Rational Design of ZnO/Sc2CF2 Heterostructure with Tunable Electronic Structure for Water Splitting: A First-Principles Study
Abstract
1. Introduction
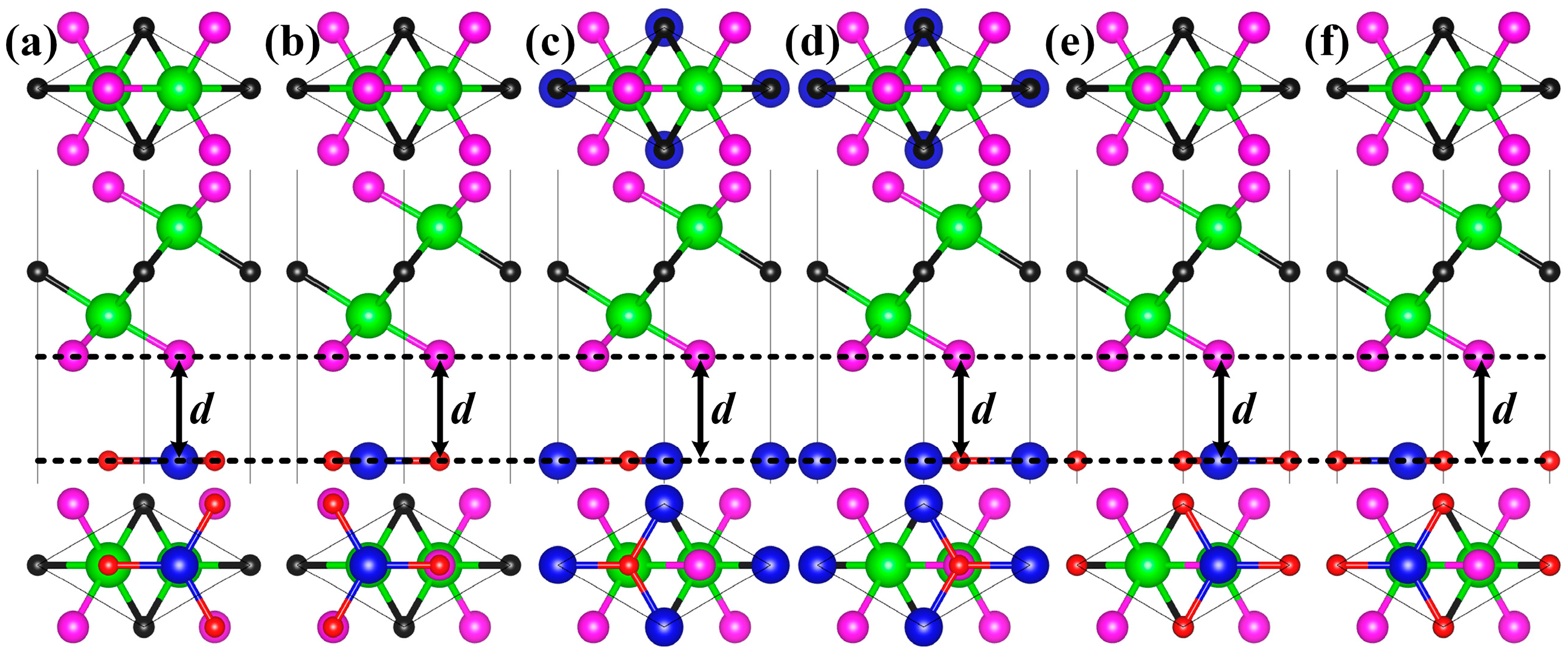
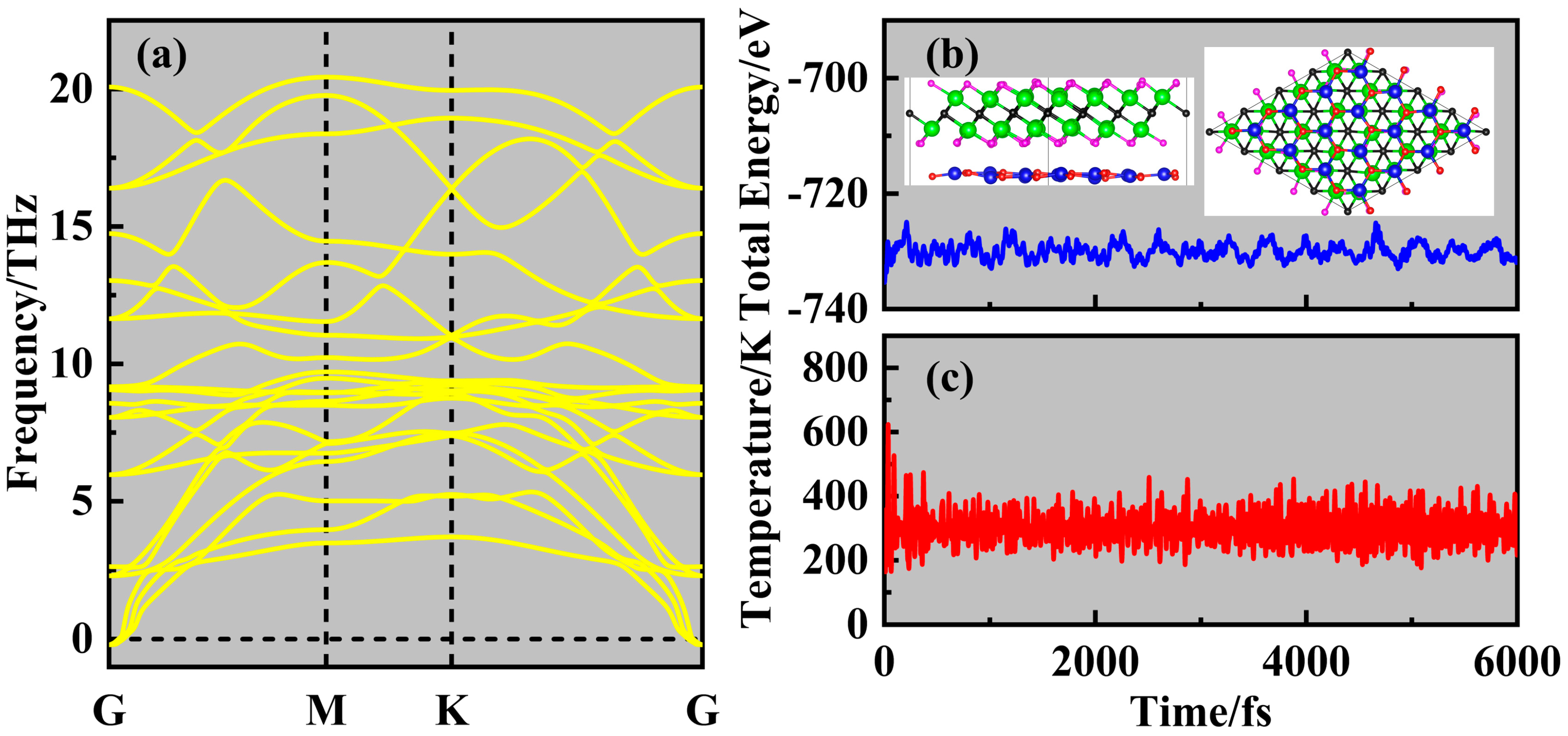
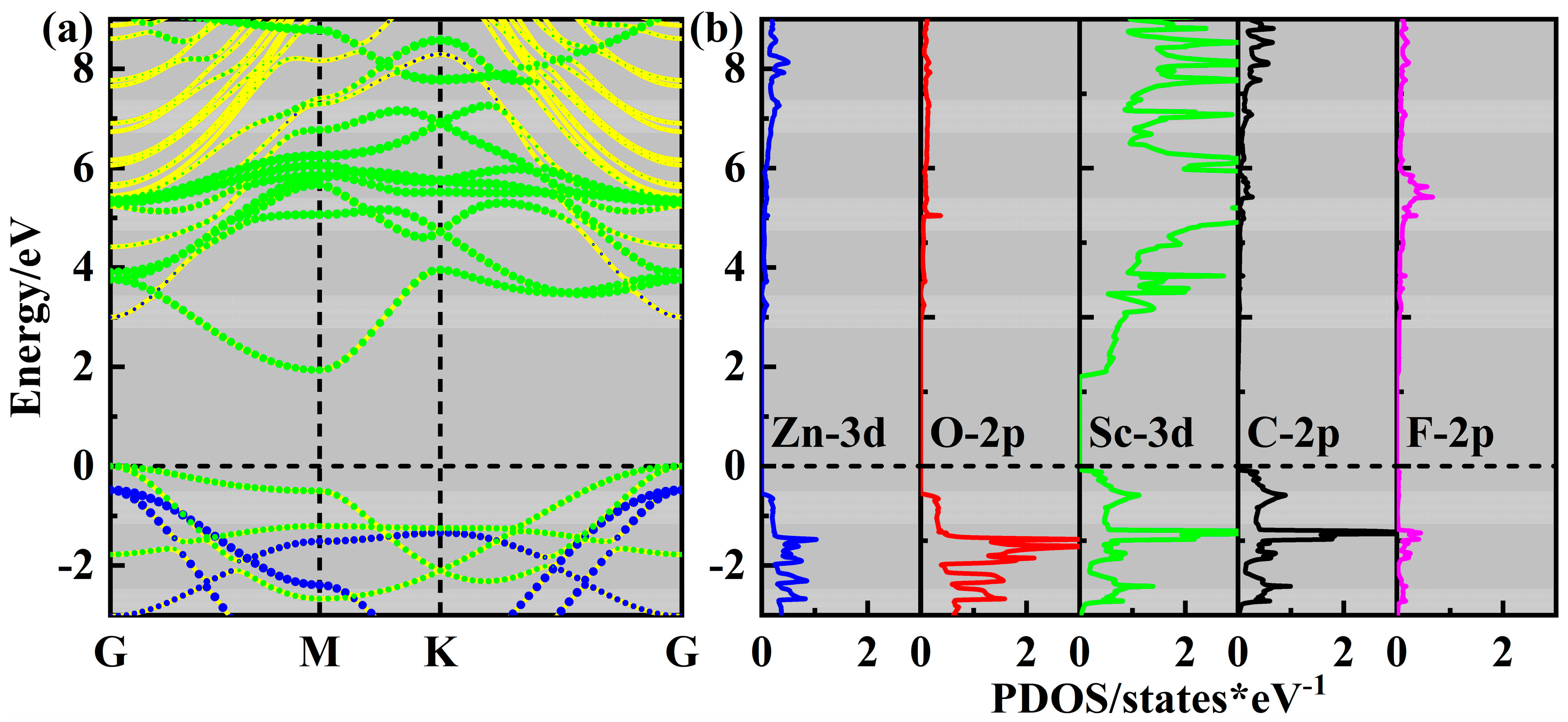
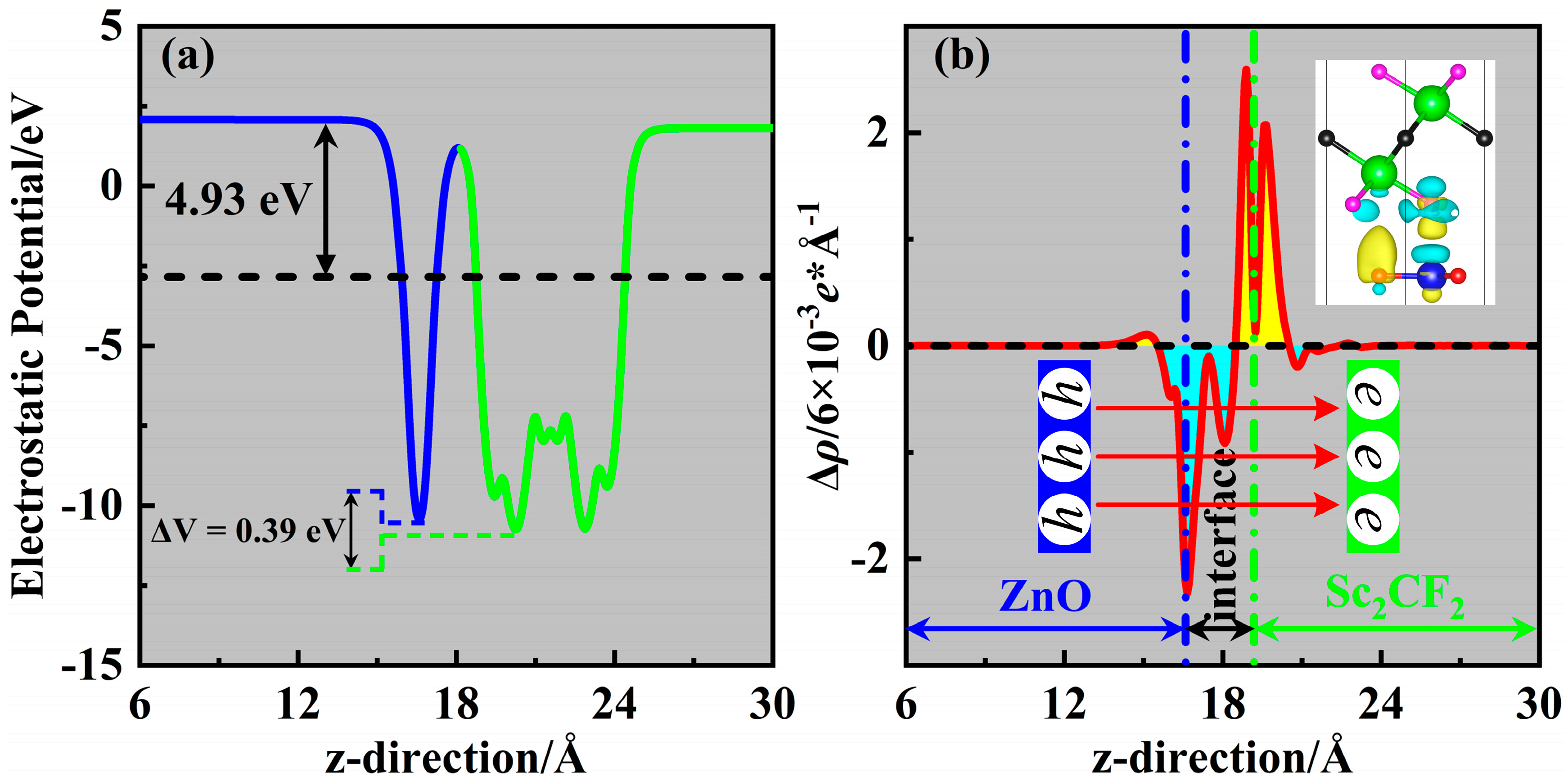
2. Results and Discussion
3. Computational Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Osterloh, F.E.; Wang, X.; Mallouk, T.E.; Maeda, K. Photocatalytic Water Splitting. Nat. Rev. Methods Primers 2023, 3, 42. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Ye, H.; Liang, F.; Wang, Z.; Li, K.; Weng, Y.; Lin, Z.; Fu, W.; Che, C.; Chen, Y. Interstitial P-doped CdS with Long-lived Photogenerated Electrons for Photocatalytic Water Splitting without Sacrificial Agents. Adv. Mater. 2018, 30, 1705941. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Nguyen, N.T.; Hwang, I.; Schmuki, P. Strongly Enhanced Water Splitting Performance of Ta3N5 Nanotube Photoanodes with Subnitrides. Adv. Mater. 2016, 28, 2432–2438. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the Performance of Cu2O Photocathodes for Unassisted Solar Water Splitting Devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Castro Neto, A.H. Phosphorene: From Theory to Applications. Nat. Rev. Mater. 2016, 1, 16061. [Google Scholar] [CrossRef]
- Lu, A.-Y.; Zhu, H.; Xiao, J.; Chuu, C.-P.; Han, Y.; Chiu, M.-H.; Cheng, C.-C.; Yang, C.-W.; Wei, K.-H.; Yang, Y.; et al. Janus Monolayers of Transition Metal Dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef]
- Barraza-Lopez, S.; Fregoso, B.M.; Villanova, J.W.; Parkin, S.S.P.; Chang, K. Colloquium: Physical Properties of Group-IV Monochalcogenide Monolayers. Rev. Mod. Phys. 2021, 93, 011001. [Google Scholar] [CrossRef]
