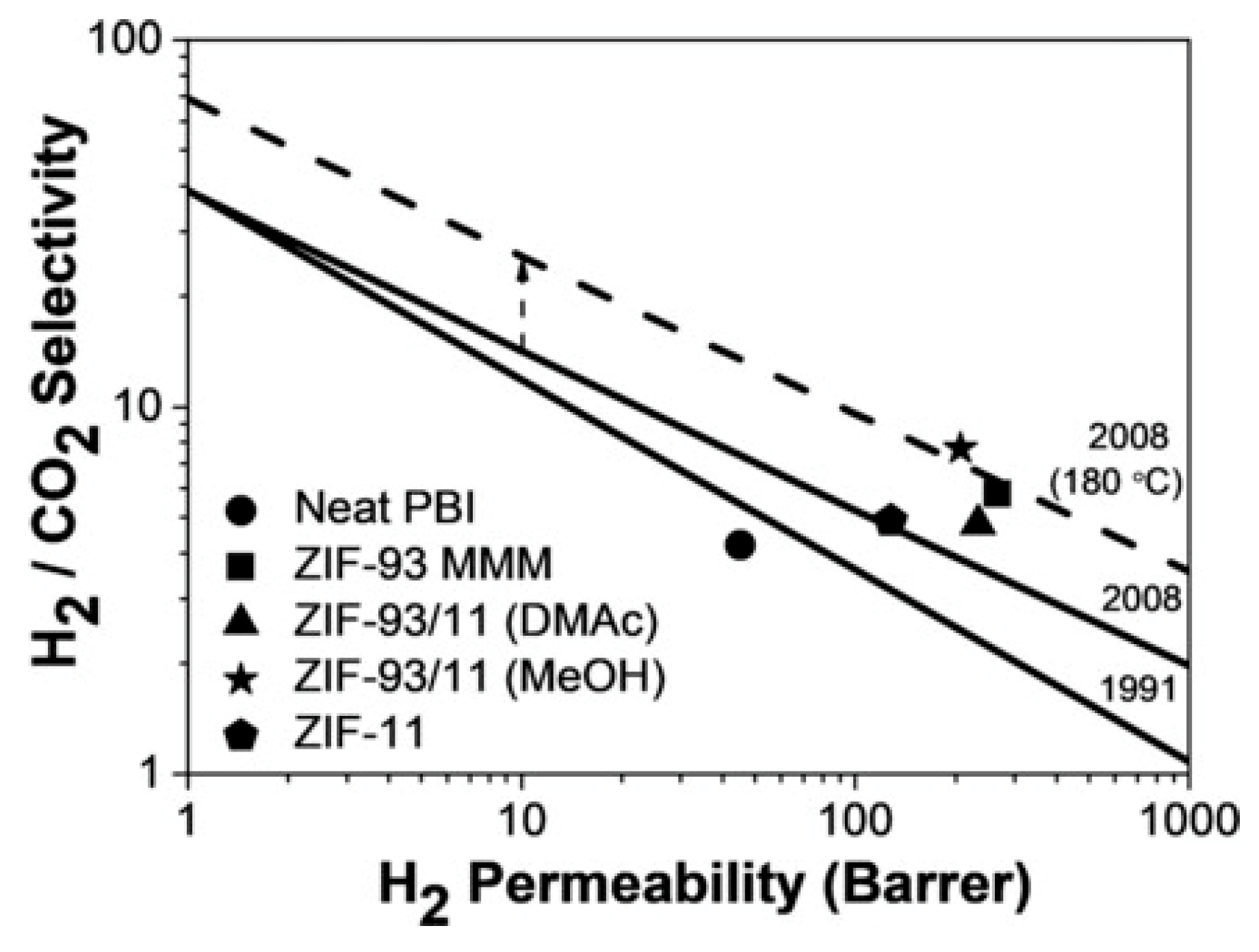
- Sánchez-Laínez, J.; Zornoza, B.; Orsi, A.F.; Łozińska, M.M.; Dawson, D.M.; Ashbrook, S.E.; Francis, S.M.; Wright, P.A.; Benoit, V.; Llewellyn, P.L.; et al. Synthesis of ZIF-93/11 Hybrid Nanoparticles via Post-Synthetic Modification of ZIF-93 and Their Use for H2/CO2 Separation. Chem. A Eur. J. 2018, 24, 11211–11219. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Makarevicius, V.; Tonkonogovas, A. GNs/MOF-Based Mixed Matrix Membranes for Gas Separations. Int. J. Hydrogen Energy 2023, 48, 19596–19604. [Google Scholar] [CrossRef]
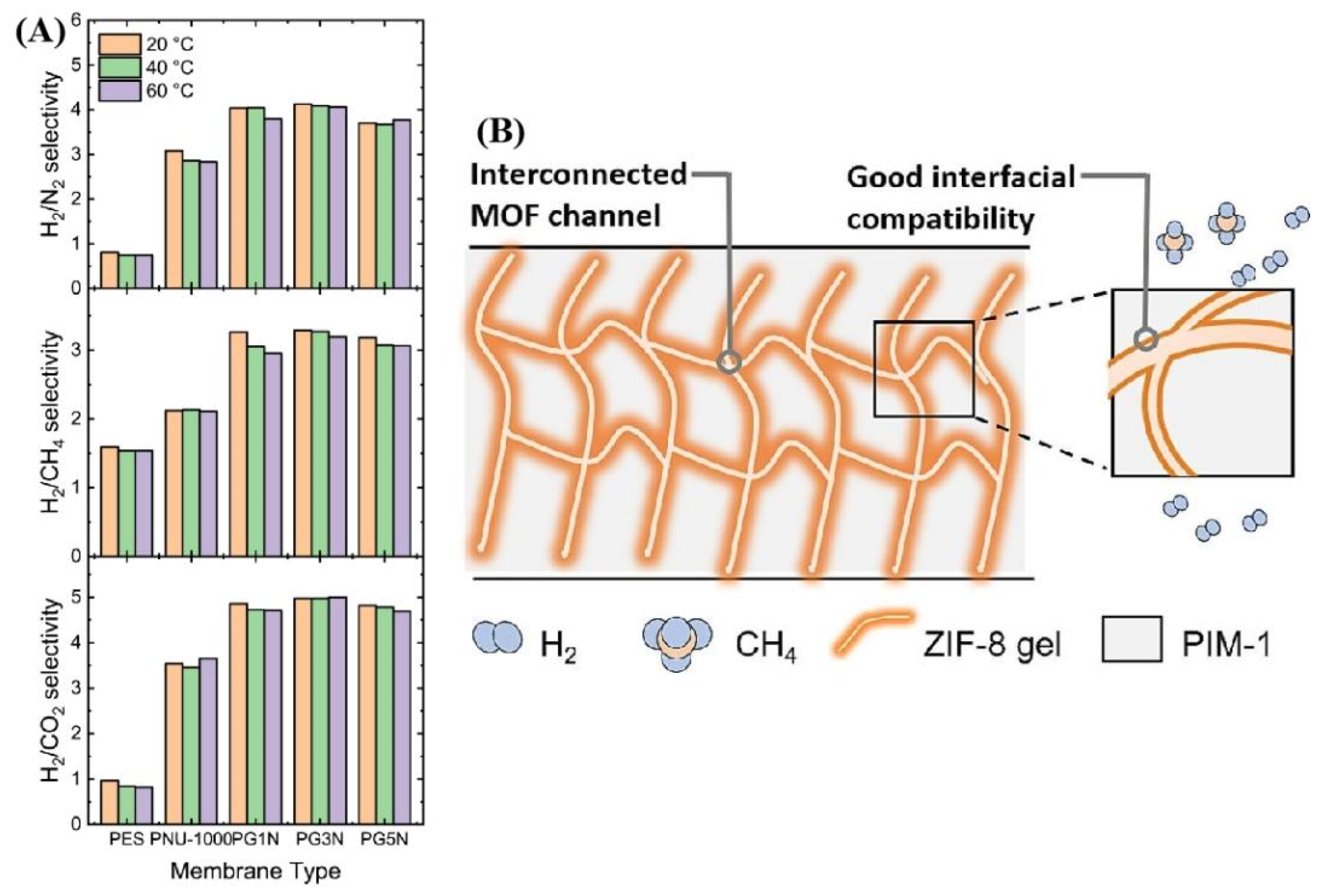
- Zhang, K.; Luo, X.; Li, S.; Tian, X.; Wang, Q.; Liu, C.; Tang, Y.; Feng, X.; Zhang, R.; Yin, S.; et al. ZIF-8 Gel/PIM-1 Mixed Matrix Membranes for Enhanced H2/CH4 Separations. Chem. Eng. J. 2024, 484, 149489. [Google Scholar] [CrossRef]
- Regmi, C.; Ashtiani, S.; Hrdlička, Z.; Friess, K. CO2/CH4 and H2/CH4 Gas Separation Performance of CTA-TNT@CNT Hybrid Mixed Matrix Membranes. Membranes 2021, 11, 862. