Development of Novel Honey- and Oat-Based Cocoa Beverages—A Comprehensive Analysis of the Impact of Drying Temperature and Mixture Composition on Physical, Chemical and Sensory Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties of the Cocoa Powder Mixtures
2.1.1. Color Measurement
2.1.2. Moisture Content and Water Activity
2.1.3. Bulk Density
2.1.4. Reconstitution Properties
2.1.5. Particle Size Distribution
2.2. Chemical Properties of the Cocoa Mixture Powder Extracts
2.3. Sensory Properties of Cocoa Mixture Powders
2.4. Optimization of Process Conditions and Mixture Composition
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation and Drying of Mixtures
3.2.2. Analysis of Physical Properties of Powders
Color Measurement
Moisture Content
Bulk Density
Water Activity
Reconstitution Properties
Particle Size Distribution
3.2.3. Analysis of Chemical Properties of Extracts
Extract Preparation
Total Dissolved Substance and Conductivity
pH Value
Sugar Content in Degrees Brix
Color Measurement
Determination of Total Polyphenolic Content (TPC)
Determination of Antioxidant Activity Using the DPPH Method
Determination of Antioxidant Activity Using the FRAP Method
3.2.4. Sensory Analysis of Cocoa Powders and Drinks
3.2.5. Statistical Processing of Data and Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shabir, I.; Dash, K.K.; Dar, A.H.; Pandey, V.K.; Fayaz, U.; Srivastava, S.; Nisha, R. Carbon footprints evaluation for sustainable food processing system development: A comprehensive review. Futur. Foods 2023, 7, 100215. [Google Scholar] [CrossRef]
- Kongor, J.E.; Muhammad, D.R.A. Processing of Cocoa and Development of Chocolate Beverages. In Natural Products in Beverages. Reference Series in Phytochemistry; Mérillon, J.M., Riviere, C., Lefèvre, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–37. [Google Scholar] [CrossRef]
- Putri, N.T.; Sukma, M. Development of Chocolate Beverage Products by Considering Consumer Preferences. In Proceedings of the Second Asia Pacific International Conference on Industrial Engineering and Operations Management, Surakarta, Indonesia, 14–16 September 2021. [Google Scholar]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Tušek, K.; Valinger, D.; Jurina, T.; Sokač Cvetnić, T.; Gajdoš Kljusurić, J.; Benković, M. Bioactives in Cocoa: Novel Findings, Health Benefits, and Extraction Techniques. Separations 2024, 11, 128. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Shim, J.; Lee, C.Y.; Lee, K.W.; Lee, H.J. Cocoa phytochemicals: Recent advances in molecular mechanisms on health. Crit. Rev. Food Sci. Nutr. 2014, 54, 1458–1472. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Al-Waili, F.S.; Akmal, M.; Ali, A.; Salom, K.Y.; Al Ghamdi, A.A. Effects of natural honey on polymicrobial culture of various human pathogens. Arch. Med. Sci. 2014, 10, 246–250. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Peternelj, T.T.; Coombes, J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E. Health benefits of oat: Current evidence and molecular mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. A Review of Extraction and Analysis of Bioactives in Oat and Barley and Scope for Use of Novel Food Processing Technologies. Molecules 2015, 20, 10884–10909. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Wu, W.; Zhao, Y.; Idehen, E.; Sang, S. Steroidal Saponins in Oat Bran. J. Agric. Food Chem. 2016, 64, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Samborska, K.; Wiktor, A.; Jedlińska, A.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Tułodziecki, M.; Błażowski, Ł.; Witrowa-Rajchert, D. Development and characterization of physical properties of honey-rich powder. Food Bioprod. Process. 2019, 115, 78–86. [Google Scholar] [CrossRef]
- Samborska, K.; Langa, E.; Kamińska-Dwórznicka, A.; Witrowa-Rajchert, D. The influence of sodium caseinate on the physical properties of spray-dried honey. Int. J. Food Sci. Technol. 2015, 50, 256–262. [Google Scholar] [CrossRef]
- Samborska, K.; Wasilewska, A.; Gondek, E.; Jakubczyk, E.; Kamińska-Dwórznicka, A. Diastase Activity Retention and Physical Properties of Honey/Arabic Gum Mixtures after Spray Drying and Storage. Int. J. Food Eng. 2017, 13, 20160320. [Google Scholar] [CrossRef]
- Shi, Q.; Fang, Z.; Bhandari, B. Effect of Addition of Whey Protein Isolate on Spray-Drying Behavior of Honey with Maltodextrin as a Carrier Material. Dry. Technol. 2013, 31, 1681–1692. [Google Scholar] [CrossRef]
- Ramly, N.S.; Shahira, I.; Sujanto, R.; Ghani, A.A.; Tang, J.; Huat, Y.; Alias, N.; Ngah, N. The Impact of Processing Methods on the Quality of Honey: A Review. Malays. J. Appl. Sci. 2021, 6, 99–110. [Google Scholar] [CrossRef]
- Salehifar, M.; Shahedi, M. Effects of Oat Flour on Dough Rheology, Texture and Organoleptic Properties of Taftoon Bread. J. Agric. Sci. Technol. 2007, 9, 227–234. [Google Scholar]
- van den Broeck, H.C.; Londono, D.M.; Timmer, R.; Smulders, M.J.M.; Gilissen, L.J.W.J.; van der Meer, I.M. Profiling of Nutritional and Health-Related Compounds in Oat Varieties. Foods 2016, 5, 2. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, R.; Hu, X.; Ren, C.; Li, Y. Oat-Based Foods: Chemical Constituents, Glycemic Index, and the Effect of Processing. Foods 2021, 10, 1304. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Zhu, S.; Luo, C.; Ma, J.; Zhong, F. The effect of alkalization on the bioactive and flavor related components in commercial cocoa powder. J. Food Compos. Anal. 2012, 25, 17–23. [Google Scholar] [CrossRef]
- Jung, H.; Lee, Y.J.; Yoon, W.B. Effect of Moisture Content on the Grinding Process and Powder Properties in Food: A Review. Processes 2018, 6, 69. [Google Scholar] [CrossRef]
- Hii, C.L.; Law, C.L.; Cloke, M.; Suzannah, S. Thin layer drying kinetics of cocoa and dried product quality. Biosyst. Eng. 2009, 102, 153–161. [Google Scholar] [CrossRef]
- Hardiyanto, Y.F.; Saputro, A.D.; Nurkholisa, Z.; Setiyadi, P.A.; Bintoro, N.; Kusuma, R.A. The effect of steaming time and types of cocoa powder on the characteristics of instantized cocoa powder made using batch-type steam jet agglomerator. IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012089. [Google Scholar] [CrossRef]
- Nakagawa, H.; Oyama, T. Molecular Basis of Water Activity in Glycerol–Water Mixtures. Front. Chem. 2019, 7, 485689. [Google Scholar] [CrossRef]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G. Food Powders: Physical Properties, Processing, and Functionality; Springer: New York, NY, USA, 2005. [Google Scholar]
- Belščak-Cvitanović, A.; Benković, M.; Komes, D.; Bauman, I.; Horžić, D.; Dujmić, F.; Matijašec, M. Physical properties and bioactive constituents of powdered mixtures and drinks prepared with cocoa and various sweeteners. J. Agric. Food Chem. 2010, 58, 7187–7195. [Google Scholar] [CrossRef]
- Kowalska, J.; Majewska, E.; Lenart, A. Sorption properties of a modified powdered cocoa beverage. Chem. Process Eng.-Inz. Chem. I Proces. 2011, 32, 21–31. [Google Scholar] [CrossRef][Green Version]
- Fu, X.; Huck, D.; Makein, L.; Armstrong, B.; Willen, U.; Freeman, T. Effect of particle shape and size on flow properties of lactose powders. Particuology 2012, 10, 203–208. [Google Scholar] [CrossRef]
- Malvern Instruments Ltd. Manual: Mastersizer 2000 Essentials Version 5.60 User Manual; Malvern Instruments Ltd.: Worcestershire, UK, 2000. [Google Scholar]
- Benković, M.; Srečec, S.; Špoljarić, I.; Mršić, G.; Bauman, I. Flow Properties of Commonly Used Food Powders and Their Mixtures. Food Bioprocess Technol. 2013, 6, 2525–2537. [Google Scholar] [CrossRef]
- Moon, J.H.; Yoon, W.B. Effect of moisture content and particle size on grinding kinetics and flowability of balloon flower (Platycodon grandiflorum). Food Sci. Biotechnol. 2018, 27, 641–650. [Google Scholar] [CrossRef]
- Buljat, A.M.; Jurina, T.; Tušek, A.J.; Valinger, D.; Kljusuric, J.G.; Benkovic, M. Applicability of foam mat drying process for production of instant cocoa powder enriched with lavender extract. Food Technol. Biotechnol. 2019, 57, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Puchol-Miquel, M.; Palomares, C.; Fernández-Segovia, I.; Barat, J.M.; Perez-Esteve, É. Effect of the type and degree of alkalization of cocoa powder on the physico-chemical and sensory properties of sponge cakes. LWT 2021, 152, 112241. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Feng, Y.; Xu, F.; Ma, J.; Zhong, F. Influence of alkalization treatment on the color quality and the total phenolic and anthocyanin contents in cocoa powder. Food Sci. Biotechnol. 2014, 23, 59–63. [Google Scholar] [CrossRef]
- Valverde, D.; Behrends, B.; Pérez-Esteve, É.; Kuhnert, N.; Barat, J.M. Functional changes induced by extrusion during cocoa alkalization. Food Res. Int. 2020, 136, 109469. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Elmaci, C.; Atalar, İ.; Toker, O.S.; Palabiyik, I.; Konar, N. Influence of process conditions of alkalization on quality of cocoa powder. Food Res. Int. 2024, 182, 114147. [Google Scholar] [CrossRef]
- Urbańska, B.; Kowalska, J. Comparison of the Total Polyphenol Content and Antioxidant Activity of Chocolate Obtained from Roasted and Unroasted Cocoa Beans from Different Regions of the World. Antioxidants 2019, 8, 283. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; Tavares, I.R.G.; Junior, O.J.F.R.; de Souza, M.V.G.; Junior, C.A.C.; Alvares, T.d.S. Evaluation of total polyphenols content and antioxidant capacity of different commercial cocoa (theobroma cacao) powders)/Avaliação do teor de polifenóis totais e capacidade antioxidante de diferentes pós comerciais de cacau (theobroma cacao). Brazilian J. Dev. 2021, 7, 39100–39109. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Jalil, A.M.M.; Ismail, A. Polyphenols in Cocoa and Cocoa Products: Is There a Link between Antioxidant Properties and Health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef]
- Jaćimović, S.; Popović-Djordjević, J.; Sarić, B.; Krstić, A.; Mickovski-Stefanović, V.; Pantelić, N. Antioxidant Activity and Multi-Elemental Analysis of Dark Chocolate. Foods 2022, 11, 1445. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Kulieva, V.A.; Sánchez-Chero, M.; Villegasyarlequé, M.; Aguilar, G.F.V.; Carrión-Barco, G.; Santa Cruz, A.G.Y.; Sánchez-Chero, J. An Overview on the Use of Response Surface Methodology to Model and Optimize Extraction Processes in the Food Industry. Curr. Res. Nutr. Food Sci. 2021, 9, 745–754. [Google Scholar] [CrossRef]
- Chicco, D.; Warrens, M.J.; Jurman, G. The coefficient of determination R-squared is more informative than SMAPE, MAE, MAPE, MSE and RMSE in regression analysis evaluation. PeerJ Comput. Sci. 2021, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Kowalska, H.; Cieślak, B.; Majewska, E.; Ciecierska, M.; Derewiaka, D.; Lenart, A. Influence of sucrose substitutes and agglomeration on volatile compounds in powdered cocoa beverages. J. Food Sci. Technol. 2020, 57, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Aribah, S.A.l.; Sanjaya, A.P.; Muhammad, D.R.A.; Praseptiangga, D. Sensorial and physical properties of chocolate beverage prepared using low fat cocoa powder. In Proceedings of the 2nd International Conference and Exhibition on Powder Technology (ICePTi) 2019, Solo, Indonesia, 20–21 August 2019; AIP Publishing: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Valinger, D.; Longin, L.; Grbeš, F.; Benković, M.; Jurina, T.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Detection of honey adulteration—The potential of UV-VIS and NIR spectroscopy coupled with multivariate analysis. LWT 2021, 145, 111316. Available online: https://www.scirp.org/reference/ReferencesPapers?ReferenceID=1929875 (accessed on 25 August 2024). [CrossRef]
- Association of Official Analytical Chemist. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Haugaard, I.S.; Krag, J.; Pisecky, J.; Westergaard, V. Analytical Methods for Dry Milk Powders. Denmark Niro Atomizer; Academic Press: San Diego, CA, USA, 1978; Available online: https://www.scirp.org/reference/referencespapers?referenceid=2528207 (accessed on 25 August 2024).