- Tusche, C.; Meyerheim, H.L.; Kirschner, J. Observation of Depolarized ZnO(0001) Monolayers: Formation of Unreconstructed Planar Sheets. Phys. Rev. Lett. 2007, 99, 026102. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Zhang, K.; Zhao, W.; Tian, X.; Huang, Y. Enhanced Photocatalytic Performance of ZnO Monolayer for Water Splitting via Biaxial Strain and External Electric Field. Appl. Surf. Sci. 2019, 481, 1064–1071. [Google Scholar] [CrossRef]
- Peng, J.; Chen, X.; Ong, W.-J.; Zhao, X.; Li, N. Surface and Heterointerface Engineering of 2D MXenes and Their Nanocomposites: Insights into Electro- and Photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef]
- Balcı, E.; Akkuş, Ü.Ö.; Berber, S. Band Gap Modification in Doped MXene: Sc2CF2. J. Mater. Chem. C 2017, 5, 5956–5961. [Google Scholar] [CrossRef]
- Zha, X.-H.; Zhou, J.; Zhou, Y.; Huang, Q.; He, J.; Francisco, J.S.; Luo, K.; Du, S. Promising Electron Mobility and High Thermal Conductivity in Sc2CT2 (T = F., OH) MXenes. Nanoscale 2016, 8, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Schwingenschlögl, U. Thermoelectric Performance of Functionalized Sc2C MXenes. Phys. Rev. B 2016, 94, 035405. [Google Scholar] [CrossRef]
- Xiong, K.; Wang, P.; Yang, G.; Liu, Z.; Zhang, H.; Jin, S.; Xu, X. Functional Group Effects on the Photoelectronic Properties of MXene (Sc2CT2, T = O, F, OH) and Their Possible Photocatalytic Activities. Sci. Rep. 2017, 7, 15095. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zeng, X.; Zhang, X. 2D/2D Heterostructured Photocatalyst: Rational Design for Energy and Environmental Applications. Sci. China Mater. 2020, 63, 2119–2152. [Google Scholar] [CrossRef]
- Su, Q.; Li, Y.; Hu, R.; Song, F.; Liu, S.; Guo, C.; Zhu, S.; Liu, W.; Pan, J. Heterojunction Photocatalysts Based on 2D Materials: The Role of Configuration. Adv. Sustain. Syst. 2020, 4, 2000130. [Google Scholar] [CrossRef]