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Caro, J.; Huang, A. Fabrication of a Flexible Hydrogen-Bonded Organic Framework Based Mixed Matrix Membrane for Hydrogen Separation. J. Membr. Sci. 2022, 643, 120021. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Effective Hydrogen Purification from Methane via Polyimide Matrimid® 5218-Deca-Dodecasil 3R Type Zeolite Mixed Matrix Membrane. Energy 2017, 141, 2100–2107. [Google Scholar] [CrossRef]
- Gorgojo, P.; Uriel, S.; Téllez, C.; Coronas, J. Development of Mixed Matrix Membranes Based on Zeolite Nu-6 for Gas Separation. Microporous Mesoporous Mater. 2008, 115, 85–92. [Google Scholar] [CrossRef]
- Japip, S.; Wang, H.; Xiao, Y.; Chung, T.S. Highly Permeable Zeolitic Imidazolate Framework (ZIF)-71 Nano-Particles Enhanced Polyimide Membranes for Gas Separation. J. Membr. Sci. 2014, 467, 162–174. [Google Scholar] [CrossRef]
- Hu, J.; Cai, H.; Ren, H.; Wei, Y.; Xu, Z.; Liu, H.; Hu, Y. Mixed-Matrix Membrane Hollow Fibers of Cu3(BTC)2 MOF and Polyimide for Gas Separation and Adsorption. Ind. Eng. Chem. Res. 2010, 49, 12605–12612. [Google Scholar] [CrossRef]