- Benković, M.; Tušek, A.J.; Belščak-Cvitanović, A.; Lenart, A.; Domian, E.; Komes, D.; Bauman, I. Artificial neural network modelling of changes in physical and chemical properties of cocoa powder mixtures during agglomeration. LWT-Food Sci. Technol. 2015, 64, 140–148. [Google Scholar] [CrossRef]
- ISO 18787:2017; Foodstuffs—Determination of Water Activity. ISO: Geneva, Switzerland, 2017. Available online: https://bbn.isolutions.iso.org/obp/ui#iso:std:iso:18787:ed-1:v1:en (accessed on 25 August 2024).
- ISO 13320:2020; Particle Size Analysis—Laser Diffraction Methods. ISO: Geneva, Switzerland, 2020; p. 59.
- Yoshikawa, H.; Hirano, A.; Arakawa, T.; Shiraki, K. Mechanistic insights into protein precipitation by alcohol. Int. J. Biol. Macromol. 2012, 50, 865–871. [Google Scholar] [CrossRef]
- Jurina, T.; Cetković, A.-M.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A.; Benković, M.; Valinger, D. Modelling and optimization of physical characteristics based on UV-VIS/NIR spectra of aqueous extracts of lavender, mint and melissa. South East Eur. J. Sustain. Dev. 2018, 2, 51–59. [Google Scholar]
- Jaywant, S.A.; Singh, H.; Arif, K.M. Sensors and Instruments for Brix Measurement: A Review. Sensors 2022, 22, 2290. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Benković, M.; Valinger, D.; Jurina, T.; Belščak-Cvitanović, A.; Gajdoš Kljusurić, J. Optimizing bioactive compounds extraction from different medicinal plants and prediction through nonlinear and linear models. Ind. Crops Prod. 2018, 126, 449–458. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Benković, M.; Pižeta, M.; Jurinjak Tušek, A.; Jurina, T.; Gajdoš Kljusurić, J.; Valinger, D. Optimization of the foam mat drying process for production of cocoa powder enriched with peppermint extract. LWT 2019, 115, 108440. [Google Scholar] [CrossRef]
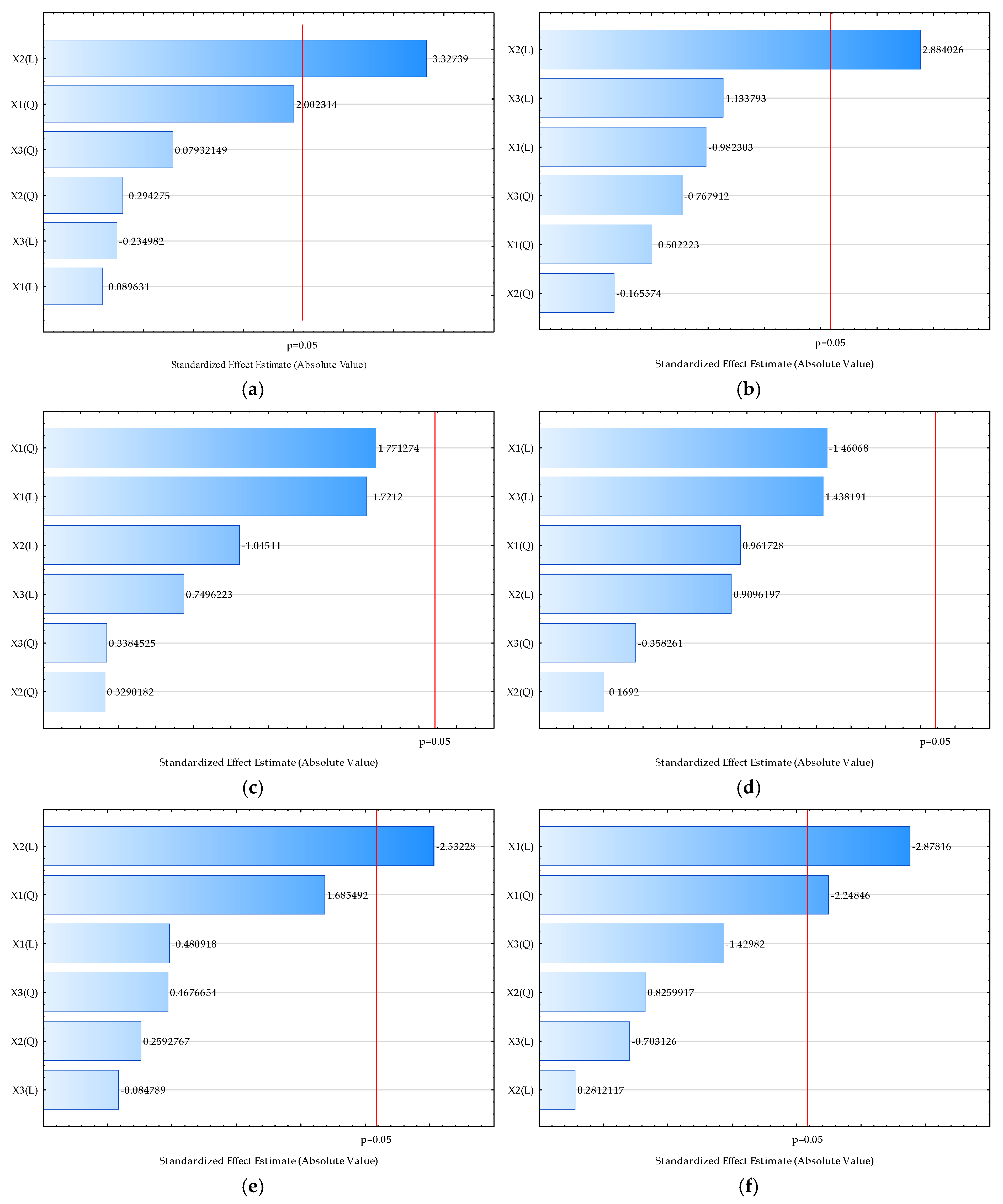
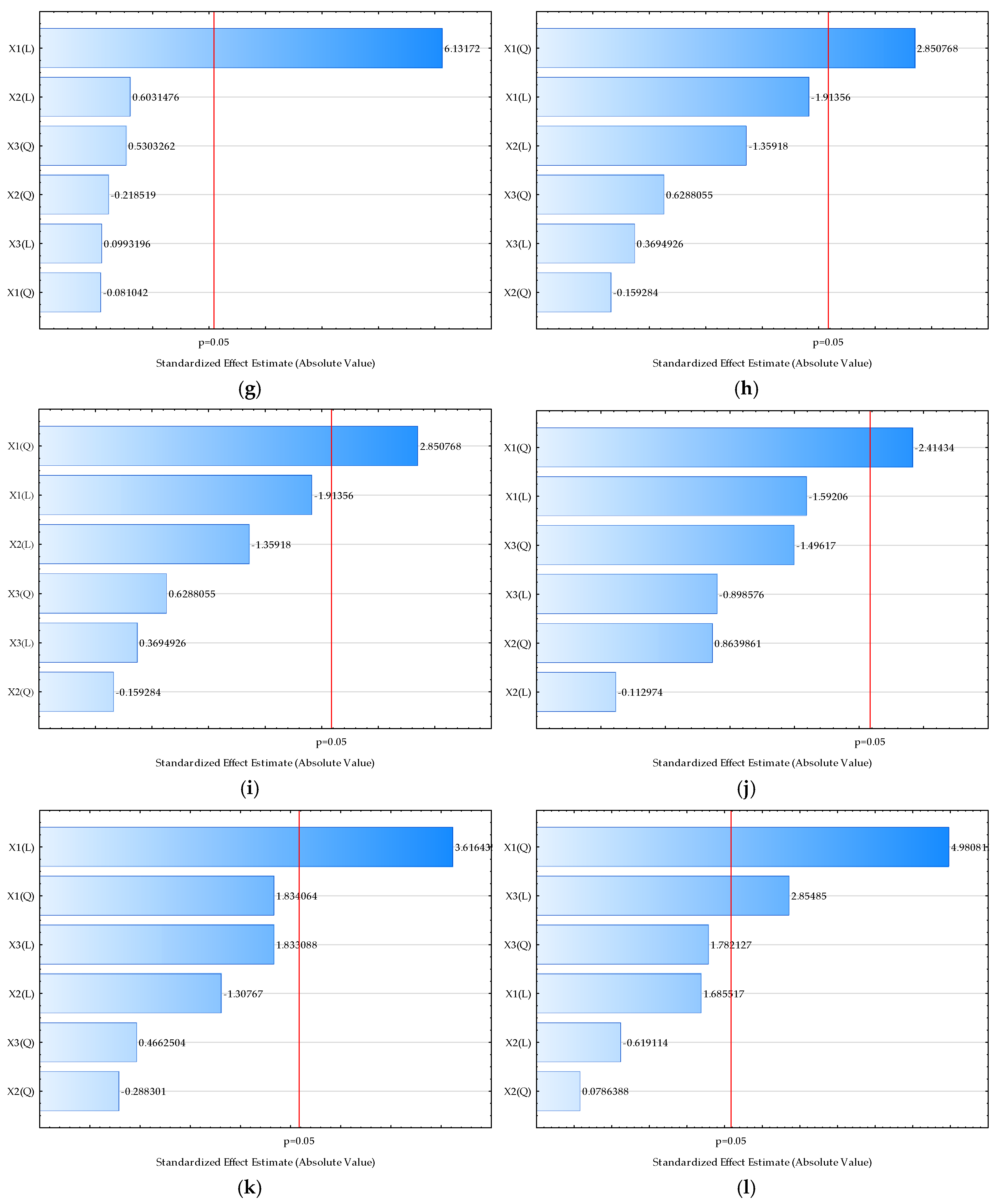
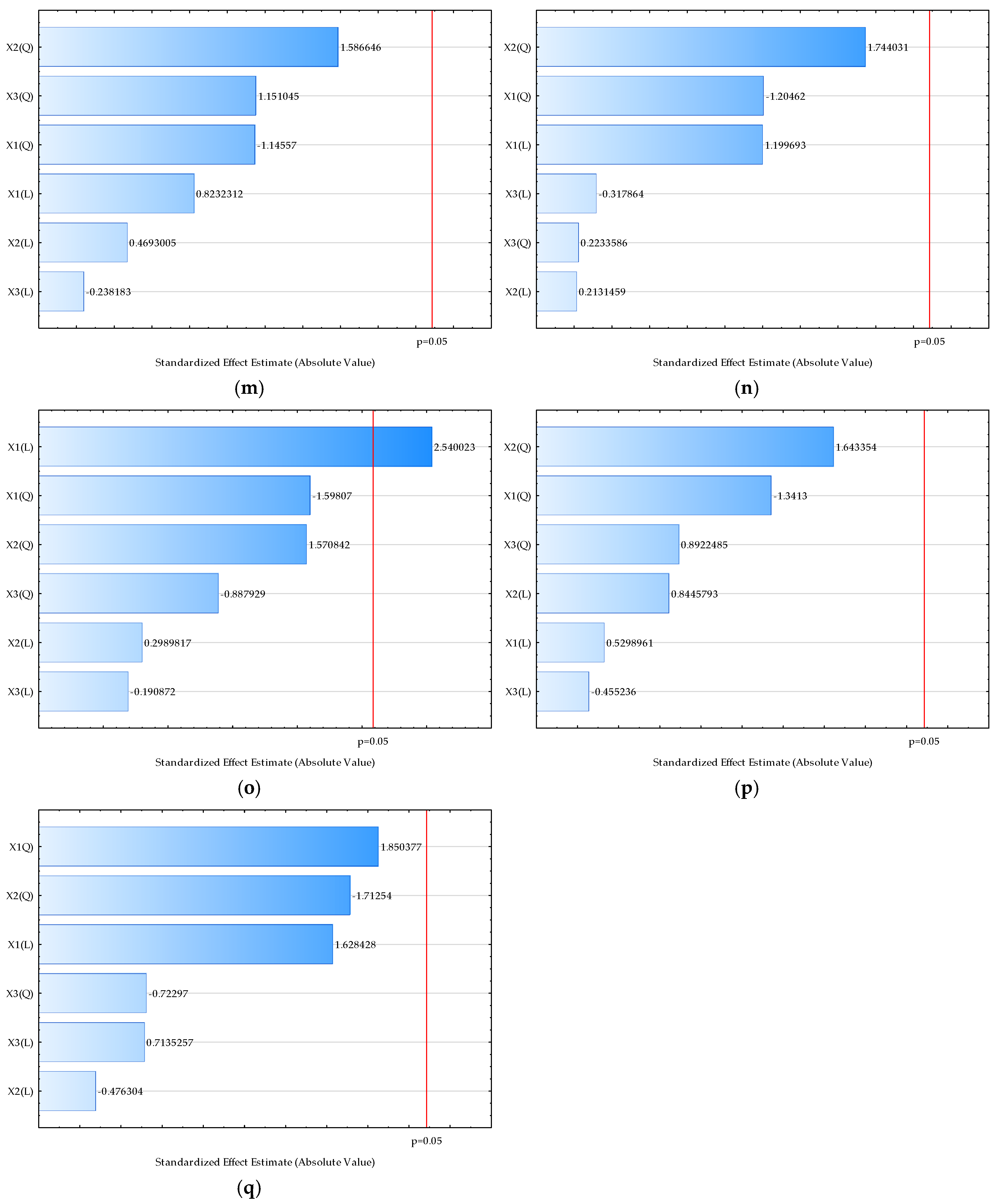

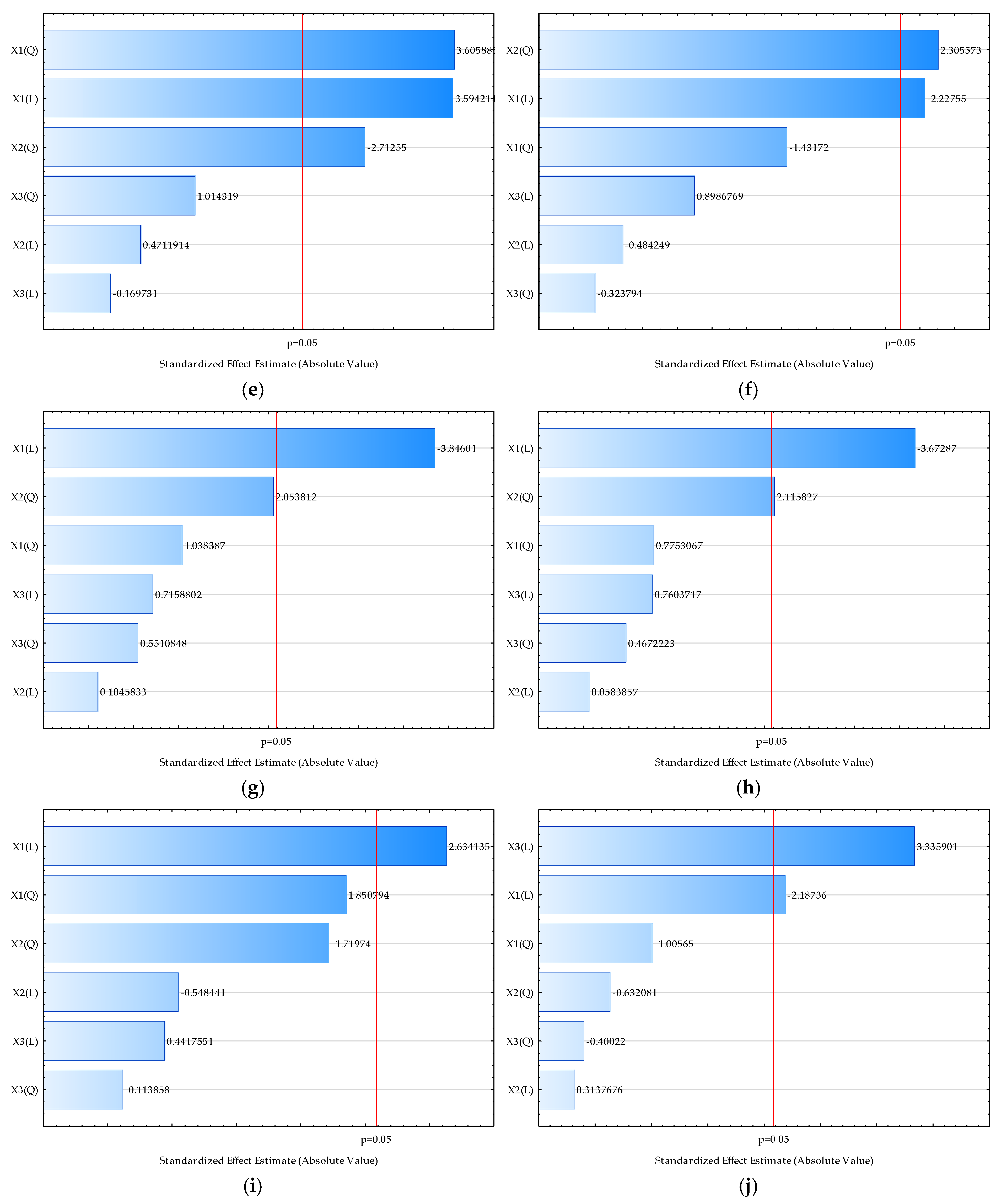
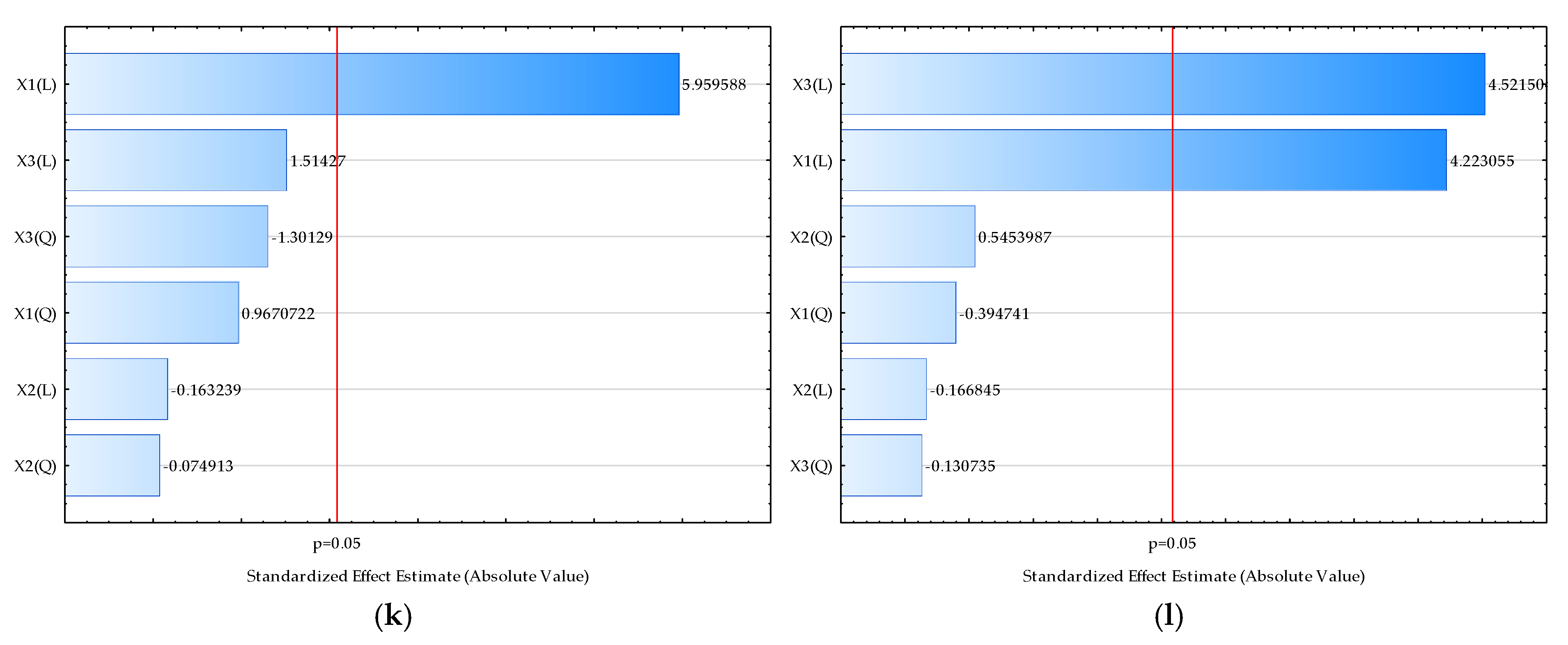
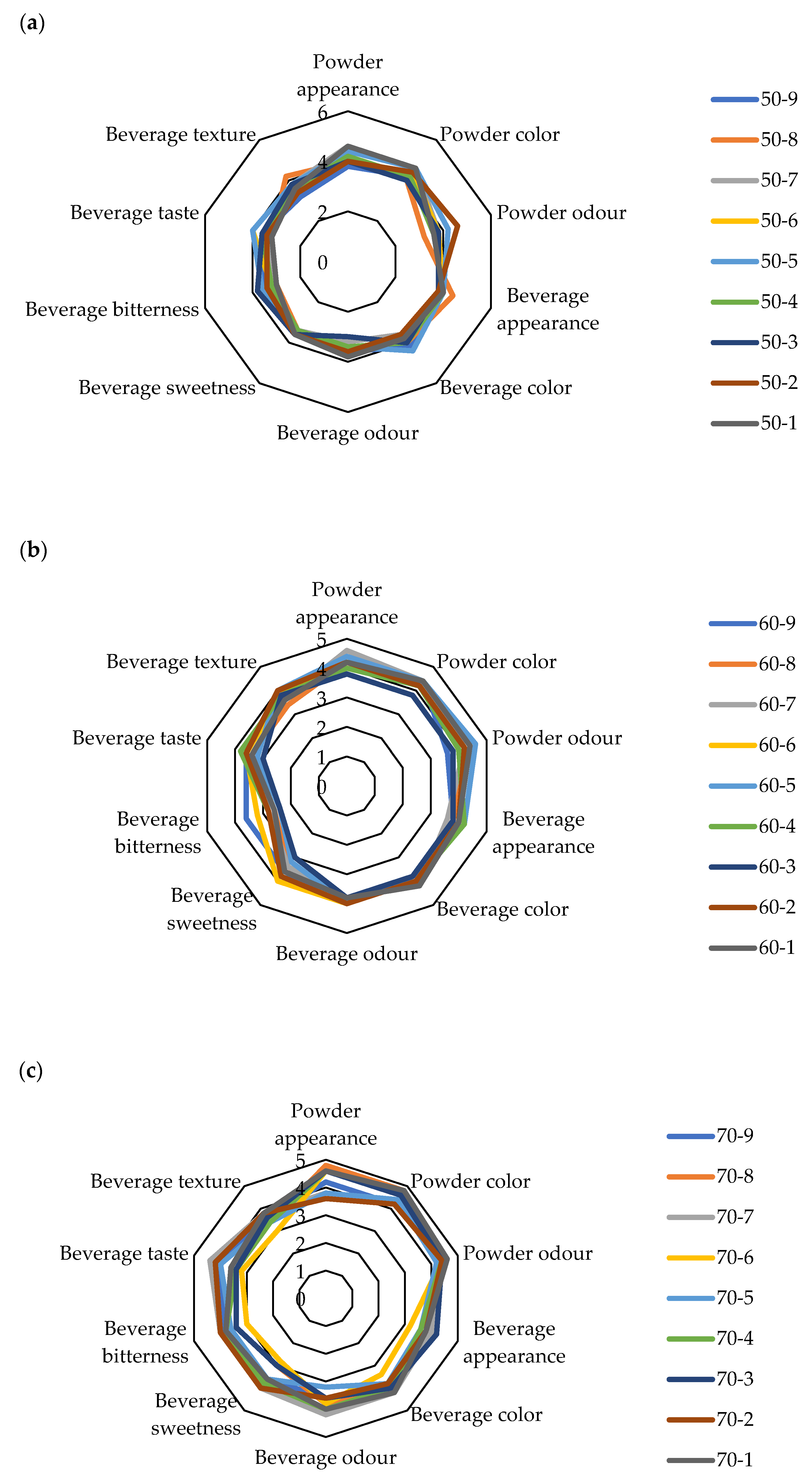
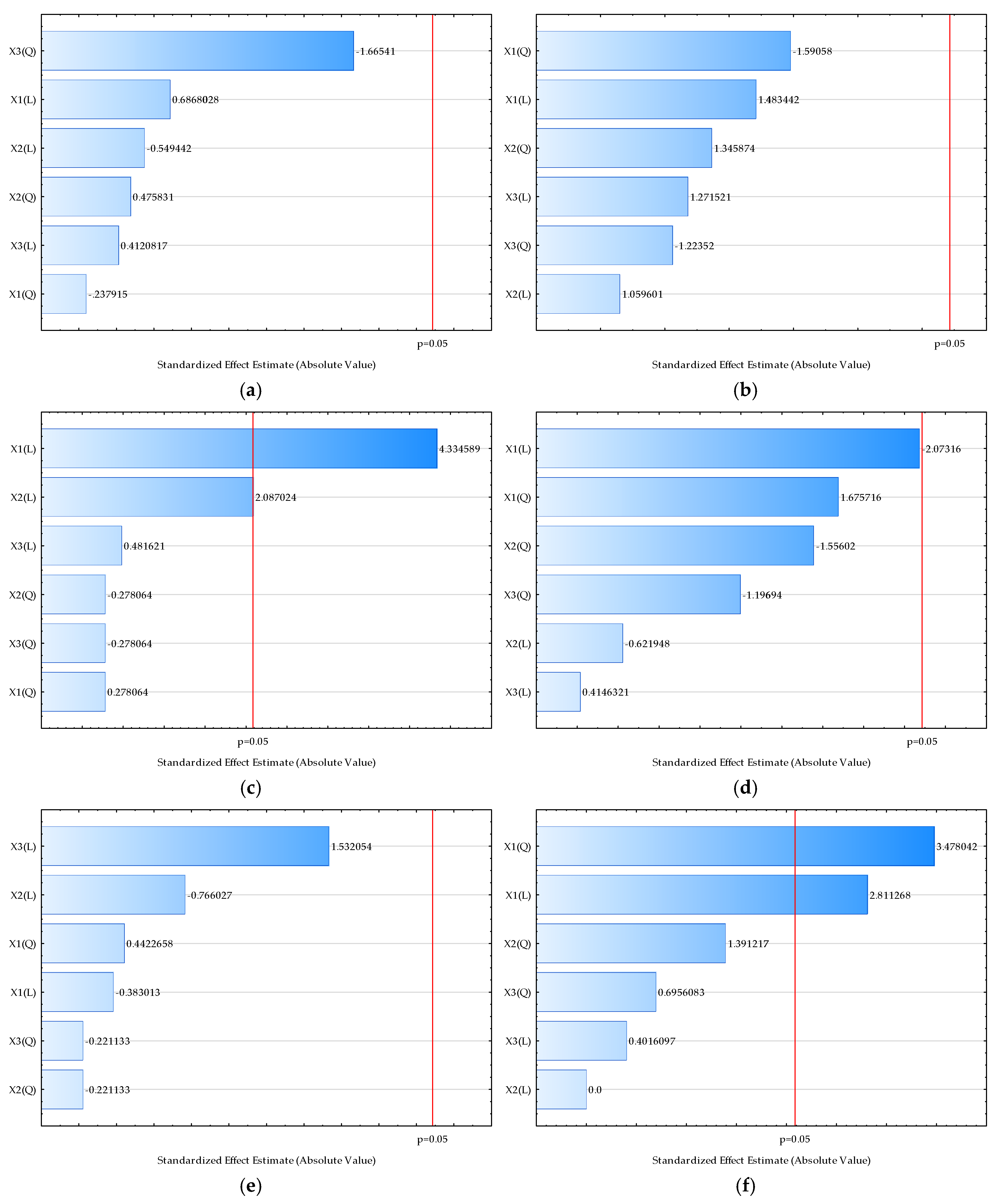
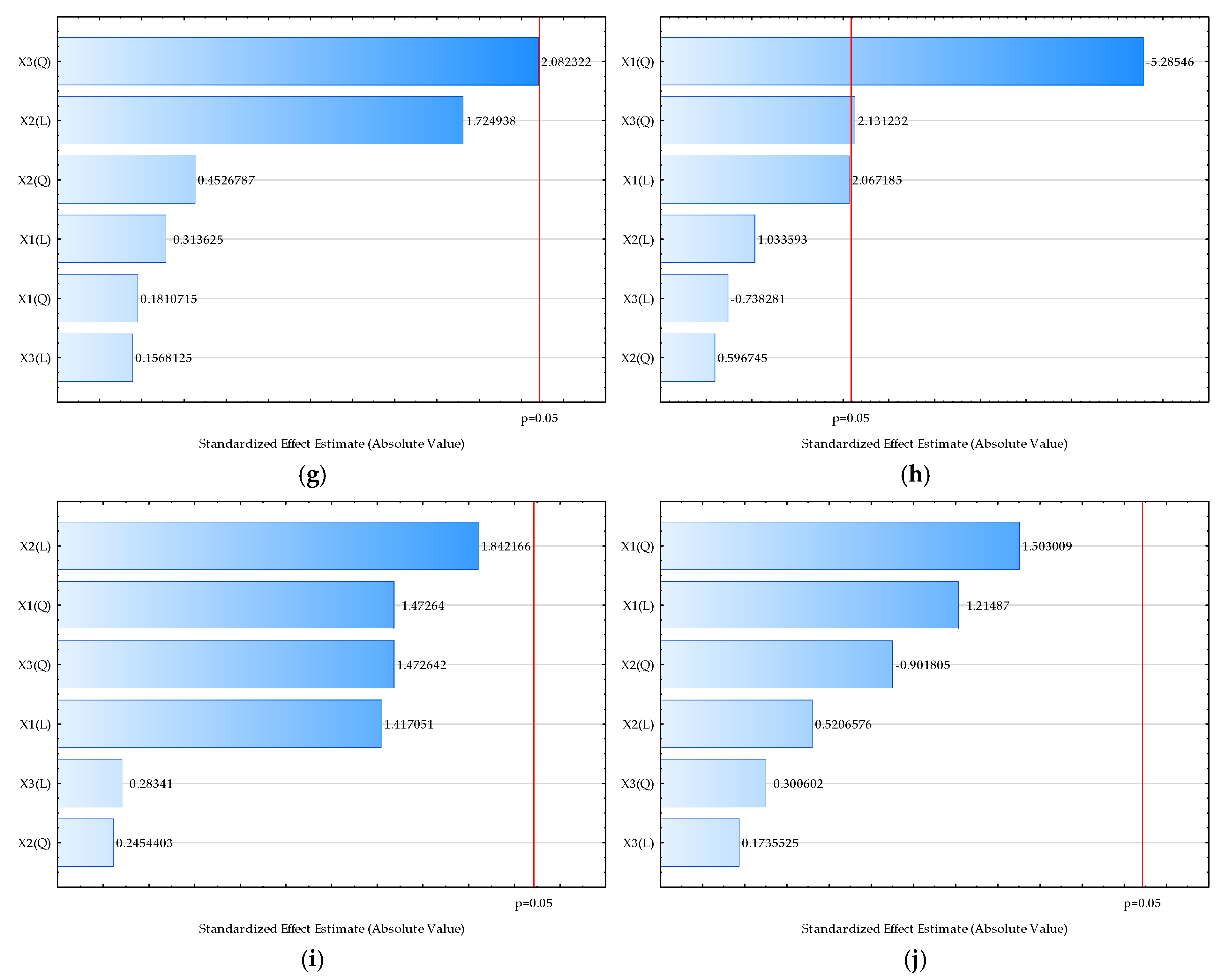
| Sample | L* | a* | b* | Chroma | Hue | Moisture (%) | Bulk Density (kgm−3) | HR | CI | Water Activity | Dispersibility (s) | Wettability (s) | d (0.1) (µm) | d (0.5) (µm) | d (0.9) (µm) | D [3,2] (µm) | Span |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50-1 | 37.94 ± 0.02 a | 16.48 ± 0.01 a | 19.20 ± 0.01 a | 25.31 ± 0.02 a | 49.36 ± 0.01 a | 11.32 ± 0.03 a | 666.49 ± 33.32 a | 1.14 ± 0.06 a | 14.45 ± 0.72 a | 0.51 ± 0.004 a | 8.88 ± 1.06 a | 16.63 ± 0.73 a | 94.22 ± 10.20 a | 304.86 ± 37.10 a | 663.03 ± 73.33 a | 186.30 ± 19.9 a | 1.87 ± 0.03 a |
| 50-2 | 31.23 ± 0.01 b | 15.54 ± 0.01 b | 14.69 ± 0.01 b | 21.38 ± 0.02 b | 43.3 ± 0.00 b | 11.13 ± 0.08 b | 720.55 ± 36.03 b | 1.18 ± 0.06 a | 18.52 ± 0.93 b | 0.49 ± 0.003 b | 7.73 ± 0.54 b | 24.75 ± 1.92 b | 169.48 ± 59.62 b | 470.11 ± 95.97 b | 889.76 ± 112.38 b | 313.48 ± 90.1 b | 1.53 ± 0.19 b |