- Fang, H.; Battaglia, C.; Carraro, C.; Nemsak, S.; Ozdol, B.; Kang, J.S.; Bechtel, H.A.; Desai, S.B.; Kronast, F.; Unal, A.A.; et al. Strong Interlayer Coupling in van Der Waals Heterostructures Built from Single-Layer Chalcogenides. Proc. Natl. Acad. Sci. USA 2014, 111, 6198–6202. [Google Scholar] [CrossRef] [PubMed]
- Dastgeer, G.; Shahzad, Z.M.; Chae, H.; Kim, Y.H.; Ko, B.M.; Eom, J. Bipolar Junction Transistor Exhibiting Excellent Output Characteristics with a Prompt Response against the Selective Protein. Adv. Funct. Mater. 2022, 32, 2204781. [Google Scholar] [CrossRef]
- Dastgeer, G.; Afzal, A.M.; Nazir, G.; Sarwar, N. p--GeSe/n--ReS2 Heterojunction Rectifier Exhibiting a Fast Photoresponse with Ultra--high Frequency--switching Applications. Adv. Mater. Interfaces 2021, 8, 2100705. [Google Scholar] [CrossRef]
- Shen, R.; Liang, G.; Hao, L.; Zhang, P.; Li, X. In Situ Synthesis of Chemically Bonded 2D/2D Covalent Organic Frameworks/O--vacancy WO3 Z-scheme Heterostructure for Photocatalytic Overall Water Splitting. Adv. Mater. 2023, 35, 2303649. [Google Scholar] [CrossRef]
- Tho, C.C.; Yu, C.; Tang, Q.; Wang, Q.; Su, T.; Feng, Z.; Wu, Q.; Nguyen, C.V.; Ong, W.; Liang, S.; et al. Cataloguing MoSi2N4 and WSi2N4 van Der Waals Heterostructures: An Exceptional Material Platform for Excitonic Solar Cell Applications. Adv. Mater. Interfaces 2023, 10, 2201856. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Ponnamma, D.; Nagi Saeed, A.M.; Ganesh, V.; Neppolian, B.; Byrappa, K. Direct Z-Scheme Cs2O–Bi2O3–ZnO Heterostructures for Photocatalytic Overall Water Splitting. J. Mater. Chem. A 2018, 6, 21379–21388. [Google Scholar] [CrossRef]
- Riffat, M.; Ali, H.; Qayyum, H.A.; Bilal, M.; Hussain, T. Enhanced Solar-Driven Water Splitting by ZnO/CdTe Heterostructure Thin Films-Based Photocatalysts. Int. J. Hydrogen Energy 2023, 48, 22069–22078. [Google Scholar] [CrossRef]