- Zornoza, B.; Irusta, S.; Téllez, C.; Coronas, J. Mesoporous Silica Sphere-Polysulfone Mixed Matrix Membranes for Gas Separation. Langmuir 2009, 25, 5903–5909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Musselman, I.H.; Ferraris, J.P.; Balkus, K.J. Gas Permeability Properties of Matrimid® Membranes Containing the Metal-Organic Framework Cu–BPY–HFS. J. Membr. Sci. 2008, 313, 170–181. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, S.; Wang, Y.; Ma, X.; Cao, K.; Peng, D.; Wu, X.; Wu, H.; Jiang, Z. Enhanced Gas Separation Performance of Mixed Matrix Membranes from Graphitic Carbon Nitride Nanosheets and Polymers of Intrinsic Microporosity. J. Membr. Sci. 2016, 514, 15–24. [Google Scholar] [CrossRef]
- Singh, Z.V.; Tan, L.L.; Cowan, M.G.; Yang, Y.W.; Zhang, W.; Gin, D.L.; Noble, R.D. Pillar[5]Arene/Matrimid™ Materials for High-Performance Methane Purification Membranes. J. Membr. Sci. 2017, 539, 224–228. [Google Scholar] [CrossRef]
- Hua, Y.; Park, S.; Jeong, H.K. Redefining Progress, Challenges, and Future Opportunities of Mixed-Matrix Membranes from an Engineering Perspective for Commercial Gas Separation Applications: A Review. J. Environ. Chem. Eng. 2024, 12, 113753. [Google Scholar] [CrossRef]