| 50-3 | 56.55 ± 0.25 c | 13.64 ± 0.03 c | 18.76 ± 0.04 c | 23.2 ± 0.05 c | 53.97 ± 0.01 c | 6.95 ± 0.06 c | 627.96 ± 31.39 c | 1.24. ± 0.06 b | 24.09 ± 1.20 c | 0.35 ± 0.005 c | 11.32 ± 1.37 c | 81.74 ± 7.66 c | 26.43 ± 1.10 c | 219.56 ± 6.35 c | 572.38 ± 22.10 c | 64.80 ± 1.73 c | 2.49 ± 0.48 c |
| 50-4 | 50.42 ± 0.01 d | 14.98 ± 0.06 d | 20.11 ± 0.02 d | 25.12 ± 0.01 d | 53.31 ± 0.02 d | 8.34 ± 0.07 d | 672.54 ± 33.63 a | 1.18. ± 0.06 a | 17.95 ± 1.20 b | 0.38 ± 0.005 d | 11.12 ± 0.84 c | 27.21 ± 2.81 d | 92.97 ± 0.80 a | 303.26 ± 6.17 a | 665.44 ± 20.92 a | 184.88 ± 1.66 a | 1.89 ± 0.48 a |
| 50-5 | 34.89 ± 0.01 e | 16.1 ± 0.01 e | 16.9 ± 0.01 e | 23.41 ± 0.01 e | 46.34 ± 0.02 e | 7.75 ± 0.12 e | 641.27 ± 32.06 a | 1.21 ± 0.06 b | 20.18 ± 1.01 d | 0.36 ± 0.001 e | 8.25 ± 0.11 a | 27.86 ± 4.87 d | 174.07 ± 5.66 b | 407.06 ± 13.28 b | 795.96 ± 21.58 d | 319.31 ± 9.10 b | 1.53 ± 0.02 b |
| 50-6 | 42.99 ± 0.01 f | 15.03 ± 0.01 f | 18.92 ± 0.01 f | 24.16 ± 0.01 f | 51.54 ± 0.01 f | 11.33 ± 0.10 f | 640.36 ± 32.02 a | 1.88 ± 0.09 c | 18.82 ± 0.94 b | 0.49 ± 0.002 f | 7.66 ± 0.51 b | 14.27 ± 2.57 a | 126.11 ± 2.57 d | 336.39 ± 5.91 d | 741.47 ± 10.43 d | 238.49 ± 4.04 d | 1.83 ± 0.01 a |
| 50-7 | 43.99 ± 0.02 g | 14.58 ± 0.01 g | 18.59 ± 0.01 g | 23.63 ± 0.01 g | 51.9 ± 0.03 g | 9.07 ± 0.08 g | 692.79 ± 34.64 a | 1.21 ± 0.06 b | 20.99 ± 1.05 d | 0.41 ± 0.002 g | 5.44 ± 0.51 d | 11.22 ± 1.38 e | 98.85 ± 3.09 g | 376.94 ± 9.77 e | 858.17 ± 16.55 b | 206.65 ± 1.57 e | 2.01 ± 0.02 d |
| 50-8 | 49.95 ± 0.01 h | 15.32 ± 0.006 h | 20.06 ± 0.01 h | 25.24 ± 0.05 h | 52.64 ± 0.01 h | 9.60 ± 0.08 h | 701.98 ± 35.10 a | 1.12 ± 0.06 a | 18.60 ± 0.93 b | 0.44 ± 0.01 h | 16.44 ± 1.19 e | 63.17 ± 2.74 f | 66.50 ± 3.31 e | 450.58 ± 8.88 f | 906.71 ± 45.41 b | 126.63 ± 68.17 f | 1.86 ± 0.20 a |
| 50-9 | 39.89 ± 0.05 i | 14.85 ± 0.04 i | 18.25 ± 0.01 i | 23.53 ± 0.01 i | 50.85 ± 0.09 i | 10.21 ± 0.01 i | 650.00 ± 32.50 a | 1.21 ± 0.06 b | 20.99 ± 1.05 d | 0.47 ± 0.005 i | 8.40 ± 0.65 a | 21.43 ± 1.28 g | 139.99 ± 2.50 d | 339.98 ± 9.79 e | 707.34 ± 36.39 d | 259.66 ± 3.26 g | 1.67 ± 0.08 b |
| 60-1 | 48.65 ± 0.02 j | 15.04 ± 0.01 j | 19.31 ± 0.01 j | 24.47 ± 0.01 j | 52.08 ± 0.01 j | 6.51 ± 0.09 j | 724.09 ± 36.20 b | 1.19 ± 0.06 a | 18.75 ± 0.37 b | 0.32 ± 0.004 j | 27.19 ± 9.08 f | 261.08 ± 12.75 h | 37.90 ± 0.26 f | 238.06 ± 2.09 g | 612.82 ± 9.11 a | 81.86 ± 0.41 h | 2.41 ± 0.04 c |
| 60-2 | 50.93 ± 0.01 d | 14.42 ± 0.02 k | 19.36 ± 0.01 k | 24.14 ± 0.01 k | 53.32 ± 0.03 d | 5.35 ± 0.06 k | 700.63 ± 35.03 a,b | 1.23 ± 0.06 b | 23.39 ± 1.17 c | 0.26 ± 0.001 k | 24.87 ± 2.45 g | 239.87 ± 14.99 i | 29.35 ± 0.44 g | 202.23 ± 4.40 h | 569.40 ± 23.06 c | 69.09 ± 0.88 i | 2.67 ± 0.05 c |
| 60-3 | 44.25 ± 0.03 k | 14.22 ± 0.01 l | 17.71 ± 0.01 l | 22.71 ± 0.01 l | 51.24 ± 0.03 k | 9.64 ± 0.03 h | 706.67 ± 35.03 b | 1.17 ± 0.06 a | 17.56 ± 0.35 b | 0.47 ± 0.007 l | 11.32 ± 1.13 c | 53.30 ± 9.67 j | 71.84 ± 2.21 e | 327.41 ± 15.81 e | 840.94 ± 76.76 b | 147.28 ± 4.77 j | 2.33 ± 0.11 c |