- Nayak, D.; Thangavel, R. Theoretical Investigation of Electronic and Photocatalytic Properties of a Trilayer vdW MoS2/ZnO/WS2 Heterojunction for Overall Water-Splitting Applications. ACS Appl. Energy Mater. 2024, 7, 2642–2652. [Google Scholar] [CrossRef]
- Zhang, W.X.; Hou, J.T.; Bai, M.; He, C.; Wen, J.R. Construction of Novel ZnO/Ga2SSe (GaSe) vdW Heterostructures as Efficient Catalysts for Water Splitting. Appl. Surf. Sci. 2023, 634, 157648. [Google Scholar] [CrossRef]
- Bao, J.; Zhu, B.; Zhang, F.; Chen, X.; Guo, H.; Qiu, J.; Liu, X.; Yu, J. Sc2CF2/Janus MoSSe Heterostructure: A Potential Z-Scheme Photocatalyst with Ultra-High Solar-to-Hydrogen Efficiency. Int. J. Hydrogen Energy 2021, 46, 39830–39843. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, M.; Zhong, X.; Qiu, K.; Bai, L.; Ma, B.; Wang, J.; Chen, Y. Theoretical Design of Sc2CF2/Ti2CO2 Heterostructure as a Promising Direct Z-Scheme Photocatalyst towards Efficient Water Splitting. Results Phys. 2024, 60, 107706. [Google Scholar] [CrossRef]
- Bayode, A.A.; Vieira, E.M.; Moodley, R.; Akpotu, S.; De Camargo, A.S.S.; Fatta-Kassinos, D.; Unuabonah, E.I. Tuning ZnO/GO p-n Heterostructure with Carbon Interlayer Supported on Clay for Visible-Light Catalysis: Removal of Steroid Estrogens from Water. Chem. Eng. J. 2021, 420, 127668. [Google Scholar] [CrossRef]
- Hu, F.; Tao, L.; Ye, H.; Li, X.; Chen, X. ZnO/WSe2 vdW Heterostructure for Photocatalytic Water Splitting. J. Mater. Chem. C 2019, 7, 7104–7113. [Google Scholar] [CrossRef]