- Gade, S.K.; Thoen, P.M.; Way, J.D. Unsupported Palladium Alloy Foil Membranes Fabricated by Electroless Plating. J. Membr. Sci. 2008, 316, 112–118. [Google Scholar] [CrossRef]
- Uemiya, S.; Sato, N.; Ando, H.; Kude, Y.; Matsuda, T.; Kikuchi, E. Separation of Hydrogen Through Palladium Thin Film Supported on a Porous Glass Tube. J. Membr. Sci. 1991, 56, 303–313. [Google Scholar] [CrossRef]
- Kuraoka, K.; Zhao, H.; Yazawa, T. Pore-Filled Palladium-Glass Composite Membranes for Hydrogen Separation by Novel Electroless Plating Technique. J. Mater. Sci. 2004, 39, 1445–1449. [Google Scholar] [CrossRef]
- Itoh, N.; Akiha, T.; Sato, T. Preparation of Thin Palladium Composite Membrane Tube by a CVD Technique and Its Hydrogen Permselectivity. Catal. Today 2005, 104, 231–237. [Google Scholar] [CrossRef]
- Jun, C.S.; Lee, K.H. Palladium and Palladium Alloy Composite Membranes Prepared by Metal-Organic Chemical Vapor Deposition Method (Cold-Wall). J. Membr. Sci. 2000, 176, 121–130. [Google Scholar] [CrossRef]