| 60-4 | 50.83 ± 0.01 d | 14.34 ± 0.16 m | 19.41 ± 0.01 m | 24.19 ± 0.01 m | 53.36 ± 0.03 l | 6.11 ± 0.03 l | 689.84 ± 34.49 a,b | 1.24 ± 0.06 b | 24.41 ± 1.22 c | 0.31 ± 0.004 m | 24.44 ± 1.95 g | 150.16 ± 15.77 k | 31.33 ± 1.57 g | 204.47 ± 10.23 h | 514.53 ± 27.73 e | 69.56 ± 3.48 k | 2.36 ± 0.56 c |
| 60-5 | 46.65 ± 0.01 l | 14.9 ± 0.01 n | 19.24± 0.01 n | 24.33 ± 0.01 n | 52.24 ± 0.02 m | 6.84 ± 0.11 m | 695.53 ± 34.78 a,b | 1.24 ± 0.06 b | 24.03 ± 0.48 c | 0.33 ± 0.001 n | 15.59 ± 1.62 e | 184.28 ± 16.69 l | 41.40 ± 1.60 h | 230.64 ± 11.47 g | 678.49 ± 34.92 a | 92.93 ± 3.90 l | 2.76 ± 0.25 c |
| 60-6 | 42.67 ± 0.03 m | 14.55 ± 0.02 o | 17.85 ± 0.03 o | 23.14 ± 0.22 c | 50.82 ± 0.04 i | 9.24 ± 0.04 n | 719.45 ± 35.97 b | 1.21 ± 0.06 b | 20.63 ± 1.03 d | 0.45 ± 0.004 o | 11.60 ± 1.56 c | 43.22 ± 5.69 m | 183.51 ± 9.17 b | 792.73 ± 39.64 i | 1454.75 ± 72.74 f | 362.58 ± 18.13 m | 1.60 ± 0.09 b |
| 60-7 | 35.68 ± 0.01 n | 17.83 ± 0.02 p | 18.31 ± 0.03 p | 25.56 ± 0.03 o | 45.76 ± 0.02 n | 9.33 ± 0.07 o | 721.65 ± 36.08 b | 1.17 ± 0.06 a | 16.92 ± 0.85 b | 0.45 ± 0.002 p | 10.70 ± 0.88 c | 30.17 ± 5.64 d | 144.04 ± 13.83 b | 409.00 ± 38.12 b | 927.72 ± 47.39 b | 278.81 ± 25.40 g | 1.92 ± 0.07 a |
| 60-8 | 51.09 ± 0.04 d | 13.87 ± 0.01 r | 18.63 ± 0.01 r | 23.23 ± 0.01 c | 53.34 ± 0.02 o | 5.43 ± 0.05 p | 684.73 ± 34.24 a,b | 1.26 ± 0.06 b | 25.60 ± 1.28 c | 0.26 ± 0.001 q | 32.11 ± 4.96 h | 247.71 ± 19.24 i | 16.01 ± 0.82 i | 185.62 ± 7.73 j | 514.80 ± 35.49 e | 47.48 ± 1.54 n | 2.69 ± 0.09 c |
| 60-9 | 54.74 ± 0.13 o | 14.17 ± 0.02 s | 19.01 ± 0.025 s | 23.71 ± 0.03 p | 53.28 ± 0.02 p | 4.45 ± 0.01 q | 675.04 ± 33.75 a,d | 1.26 ± 0.06 b | 26.56 ± 1.33 c | 0.23 ± 0.003 r | 22.95 ± 3.52 g | 201.91 ± 21.76 n | 19.27 ± 0.33 j | 201.76 ± 20.93 h | 560.63 ± 52.89 c | 52.89 ± 0.49 o | 2.68 ± 0.02 c |
| 70-1 | 44.68 ± 0.01 p | 14.56 ± 0.01 n | 17.07 ± 0.01 t | 22.44 ± 0.02 q | 49.55 ± 0.01 q | 7.14 ± 0.05 r | 736.37 ± 36.82 e | 1.23 ± 0.06 b | 22.69 ± 1.13 c | 0.37 ± 0.002 s | 16.12 ± 6.80 e | 84.78 ± 12.32 c | 91.53 ± 4.58 a | 535.43 ± 26.77 b | 1379.74 ± 431.67 f | 172.62 ± 14.63 p | 2.41 ± 0.10 c |
| 70-2 | 41.51 ± 0.01 r | 15.58 ± 0.01 g | 18.39 ± 0.01 u | 24.10 ± 0.01 r | 49.67 ± 0.08 r | 7.01 ± 0.04 s | 725.14 ± 36.26 a,c | 1.12 ± 0.06 b | 12.31 ± 0.61 e | 0.36 ± 0.001 t | 14.97 ± 5.16 e | 115.40 ± 24.29 o | 65.01 ± 5.57 e | 290.79 ± 35.36 a | 818.80 ± 149.08 b | 141.18 ± 12.00 j | 2.59 ± 0.17 c |
| 70-3 | 47.39 ± 0.02 s | 14.48 ± 0.13 k | 17.21 ± 0.16 v | 22.49 ± 0.21 s | 49.92 ± 0.01 s | 7.00 ± 0.07 c | 716.63 ± 35.83 a,c | 1.21 ± 0.06 b | 21.01 ± 1.05 c | 0.38 ± 0.001 u | 33.64 ± 5.29 h | 69.78 ± 8.99 f | 71.53 ± 10.07 e | 369.89 ± 81.69 e | 958.29 ± 142.13 b | 132.16 ± 6.62 k | 2.40 ± 0.11 c |
| 70-4 | 48.42 ± 0.01 t | 13.97 ± 0.01 t | 17.29 ± 0.01 w | 22.24 ± 0.01 t | 51.06 ± 0.02 t | 6.25 ± 0.05 t | 769.75 ± 38.49 a,b,c | 1.21 ± 0.06 b | 20 ± 1.00 c | 0.35 ± 0.001 c | 17.54 ± 3.08 e | 11.77 ± 16.06 e | 49.38 ± 3.07 k | 274.81 ± 17.38 k | 750.28 ± 95.31 d | 95.86 ± 4.72 l | 2.55 ± 0.17 c |