- Khang, N.D.; Nguyen, C.Q.; Duc, L.M.; Nguyen, C.V. First-Principles Investigation of a Type-II BP/Sc2CF2 van Der Waals Heterostructure for Photovoltaic Solar Cells. Nanoscale Adv. 2023, 5, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Wang, B.-J.; Wang, G.-D.; Ke, S.-H. Blue Phosphorene/Sc2CX2 (X = O, F) van Der Waals Heterostructures as Suitable Candidates for Water-Splitting Photocatalysts and Solar Cells. Sustain. Energy Fuels 2020, 4, 5277–5283. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Lu, N.; Kan, E.; Zeng, X.C.; Wu, X.; Yang, J. Tunable Magnetism in a Nonmetal-Substituted ZnO Monolayer: A First-Principles Study. J. Phys. Chem. C 2012, 116, 11336–11342. [Google Scholar] [CrossRef]
- Björkman, T.; Gulans, A.; Krasheninnikov, A.V.; Nieminen, R.M. Van Der Waals Bonding in Layered Compounds from Advanced Density-Functional First-Principles Calculations. Phys. Rev. Lett. 2012, 108, 235502. [Google Scholar] [CrossRef] [PubMed]
- Cahangirov, S.; Topsakal, M.; Aktürk, E.; Şahin, H.; Ciraci, S. Two- and One-Dimensional Honeycomb Structures of Silicon and Germanium. Phys. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.-F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of Borophenes: Anisotropic, Two-Dimensional Boron Polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef]
- Zólyomi, V.; Drummond, N.D.; Fal’ko, V.I. Electrons and Phonons in Single Layers of Hexagonal Indium Chalcogenides from Ab Initio Calculations. Phys. Rev. B 2014, 89, 205416. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.-X. Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved Grid--based Algorithm for Bader Charge Allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear Optical Properties in the Projector-Augmented Wave Methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]