- Nair, B.K.R.; Choi, J.; Harold, M.P. Electroless Plating and Permeation Features of Pd and Pd/Ag Hollow Fiber Composite Membranes. J. Membr. Sci. 2007, 288, 67–84. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ghasemzadeh, K.; Marelli, M.; Evangelisti, C. A Supported Pd-Cu/Al2O3 Membrane from Solvated Metal Atoms for Hydrogen Separation/Purification. Fuel Process. Technol. 2019, 195, 106141. [Google Scholar] [CrossRef]
- Fernandez, E.; Medrano, J.A.; Melendez, J.; Parco, M.; Viviente, J.L.; Van Sint Annaland, M.; Tanaka, D.P. Preparation and Characterization of Metallic Supported Thin Pd–Ag Membranes for Hydrogen Separation. Chem. Eng. J. 2016, 305, 182–190. [Google Scholar] [CrossRef]
- Kim, D.H.; Kong, S.Y.; Lee, G.H.; Yoon, C.W.; Ham, H.C.; Han, J.; Song, K.H.; Henkensmeier, D.; Choi, S.H. Effect of PBI-HFA Surface Treatments on Pd/PBI-HFA Composite Gas Separation Membranes. Int. J. Hydrogen Energy 2017, 42, 22915–22924. [Google Scholar] [CrossRef]




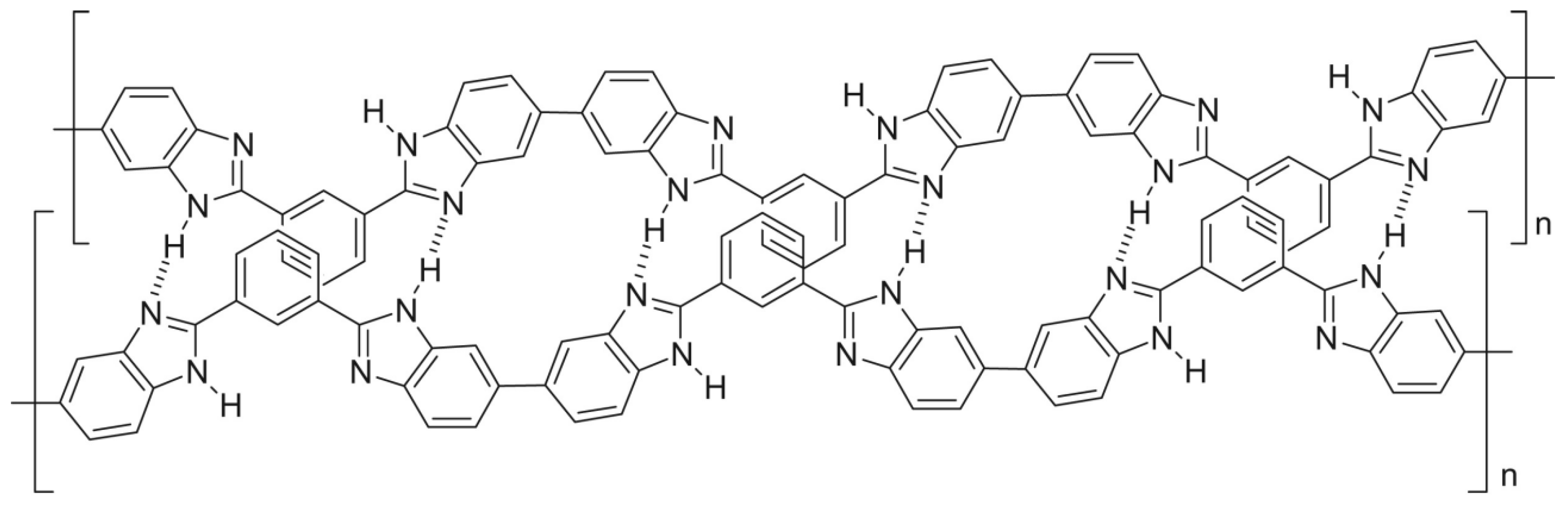
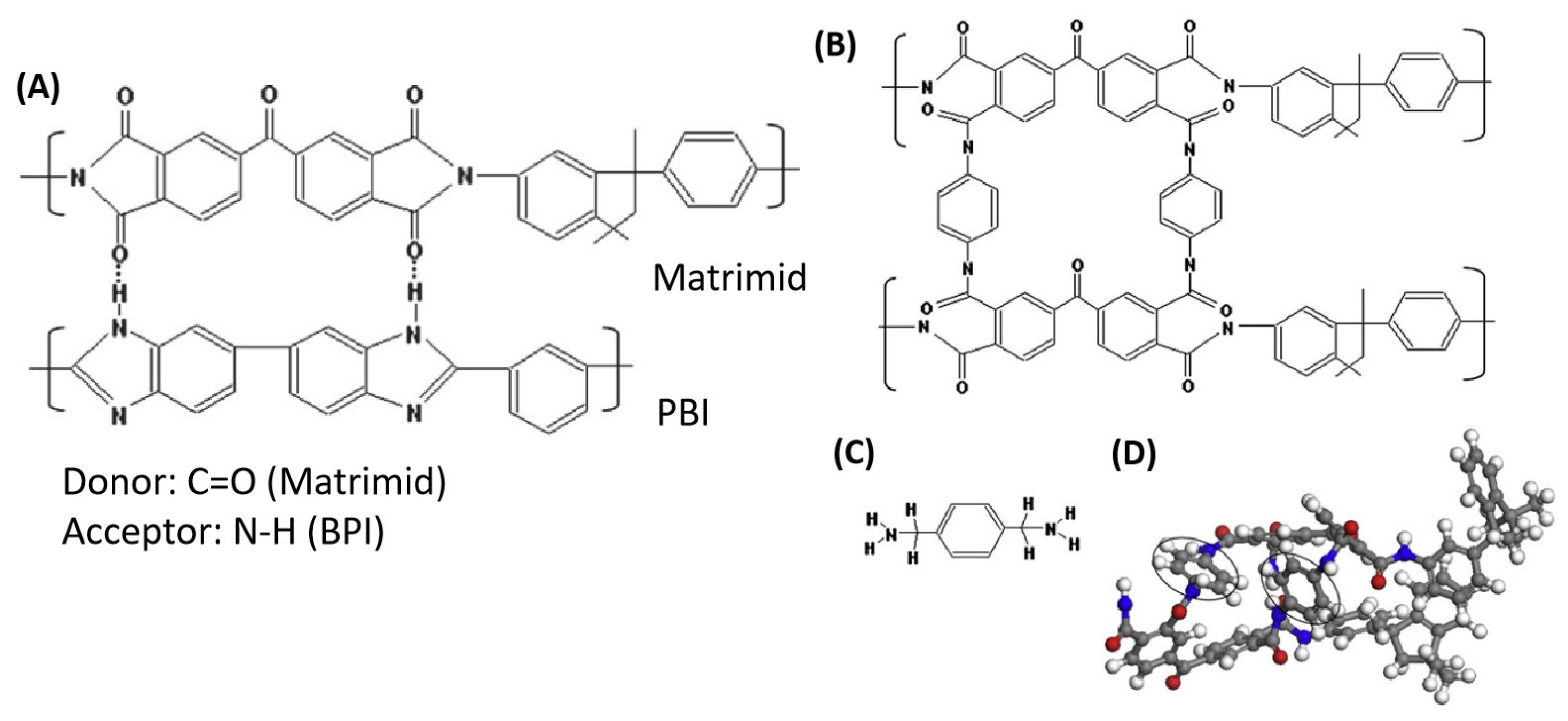
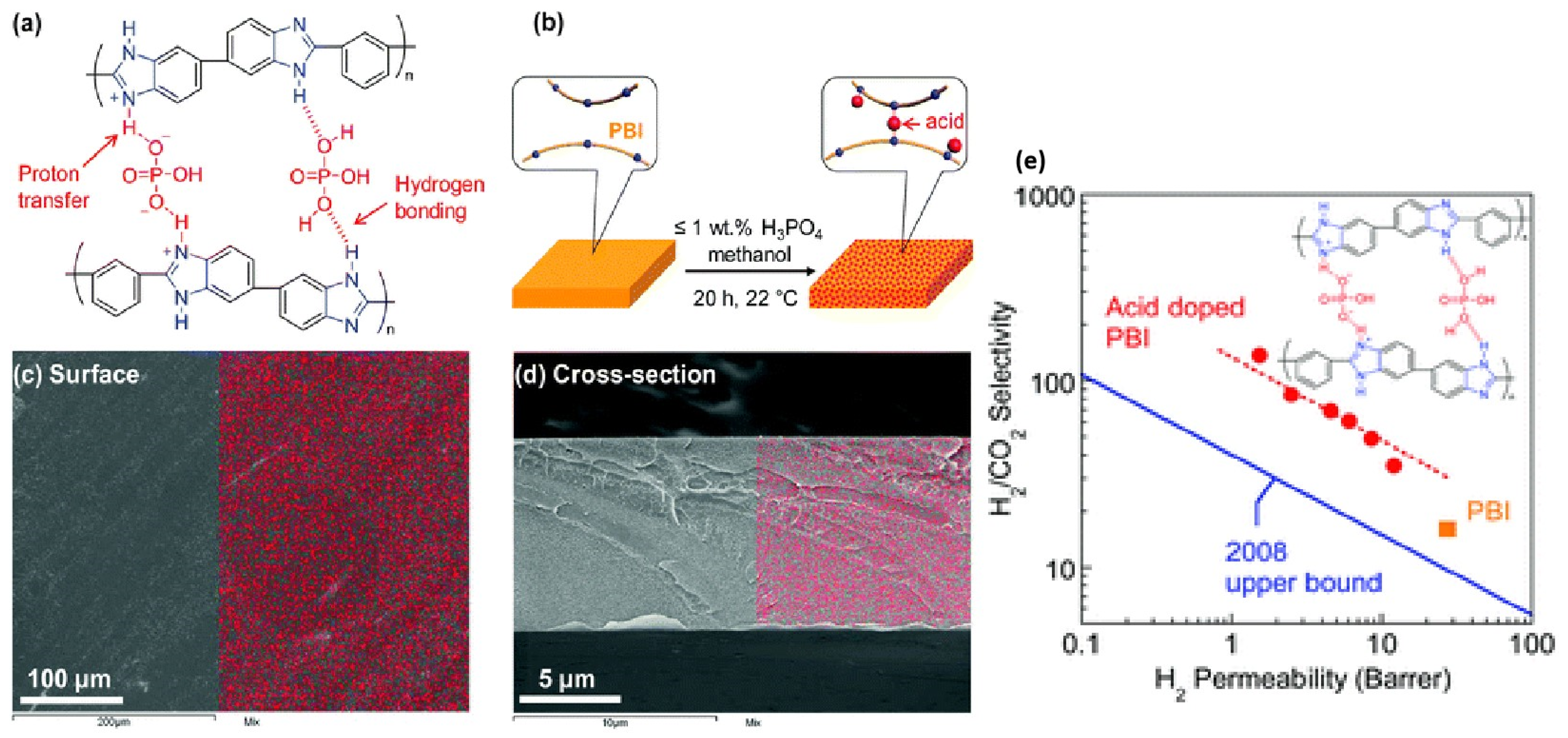
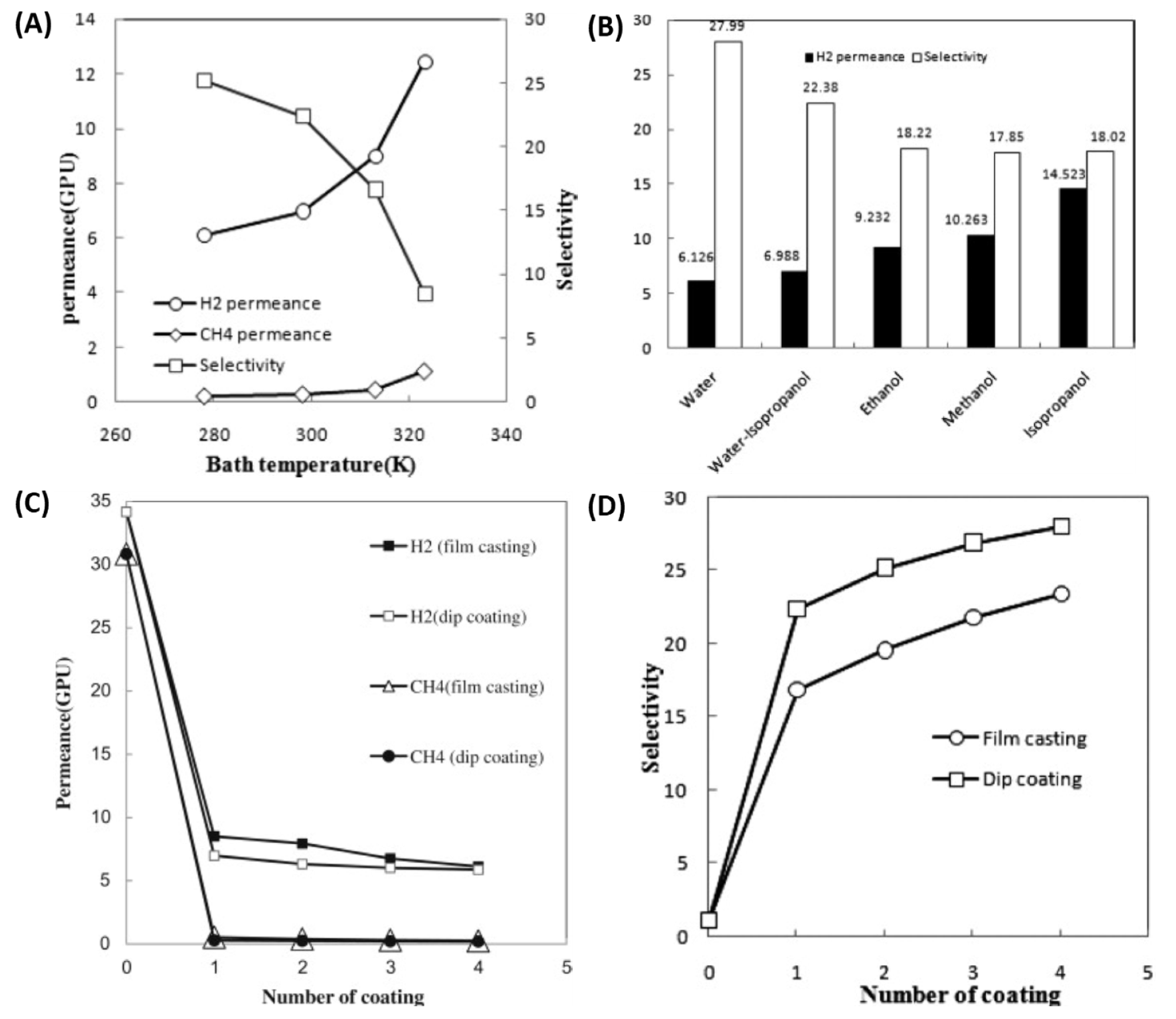
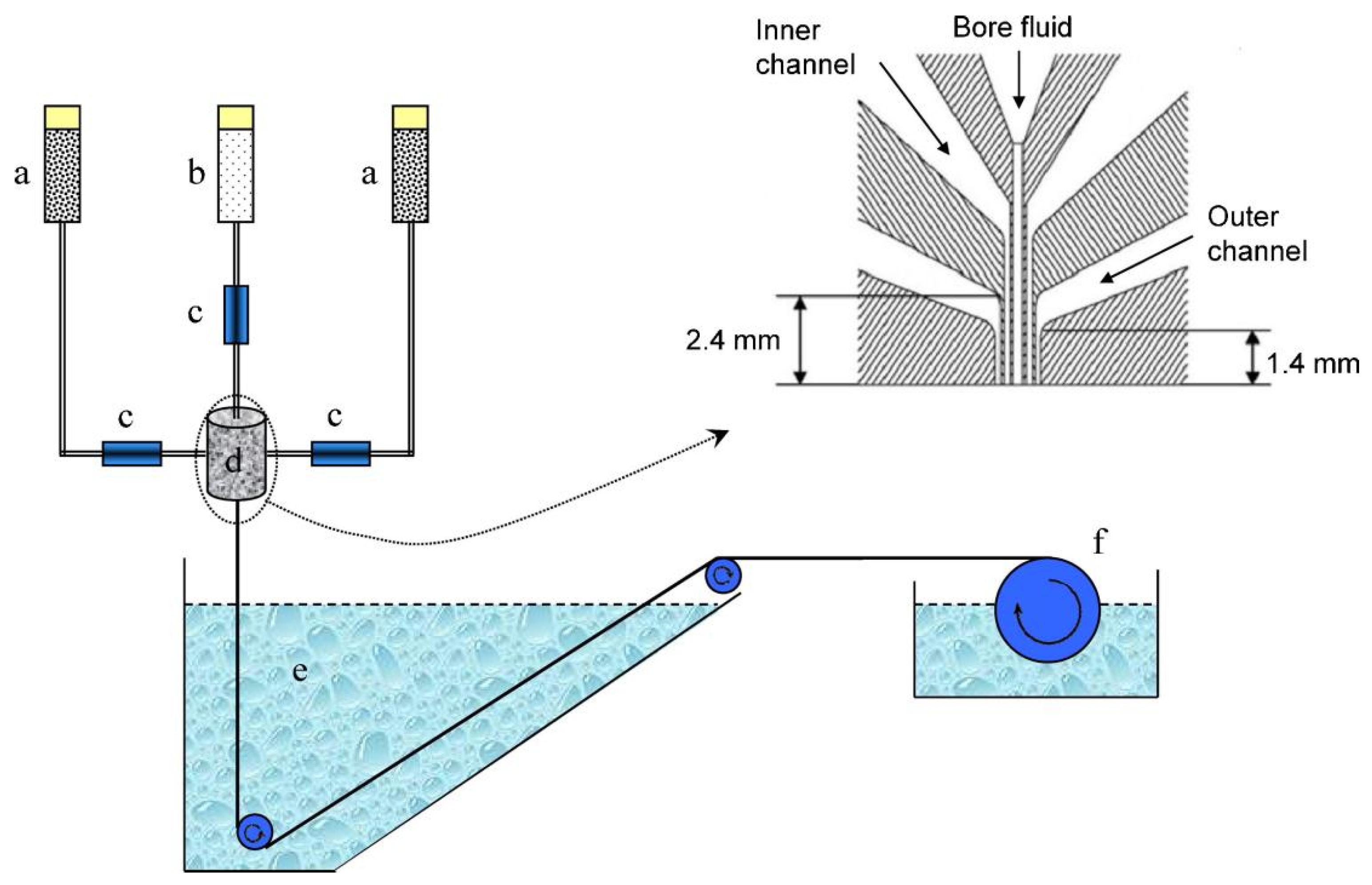




| Polymer | H2 (Barrer) | CO2 (Barrer) | N2 (Barrer) | CH4 (Barrer) |