| 70-5 | 38.3 ± 0.02 u | 16.15 ± 0.01 u | 18.01± 0.01 x | 26.18 ± 1.72 a,d,h | 49.67 ± 0.00 u | 7.69 ± 0.22 e | 742.74 ± 37.14 a,b | 1.35 ± 0.07 d | 13.49 ± 0.67 a | 0.39 ± 0.001 v | 33.71 ± 5.07 h | 55.73 ± 35.88 j | 76.19 ± 19.90 e | 346.80 ± 91.05 e | 1021.62 ± 282.02 b | 161.71 ± 9.13 p | 2.73 ± 0.08 c |
| 70-6 | 42.38 ± 0.01 v | 14.41 ± 0.01 k | 16.89 ± 0.03 y | 22.21 ± 0.01 u | 49.56 ± 0.02 v | 7.42 ± 0.01 u | 755.54 ± 37.77 a,c | 1.14 ± 0.06 a | 14.4 ± 0.72 a | 0.39 ± 0.001 w | 15.91 ± 5.35 e | 34.85 ± 6.48 d | 83.33 ± 1.63 l | 363.59 ± 10.70 e | 935.677 ± 38.06 b | 169.65 ± 2.93 p | 2.35 ± 0.11 c |
| 70-7 | 41.34 ± 0.03 x | 14.41 ± 0.01 k, | 17.26 ± 0.01 z | 22.48 ± 0.05 v | 50.13 ± 0.02 w | 6.82 ± 0.01 m | 729.29 ± 36.46 a,c | 1.33 ± 0.07 d | 13.28 ± 0.66 a | 0.35 ± 0.002 c | 13.14 ± 3.01 e | 23.52 ± 2.09 b | 88.18 ± 5.06 l | 339.06 ± 20.80 e | 801.867 ± 35.06 b | 181.35 ± 10.16 a | 2.10 ± 0.07 e |
| 70-8 | 39.81 ± 0.01 y | 14.64 ± 0.01 v | 16.71 ± 0.01 q | 22.22 ± 0.01 w | 48.78 ± 0.02 x | 9.75 ± 0.22 h | 727.53 ± 36.37 a,c | 1.15 ± 0.06 a | 14.84 ± 0.74 a | 0.49 ± 0.001 e | 14.69 ± 1.57 e | 42.81 ± 3.58 m | 126.38 ± 6.32 d | 536.66 ± 26.83 l | 1305.77 ± 97.36 f | 238.68 ± 13.92 d | 2.20 ± 0.25 e |
| 70-9 | 41.95 ± 0.01 z | 14.93 ± 0.01 m | 18.25 ± 0.57 g | 23.23 ± 0.17 c | 50.21 ± 0.06 y | 7.23 ± 0.03 v | 751.01 ± 37.55 a,b,c | 1.14 ± 0.06 a | 14.52 ± 0.73 a | 0.38 ± 0.005 x | 27.38 ± 4.55 f | 116.87 ± 20.42 o | 922.78 ± 61.51 m | 1279.54 ± 24.30 m | 1653.99 ± 34.19 g | 1091.32 ± 98.58 q | 0.57 ± 0.07 f |
| Sample | TDS (mg L−1) | Conductivity (µS cm−1) | pH | Brix (°) | L* | a* | b* | Chroma | Hue | TPC (mg GAE gdm−1) | DPPH (mmol TE gdm−1) | FRAP (mmol FeSO4 gdm−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50-1 | 64.30 ± 0.56 a | 128.67 ± 1.07 a | 6.30 ± 0.01 a | 18.42 ± 0.14 a | 33.35 ± 0.38 a | 1.28 ± 0.06 a | 4.59 ± 0.04 a | 4.76 ± 0.50 a | 74.44 ± 0.68 a | 3.383 ± 0.563 a | 0.0238 ± 0.0005 a | 0.0333 ± 0.0001 a |
| 50-2 | 52.63 ± 0.99 b | 105.23 ± 2.03 b | 6.28 ± 0.01 b | 19.08 ± 0.52 b | 36.45 ± 0.03 b | 1.22 ± 0.05 a | 5.28 ± 0.01 b | 5.42 ± 0.01 b | 53.64 ± 1.71 b | 4.061 ± 0.323 a | 0.0115 ± 0.0015 b | 0.0281 ± 0.0004 b |
| 50-3 | 57.97 ± 2.06 c | 115.73 ± 3.93 c | 6.09 ± 0.02 c | 18.92 ± 0.38 c | 34.43 ± 0.02 c | 0.96 ± 0.06 b | 4.77 ± 0.01 c | 4.87 ± 0.01 a | 78.59 ± 0.08 c | 3.452 ± 0.215 a | 0.0153 ± 0.0007 c | 0.0282 ± 0.0001 b |
| 50-4 | 63.23 ± 1.35 a | 126.43 ± 2.65 a | 6.23 ± 0.01 d | 18.75 ± 0.25 c | 35.22 ± 0.01 d | 1.08 ± 0.06 c | 4.78 ± 0.01 c | 4.91 ± 0.01 a | 77.17 ± 0.06 c | 3.052 ± 0.313 b | 0.0180 ± 0.0017 d | 0.0242 ± 0.0001 c |
| 50-5 | 56.30 ± 1.04 c | 112.60 ± 2.08 c | 6.34 ± 0.06 e | 19.33 ± 0.14 b | 34.88 ± 0.08 e | 1.12 ± 0.02 c | 5.16 ± 0.06 d | 5.25 ± 0.12 c | 77.79 ± 0.11 c | 3.759 ± 0.528 a | 0.0192 ± 0.0004 d | 0.0300 ± 0.0001 d |
| 50-6 | 56.87 ± 0.32 c | 113.70 ± 2.07 c | 6.11 ± 0.01 c | 19.00 ± 0.25 b | 37.19 ± 0.02 f | 0.67 ± 0.01 d | 4.55 ± 0.01 a | 4.60 ± 0.01 d | 57.29 ± 0.14 d | 2.617 ± 0.380 c | 0.0142 ± 0.0009 e | 0.0262 ± 0.0002 e |