- Hong, X.; Kim, J.; Shi, S.-F.; Zhang, Y.; Jin, C.; Sun, Y.; Tongay, S.; Wu, J.; Zhang, Y.; Wang, F. Ultrafast Charge Transfer in Atomically Thin MoS2/WS2 Heterostructures. Nat. Nanotechnol. 2014, 9, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Huang, M.; Zhang, Z.; Wang, J.; Zhao, D.; Guo, X.; Liu, X. First-Principles Study on the Electronic Structure and Catalytic Properties of Two-Dimensional MX2N4 Systems (M = Ti, Zr; X = Si, Ge). Results Phys. 2023, 52, 106820. [Google Scholar] [CrossRef]
- Liu, M.; Tang, Y.; Yao, H.; Bai, L.; Song, J.; Ma, B. Theoretical Study on Photocatalytic Performance of ZnO/C2N Heterostructure towards High Efficiency Water Splitting. Front. Chem. 2022, 10, 1048437. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Song, J.; Ma, B.; Qiu, K.; Bai, L.; Wang, Y.; Chen, Y.; Tang, Y. First-Principles Investigation on the Tunable Electronic Structures and Photocatalytic Properties of AlN/Sc2CF2 and GaN/Sc2CF2 Heterostructures. Molecules 2024, 29, 3303. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, T.; Zhang, M.; Tong, Y.; Zhong, C.; Zhang, N.; Zhang, L.; Wu, C.; Xie, Y. 3D Nitrogen-anion-decorated Nickel Sulfides for Highly Efficient Overall Water Splitting. Adv. Mater. 2017, 29, 1701584. [Google Scholar] [CrossRef]
- Shen, Y.; Yuan, Z.; Cui, Z.; Ma, D.; Yuan, P.; Cheng, F.; Yang, K.; Dong, Y.; Li, E. The G-ZnO/PtSe2 S-Scheme Heterojunction with Controllable Band Structure for Catalytic Hydrogen Production. Int. J. Hydrogen Energy 2024, 56, 807–816. [Google Scholar] [CrossRef]
- Khamdang, C.; Singsen, S.; Ngoipala, A.; Fongkaew, I.; Junkaew, A.; Suthirakun, S. Computational Design of a Strain-Induced 2D/2D g-C3N4/ZnO S-Scheme Heterostructured Photocatalyst for Water Splitting. ACS Appl. Energy Mater. 2022, 5, 13997–14007. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, X.; Cong, B.; Hong, W.; Chen, G. Tailoring the D-Band Centers Endows (NixFe1-x)2 P Nanosheets with Efficient Oxygen Evolution Catalysis. ACS Catal. 2020, 10, 9086–9097. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, X.; Qin, J.; Liu, R. Constructing MoS2/g-C3N4 Heterojunction with Enhanced Oxygen Evolution Reaction Activity: A Theoretical Insight. Appl. Surf. Sci. 2020, 510, 145489. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.H.; Zhang, W.X.; Li, T.T. Type-II InSe/g-C3N4 Heterostructure as a High-Efficiency Oxygen Evolution Reaction Catalyst for Photoelectrochemical Water Splitting. J. Phys. Chem. Lett. 2019, 10, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-Q.; Yang, C.-L.; Li, X.-H.; Wang, M.-S.; Ma, X.-G. Insights into Photogenerated Carrier Dynamics and Overall Water Splitting of the CrS3/GeSe Heterostructure. J. Phys. Chem. Lett. 2023, 14, 9126–9135. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, S.; Shu, H.; Chou, J.-P.; Ren, K.; Yu, J.; Sun, M. A MoSSe/Blue Phosphorene Vdw Heterostructure with Energy Conversion Efficiency of 19.9% for Photocatalytic Water Splitting. Semicond. Sci. Technol. 2020, 35, 125008. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, C.; Zhou, M.; He, C.; Li, J.; Ouyang, T.; Tang, C.; Zhong, J. Highly Efficient Water Splitting in Step-Scheme PtS2/GaSe van Der Waals Heterojunction. J. Appl. Phys. 2022, 132, 055001. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Nosé, S. A Unified Formulation of the Constant Temperature Molecular Dynamics Methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Baroni, S.; De Gironcoli, S.; Dal Corso, A.; Giannozzi, P. Phonons and Related Crystal Properties from Density-Functional Perturbation Theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Toroker, M.C.; Kanan, D.K.; Alidoust, N.; Isseroff, L.Y.; Liao, P.; Carter, E.A. First Principles Scheme to Evaluate Band Edge Positions in Potential Transition Metal Oxide Photocatalysts and Photoelectrodes. Phys. Chem. Chem. Phys. 2011, 13, 16644. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]