|---|---|---|---|---|
| Cellulose acetate | 2.63 | 6.3 | 0.21 | 0.21 |
| Ethyl cellulose | 87 | 26.5 | 3.2 | 19 |
| Polycarbonate, brominated | NA | 4.23 | 0.18 | 0.13 |
| Polydimethylsiloxane | 550 | 2700 | 250 | 800 |
| Polyimide (Matrimid) | 28.1 | 10.7 | 0.32 | 0.25 |
| Polymethylpentene | 125 | 84.6 | 6.7 | 14.9 |
| Polyphenyleneoxide | 113 | 75.8 | 3.81 | 11 |
| Polysulfone | 14 | 5.6 | 0.25 | 0.25 |
| Polyetherimide | 7.8 | 1.32 | 0.047 | 0.035 |
| Polyethersulfone | 8.96 | 3.38 | 0.129 | 0.112 |
| Polystyrene (PS) | 23.8 | 10.4 | 0.6 | 0.8 |
| Poly (vinylidene fluoride) (Kynar) | 2.4 | 1.2 | 0.7 | 1.3 |
| Poly (methyl methacrylate) | 2.4 | 0.6 | 1.2 | 0.6 |
| Polymer | Operating Conditions Temp/Pressure | Permeability P(H2) Barrers | Selectivity α (H2/CH4) | Year of Development | Reference |
|---|---|---|---|---|---|
| Hyflon® AD60X | 25 °C/1 bar | 187 | 61.7 | 2007 | [159] |
| Teflon AF-2400 | 25 °C/50 psig | 3300 | 5.5 | 1996 | [160] |
| Polyimide (6FDA-mMPD) | 35 °C/10 atm | 106 | 121 | 1992 | [161] |
| Polyimide (6FDA-DDBT) | 35 °C/10 atm | 179 | 71 | 1995 | [162] |