| 50-7 | 39.30 ± 0.92 d | 75.87 ± 1.85 d | 6.09 ± 0.02 c | 17.92 ± 0.14 d | 35.61 ± 0.01 g | 0.86 ± 0.02 d | 4.29 ± 0.01 e | 4.38 ± 0.01 e | 78.53 ± 0.32 c | 2.736 ± 0.302 c | 0.0147 ± 0.0022 e | 0.0228 ± 0.0001 f |
| 50-8 | 55.57 ± 1.45 c | 111.10 ± 2.95 c | 6.18 ± 0.01 f | 18.75 ± 0.43 c | 36.03 ± 0.13 h | 1.36 ± 0.01 f | 5.70 ± 0.02 f | 5.87 ± 0.02 f | 76.52 ± 0.07 d | 3.914 ± 0.289 a | 0.0169 ± 0.0004 f | 0.0322 ± 0.0004 a |
| 50-9 | 45.03 ± 1.19 e | 90.00 ± 2.33 e | 6.36 ± 0.01 e | 19.50 ± 0.25 b | 35.18 ± 0.10 d | 0.87 ± 0.03 d | 4.49 ± 0.12 a | 5.01 ± 0.13 g | 79.97 ± 0.20 c | 3.626 ± 0.237 a | 0.0146 ± 0.0007 e | 0.0248 ± 0.0007 g |
| 60-1 | 69.90 ± 0.30 f | 139.83 ± 0.60 f | 6.10 ± 0.02 c | 20.08 ± 0.72 e | 40.45 ± 0.01 i | 0.79 ± 0.01 e | 4.65 ± 0.01 g | 4.72 ± 0.01 a | 80.25 ± 0.09 e | 3.670 ± 0.283 a | 0.0183 ± 0.0002 g | 0.0391 ± 0.0002 h |
| 60-2 | 51.43 ± 0.31 g | 102.9 ± 0.70 b | 6.19 ± 0.02 f | 19.67 ± 0.14 b | 40.91 ± 0.06 j | 0.57 ± 0.01 f | 4.48 ± 0.04 a | 4.51 ± 0.04 h | 82.79 ± 0.07 f | 2.474 ± 0.384 c | 0.0224 ± 0.0001 h | 0.0332 ± 0.0001 a |
| 60-3 | 56.43 ± 0.21 c | 112.83 ± 0.40 c | 6.16 ± 0.01 g | 19.92 ± 0.29 e | 42.45 ± 0.01 k | 0.40 ± 0.01 g | 4.25 ± 0.01 h | 4.27 ± 0.01 i | 64.62 ± 0.08 g | 2.792 ± 0.256 c | 0.0273 ± 0.0001 i | 0.0241 ± 0.0001 c |
| 60-4 | 66.77 ± 0.68 h | 133.53 ± 1.36 g | 6.06 ± 0.02 h | 19.83 ± 0.14 e | 41.66 ± 0.01 l | 0.95 ± 0.05 b | 5.04 ± 0.01 i | 5.13 ± 0.01 j | 79.34 ± 0.05 e | 3.055 ± 0.054 b | 0.0226 ± 0.0011 h | 0.0310 ± 0.0002 i |
| 60-5 | 44.20 ± 0.95 e | 88.40 ± 1.91 h | 6.27 ± 0.02 b | 20.33 ± 0.28 e | 42.58 ± 0.02 k | 0.16 ± 0.01 h | 4.29 ± 0.12 e | 4.29 ± 0.12 i | 87.86 ± 0.79 h | 3.151 ± 0.326 b | 0.0234 ± 0.0007 a | 0.0295 ± 0.0001 j |
| 60-6 | 60.17 ± 0.23 i | 120.30 ± 0.43 i | 6.03 ± 0.02 i | 20.08 ± 0.14 e | 42.48 ± 0.01 k | 0.73 ± 0.01 i | 4.76 ± 0.01 c | 4.81 ± 0.01 a | 81.25 ± 0.11 e | 2.685 ± 0.162 c | 0.0267 ± 0.0015 j | 0.0276 ± 0.0001 k |
| 60-7 | 53.27 ± 0.15 b | 106.53 ± 0.38 b | 6.13 ± 0.01 c | 19.33 ± 0.14 b | 42.24 ± 0.01 k | 0.83 ± 0.01 j | 5.04 ± 0.01 i | 5.10 ± 0.01 j | 80.59 ± 0.13 e | 2.795 ± 0.188 c | 0.0249 ± 0.0005 k | 0.0285 ± 0.0001 b |
| 60-8 | 73.20 ± 0.43 j | 146.43 ± 0.97 j | 6.09 ± 0.01 c | 19.58 ± 0.14 e | 41.39 ± 0.01 l | 0.67 ± 0.01 d | 4.53 ± 0.01 a | 4.58 ± 0.02 d | 81.63 ± 0.05 e | 3.198 ± 0.290 b | 0.0219 ± 0.0004 l | 0.0311 ± 0.0001 i |
| 60-9 | 58.33 ± 3.76 c | 116.73 ± 7.54 c | 6.07 ± 0.01 h | 19.33 ± 0.14 b | 43.48 ± 0.01 k | 0.84 ± 0.01 j | 4.72 ± 0.01 c | 4.79 ± 0.01 a | 79.92 ± 0.09 c | 3.284 ± 0.176 b | 0.0117 ± 0.0002 b | 0.0385 ± 0.0010 j |
| 70-1 | 76.33 ± 0.01 k | 152.73 ± 0.30 k | 6.63 ± 0.03 j | 19.25 ± 0.29 b | 33.09 ± 0.05 a | 1.60 ± 0.01 k | 5.15 ± 0.04 d | 5.39 ± 0.03 b | 72.73 ± 0.17 i | 3.278 ± 0.175 b | 0.0312 ± 0.0022 m | 0.0398 ± 0.0018 j |
| 70-2 | 51.03 ± 0.95 g | 100.93 ± 1.01 l | 6.73 ± 0.06 k | 20.17 ± 0.14 e | 45.24 ± 0.03 m | -0.01 ± 0.02 l | 3.67 ± 0.01 j | 3.67 ± 0.01 k | 90.22 ± 0.22 j | 2.438 ± 0.094 c | 0.0242 ± 0.0001 k | 0.0312 ± 0.0001 i |
| 70-3 | 66.80 ± 0.15 h | 133.70 ± 0.36 g | 6.51 ± 0.01 l | 19.00 ± 0.43 c | 42.07 ± 0.05 k | 0.03 ± 0.35 m | 3.16 ± 0.17 k | 3.17 ± 0.17 l | 84.53 ± 0.65 k | 2.903 ± 0.054 c | 0.0224 ± 0.0002 l | 0.0339 ± 0.0010 a |