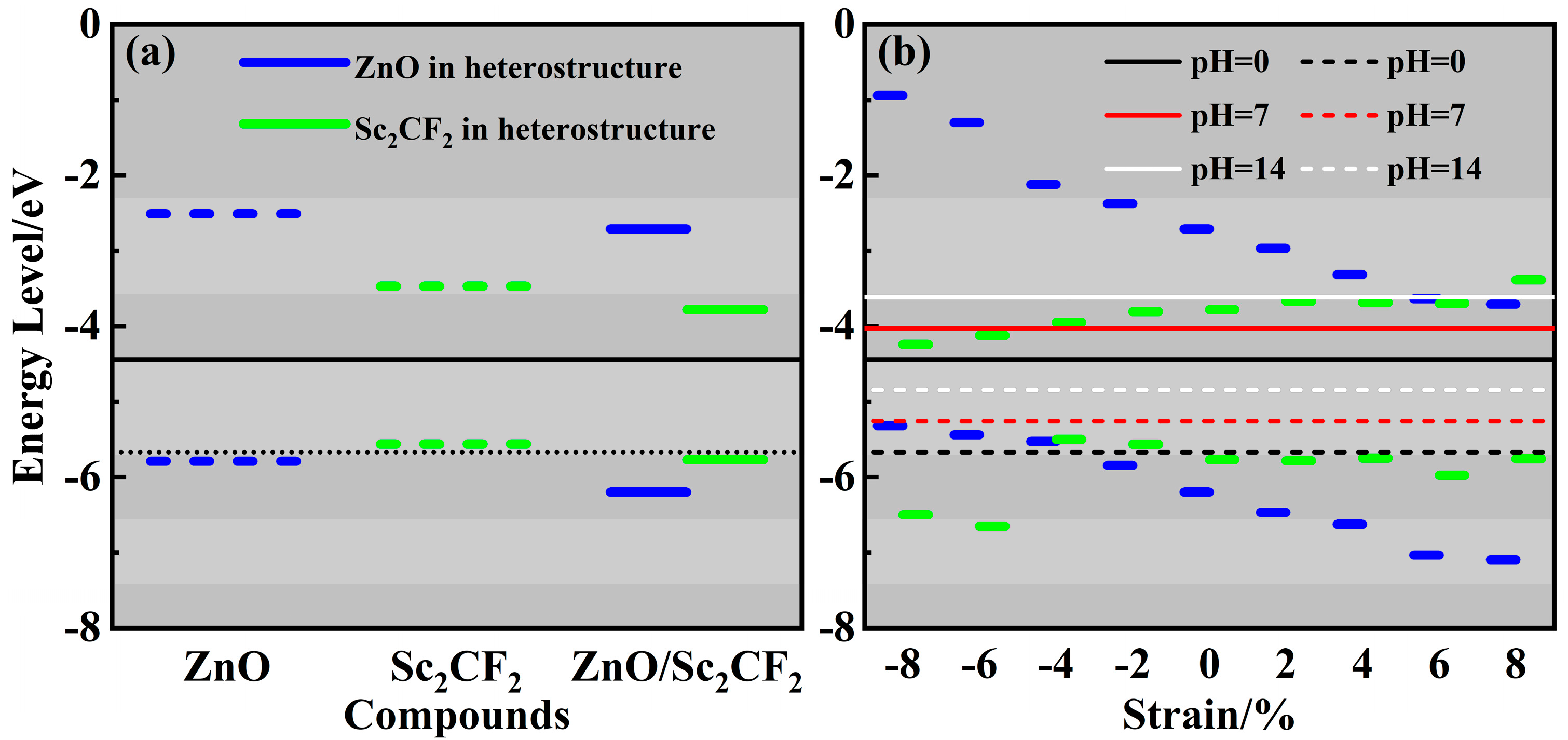
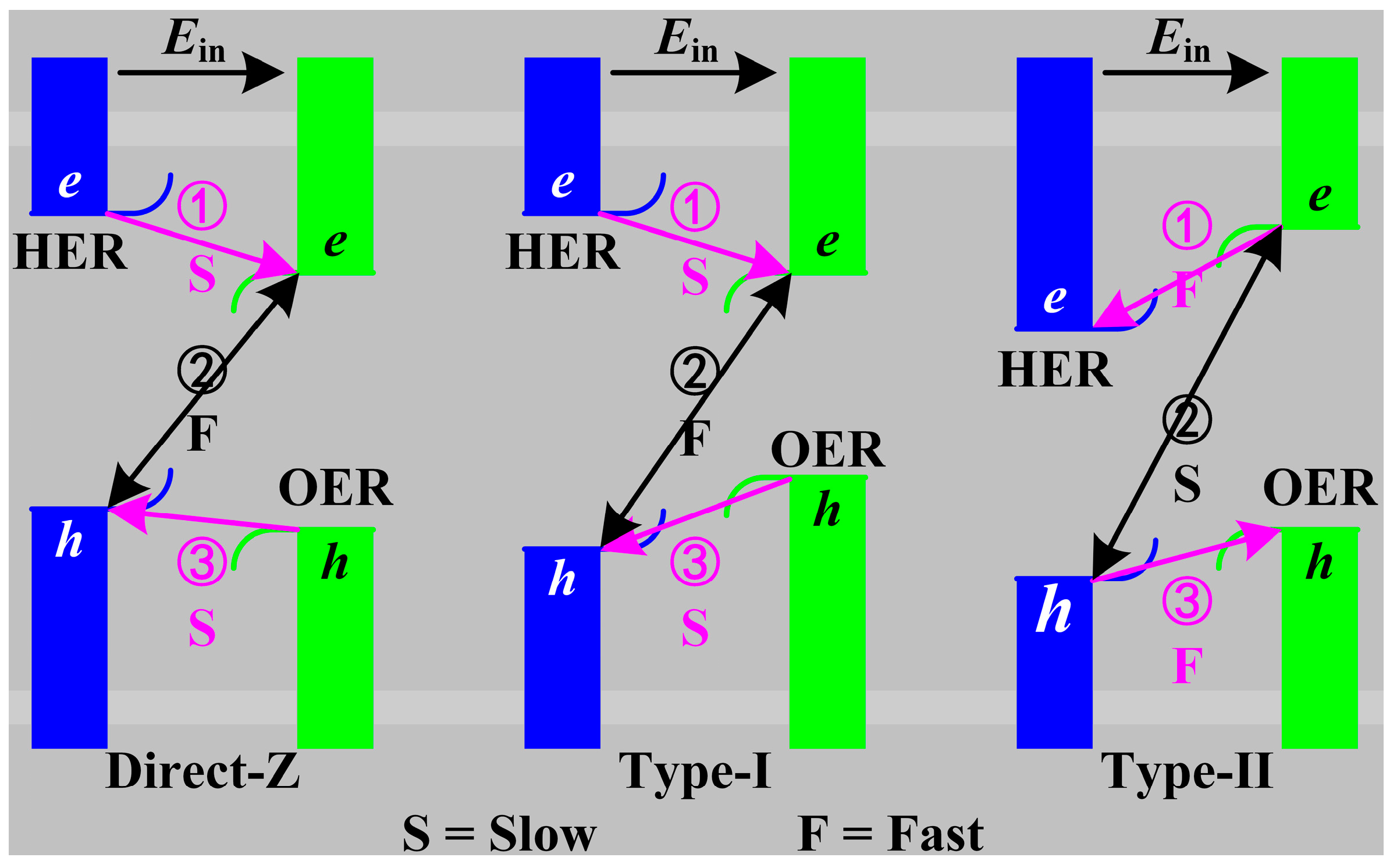
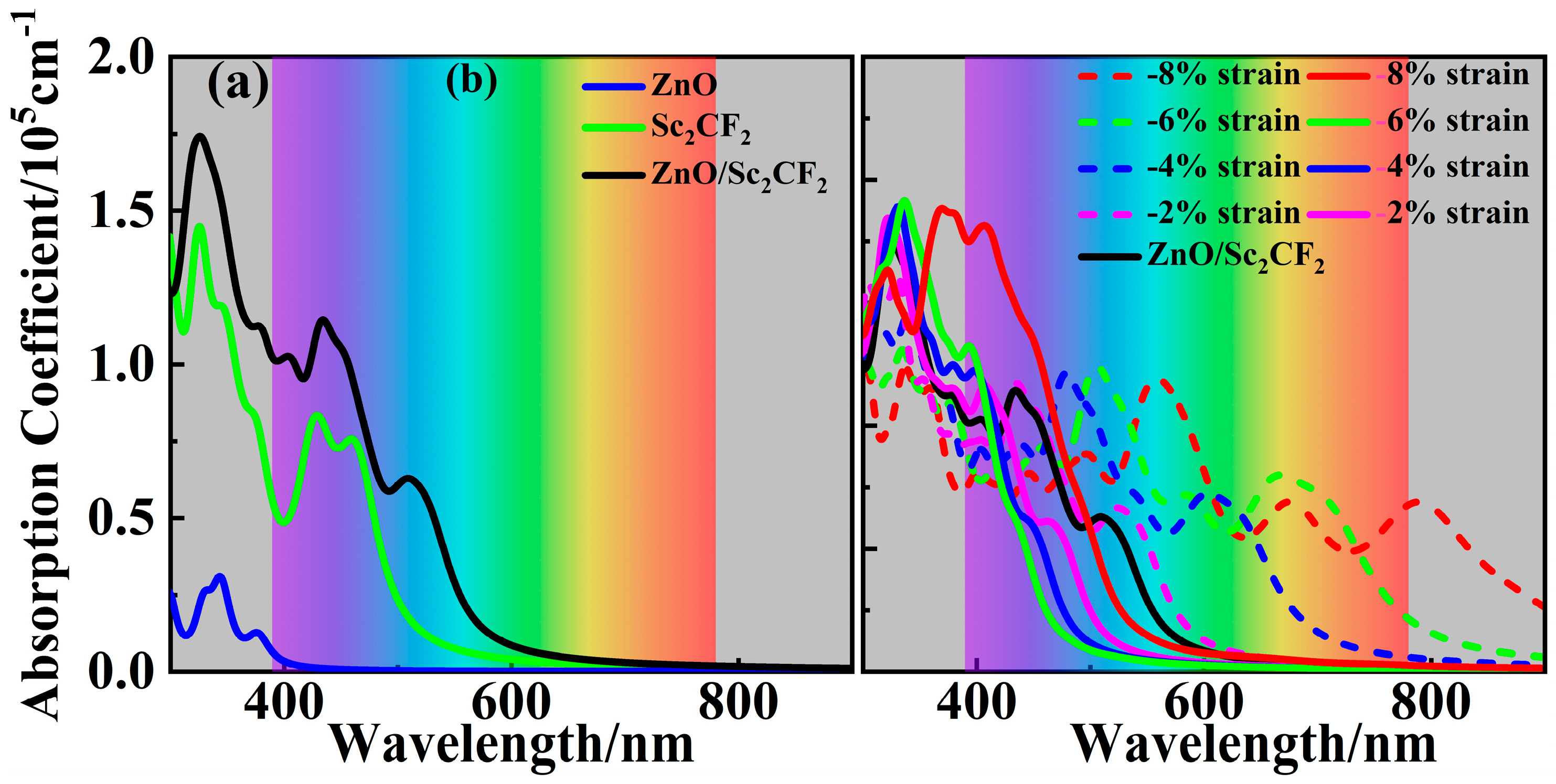

| Items | a | lZn-O | lSc-C | lSc-F | Eg | VBM | CBM | Wf |
|---|---|---|---|---|---|---|---|---|
| ZnO | 3.29 | 2.0 | - | - | 3.28 | −5.79 | −2.51 | 4.82 |
| Sc2CF2 | 3.22 | - | 2.27 | 2.21 | 2.09 | −5.56 | −3.47 | 5.02 |
| SC | SC-I | SC-II | SC-III | SC-IV | SC-V | SC-VI |
|---|---|---|---|---|---|---|
| a | 3.27 | 3.26 | 3.27 | 3.27 | 3.27 | 3.26 |
| d | 2.93 | 3.42 | 3.12 | 3.32 | 3.01 | 3.24 |
| Eb | −34.59 | −19.14 | −31.19 | −19.89 | −28.28 | −26.22 |
| Eg | 1.93 | 1.90 | 1.92 | 1.90 | 1.92 | 1.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Lu, Y.; Ma, B.; Song, J.; Bai, L.; Wang, Y.; Chen, Y.; Liu, M. Rational Design of ZnO/Sc2CF2 Heterostructure with Tunable Electronic Structure for Water Splitting: A First-Principles Study. Molecules 2024, 29, 4638. https://doi.org/10.3390/molecules29194638
Tang Y, Lu Y, Ma B, Song J, Bai L, Wang Y, Chen Y, Liu M. Rational Design of ZnO/Sc2CF2 Heterostructure with Tunable Electronic Structure for Water Splitting: A First-Principles Study. Molecules. 2024; 29(19):4638. https://doi.org/10.3390/molecules29194638
Chicago/Turabian StyleTang, Yong, Yidan Lu, Benyuan Ma, Jun Song, Liuyang Bai, Yinling Wang, Yuanyuan Chen, and Meiping Liu. 2024. "Rational Design of ZnO/Sc2CF2 Heterostructure with Tunable Electronic Structure for Water Splitting: A First-Principles Study" Molecules 29, no. 19: 4638. https://doi.org/10.3390/molecules29194638
APA StyleTang, Y., Lu, Y., Ma, B., Song, J., Bai, L., Wang, Y., Chen, Y., & Liu, M. (2024). Rational Design of ZnO/Sc2CF2 Heterostructure with Tunable Electronic Structure for Water Splitting: A First-Principles Study. Molecules, 29(19), 4638. https://doi.org/10.3390/molecules29194638