| Sulfonated polyimide (DAPHFDS(H)) | 35 °C/1 atm | 52 | 330 | 2006 | [163] |
| Membrane/ Fabrication Technique | Polymer | Filler | Gas Pair | Selectivity | Year of Development | Ref. |
|---|---|---|---|---|---|---|
| NS@PBI-20 | PBI | Cu MOF [Cu2(ndc)2(dabco)]n | H2/CO2 | 26.7 | 2015 | [171] |
| TpPa-1(40)@PBI-BuI | PBI | COF [TpPa-1] | H2/CH4 | 165.5 | 2016 | [172] |
| H2/N2 | 79 | |||||
| MMMs (20 wt.% of NUS-2@PBI) | PBI | COF [NUS-2] | H2/CO2 | 31.4 | 2016 | [173] |
| 4 wt.% UZAR-S1-PSF MMM | Psf (Udel® P-3500) | UZAR-S1 | H2/CH4 | 69.2 | 2011 | [177] |
| 6FDA-DAM-ZIF-11 at 20 wt.% | 6FDA-DAM | ZIF-11 | H2/CH4 | 32.8 | 2017 | [178] |
| HOF-30@PI MMM | Matrimid@5218 | HOF-30 | H2/CH4 | 61.7 | 2022 | [184] |
| TR-PNC | HAB-6FDA polyimide | Silica | H2/CO2 | 86.4 | 2020 | [51] |
| H2/N2 | 43.2 | |||||
| Matrimid® 5218/20% of DDR | Matrimid® 5218 | Deca-dodecasil 3R (DDR) | H2/CH4 | 375.27 | 2017 | [185] |
| Udel®-Nu-6(2) | Psf | Zeolite Nu-6(2) | H2/CH4 | 398 | 2008 | [186] |
| 6FDA-Durene-ZIF-71—20% | 6FDA-Durene | ZIF-71 | H2/CH4 | 7.4 | 2014 | [187] |
| (PI–6 wt.% of Cu3(BTC)2) Hollow fiber by dry/wet spinning | PI | Cu3(BTC)2 | H2/CH4 | 240 | 2010 | [188] |