| 70-4 | 74.93 ± 0.06 k | 149.23 ± 0.12 m | 6.06 ± 0.02 h | 19.75 ± 0.43 e | 43.72 ± 0.02 k | 0.04 ± 0.01 m | 3.43 ± 0.01 l | 3.47 ± 0.01 m | 83.27 ± 1.81 k | 3.041 ± 0.093 b | 0.0270 ± 0.0009 j | 0.0375 ± 0.0023 l |
| 70-5 | 73.10 ± 0.47 j | 146.23 ± 7.05 m | 6.70 ± 0.01 k | 19.50 ± 0.50 b | 44.04 ± 0.02 n | 0.65 ± 0.01 d | 3.51 ± 0.03 m | 3.57 ± 0.03 n | 79.54 ± 0.15 c | 4.206 ± 0.135 d | 0.0321 ± 0.0009 m | 0.0502 ± 0.0013 m |
| 70-6 | 64.87 ± 0.21 a | 129.77 ± 0.50 a | 6.56 ± 0.02 m | 19.50 ± 0.50 b | 30.71 ± 0.09 o | 1.44 ± 0.16 f | 4.95 ± 0.36 n | 5.16 ± 0.36 j | 73.71 ± 2.07 i | 2.273 ± 0.107 c | 0.0220 ± 0.0002 l | 0.0264 ± 0.0002 e |
| 70-7 | 64.10 ± 0.17 a | 128.13 ± 0.32 a | 6.60 ± 0.01 j | 19.75 ± 0.43 e | 42.87 ± 0.05 k | 0.43 ± 0.01 n | 3.45 ± 0.01 l | 3.47 ± 0.01 m | 82.85 ± 0.11 k | 3.106 ± 0.187 b | 0.0235 ± 0.0009 a | 0.0282 ± 0.0007 b |
| 70-8 | 64.97 ± 0.31 a | 129.90 ± 0.53 a | 6.78 ± 0.02 n | 20.33 ± 0.29 e | 42.17 ± 0.02 k | 0.34 ± 0.01 o | 3.26 ± 0.03 k | 3.28 ± 0.03 n | 83.97 ± 0.14 k | 2.757 ± 0.249 c | 0.0268 ± 0.0010 j | 0.0433 ± 0.0026 n |
| 70-9 | 56.13 ± 1.99 c | 112.27 ± 3.97 c | 6.71 ± 0.03 k | 20.33 ± 0.38 e | 37.75 ± 0.28 f | 0.91 ± 0.01 b | 4.77 ± 0.02 c | 4.86 ± 0.02 a | 79.18 ± 0.11 c | 2.673 ± 0.525 c | 0.0277 ± 0.0018 i | 0.0364 ± 0.0005 l |
| Optimal Process Parameters for Physical Properties | ||
|---|---|---|
| T (°C) | Oat/honey ratio (%) | Cocoa content (g/100 g) |
| 65 | 60 | 6.875 |
| Model-predicted values of physical properties at optimal conditions | ||
| Property | Value | Confidence interval (95%) |
| L* | 42.80 | 37.93–47.66 |
| a* | 15.34 | 14.48–16.11 |
| b* | 18.15 | 17.08–19.21 |
| Chroma | 24.03 | 22.93–25.13 |
| Hue | 49.86 | 47.69–52.03 |
| Moisture (%) | 6.22 | 4.75–7.69 |
| Bulk density (kg m−3) | 727.68 | 704.92–750.44 |
| HR | 1.19 | 1.16–1.23 |
| IC | 19.64 | 16.37–22.90 |
| aw | 0.31 | 0.25–0.37 |
| Dispersibility (s) | 21.86 | 15.66–28.06 |
| Wettability (s) | 165.75 | 118.24–213.27 |
| d (0.1) (µm) | 110.24 | 45.08–265.56 |
| d (0.5) (µm) | 327.44 | 122.42–532.47 |
| d (0.9) (µm) | 779.68 | 537.05–1022.31 |
| D [3,2] (µm) | 183.66 | 13.96–353.37 |
| span | 2.42 | 1.99–2.85 |
| Optimal process parameters for chemical properties | ||
| T (°C) | Oat/honey ratio (%) | Cocoa content (g/100 g) |
| 70 | 50 | 7.5 |
| Model-predicted values of chemical properties at optimal conditions | ||
| Property | Value | Confidence interval (95%) |
| TDS (mg L−1) | 71.55 | 64.02–79.08 |
| Conductivity (µS cm−1) | 143.09 | 127.98–158.21 |
| pH | 6.68 | 6.61–6.76 |
| Brix (°) | 19.82 | 19.34–20.30 |
| L* | 37.58 | 34.56–40.60 |
| a* | 0.99 | 0.62–1.36 |
| b* | 4.28 | 3.71–4.84 |
| Chroma | 4.41 | 3.79–5.02 |
| Hue | 78.74 | 71.51–85.97 |
| TPC (mg GAE gdm−1) | 3.25 | 2.80–3.70 |
| DPPH (mmol TE gdm−1) | 0.03 | 0.02–0.03 |
| FRAP (mmol FeSO4 gdm−1) | 0.04 | 0.04–0.05 |
| Optimal process parameters for sensory properties | ||
| T (°C) | Oat/honey ratio (%) | Cocoa content (g/100 g) |