| Sample M3 | Psf | Mesoporous silica spheres (MSSs) | H2/CH4 | 79.2 | 2009 | [189] |
| 40 wt.% of Cu–BPY–HFS/Matrimid® | Matrimid® | Cu-BPY-HFS | H2/CH4 | 45.4 | 2008 | [190] |
| PIM-1–g-C3N4(2.0) | PIM-1 | g-C3N4 Prepared by the thermal oxidation etching method | H2/CH4 | 11.9 | 2016 | [191] |
| 50 wt.% of P5-SOF MMM | Matrimid 5218™ | Pillar[5]arene (P5-SOF) | H2/CH4 | 600 | 2017 | [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhalani, D.V.; Lim, B. Hydrogen Separation Membranes: A Material Perspective. Molecules 2024, 29, 4676. https://doi.org/10.3390/molecules29194676
Bhalani DV, Lim B. Hydrogen Separation Membranes: A Material Perspective. Molecules. 2024; 29(19):4676. https://doi.org/10.3390/molecules29194676
Chicago/Turabian StyleBhalani, Dixit V., and Bogyu Lim. 2024. "Hydrogen Separation Membranes: A Material Perspective" Molecules 29, no. 19: 4676. https://doi.org/10.3390/molecules29194676
APA StyleBhalani, D. V., & Lim, B. (2024). Hydrogen Separation Membranes: A Material Perspective. Molecules, 29(19), 4676. https://doi.org/10.3390/molecules29194676