| 70 | 60 | 7.5 |
| Model-predicted values of sensory properties at optimal conditions | ||
| Property | Value | Confidence interval (95%) |
| Powder–appearance | 4.33 | 3.97–4.70 |
| Powder–color | 4.61 | 4.37–4.84 |
| Powder–odour | 4.62 | 4.31–4.93 |
| Beverage–appearance | 3.81 | 3.56–4.05 |
| Beverage–color | 3.99 | 3.73–4.25 |
| Beverage–odour | 3.71 | 3.46–3.96 |
| Beverage–sweetness | 3.52 | 3.20–3.84 |
| Beverage–bitterness | 3.55 | 3.21–3.89 |
| Beverage–taste | 3.80 | 3.45–4.15 |
| Beverage–texture | 3.62 | 3.33–3.91 |
| Sample | X1 Temperature (°C) | X2 Honey/Oat Flour Ratio (%) | X3 Cocoa Powder Contents (g) |
|---|---|---|---|
| 50-1 | 50 (−1) | 50:50 (0) | 7.50 (+1) |
| 50-2 | 50 (−1) | 60:40 (+1) | 6.25 (0) |
| 50-3 | 50 (−1) | 40:60 (−1) | 5.00 (−1) |
| 50-4 | 50 (−1) | 40:60 (−1) | 6.25 (0) |
| 50-5 | 50 (−1) | 60:40 (+1) | 7.50 (+1) |
| 50-6 | 50 (−1) | 50:50 (0) | 5.00 (−1) |
| 50-7 | 50 (−1) | 60:40 (+1) | 5.00 (−1) |
| 50-8 | 50 (−1) | 40:60 (−1) | 7.50 (+1) |
| 50-9 | 50 (−1) | 50:50 (0) | 6.25 (0) |
| 60-1 | 60 (0) | 50:50 (0) | 7.50 (+1) |
| 60-2 | 60 (0) | 60:40 (+1) | 6.25 (0) |
| 60-3 | 60 (0) | 40:60 (−1) | 5.00 (−1) |
| 60-4 | 60 (0) | 40:60 (−1) | 6.25 (0) |
| 60-5 | 60 (0) | 60:40 (+1) | 7.50 (+1) |
| 60-6 | 60 (0) | 50:50 (0) | 5.00 (−1) |
| 60-7 | 60 (0) | 60:40 (+1) | 5.00 (−1) |
| 60-8 | 60 (0) | 40:60 (−1) | 7.50 (+1) |
| 60-9 | 60 (0) | 50:50 (0) | 6.25 (0) |
| 70-1 | 70 (+1) | 50:50 (0) | 7.50 (+1) |
| 70-2 | 70 (+1) | 60:40 (+1) | 6.25 (0) |
| 70-3 | 70 (+1) | 40:60 (−1) | 5.00 (−1) |
| 70-4 | 70 (+1) | 40:60 (−1) | 6.25 (0) |
| 70-5 | 70 (+1) | 60:40 (+1) | 7.50 (+1) |
| 70-6 | 70 (+1) | 50:50 (0) | 5.00 (−1) |
| 70-7 | 70 (+1) | 60:40 (+1) | 5.00 (−1) |
| 70-8 | 70 (+1) | 40:60 (−1) | 7.50 (+1) |
| 70-9 | 70 (+1) | 50:50 (0) | 6.25 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tušek, K.; Benković, M. Development of Novel Honey- and Oat-Based Cocoa Beverages—A Comprehensive Analysis of the Impact of Drying Temperature and Mixture Composition on Physical, Chemical and Sensory Properties. Molecules 2024, 29, 4665. https://doi.org/10.3390/molecules29194665
Tušek K, Benković M. Development of Novel Honey- and Oat-Based Cocoa Beverages—A Comprehensive Analysis of the Impact of Drying Temperature and Mixture Composition on Physical, Chemical and Sensory Properties. Molecules. 2024; 29(19):4665. https://doi.org/10.3390/molecules29194665
Chicago/Turabian StyleTušek, Kristina, and Maja Benković. 2024. "Development of Novel Honey- and Oat-Based Cocoa Beverages—A Comprehensive Analysis of the Impact of Drying Temperature and Mixture Composition on Physical, Chemical and Sensory Properties" Molecules 29, no. 19: 4665. https://doi.org/10.3390/molecules29194665
APA StyleTušek, K., & Benković, M. (2024). Development of Novel Honey- and Oat-Based Cocoa Beverages—A Comprehensive Analysis of the Impact of Drying Temperature and Mixture Composition on Physical, Chemical and Sensory Properties. Molecules, 29(19), 4665. https://doi.org/10.3390/molecules29194665






