Analytical Insights into Methods for Measuring Ischemia-Modified Albumin
Abstract
1. Introduction
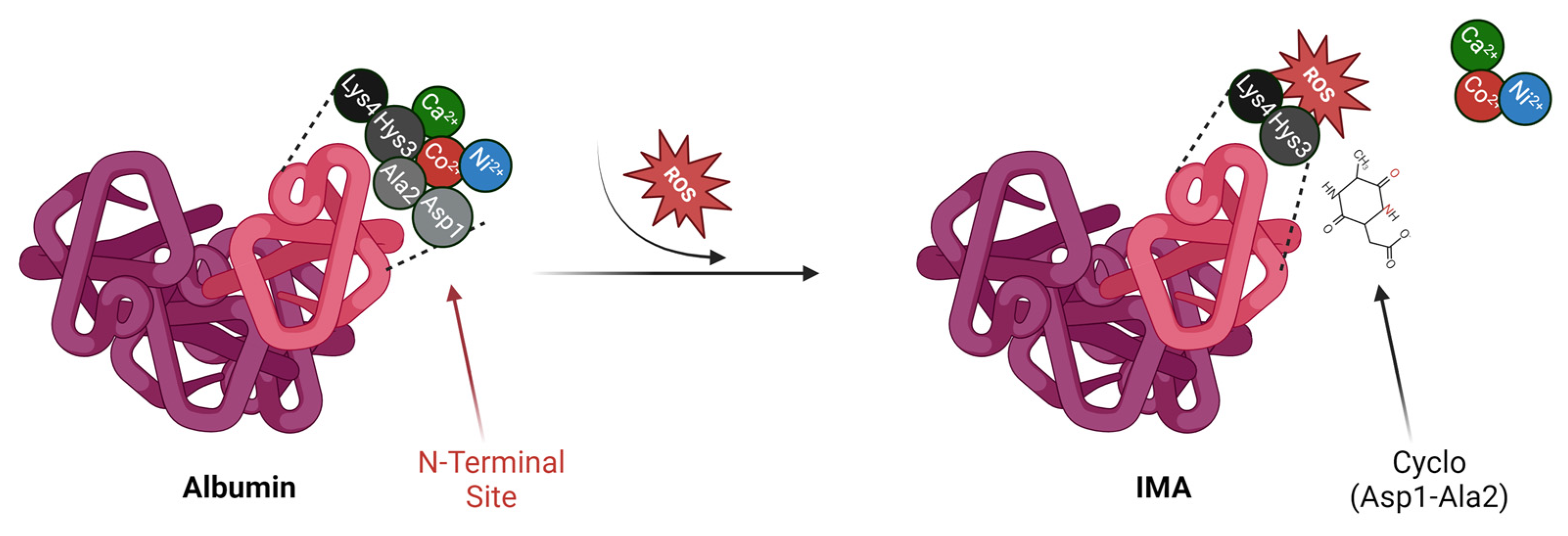
2. Albumin vs. IMA
3. Analytical Methods for the Measurement of IMAs
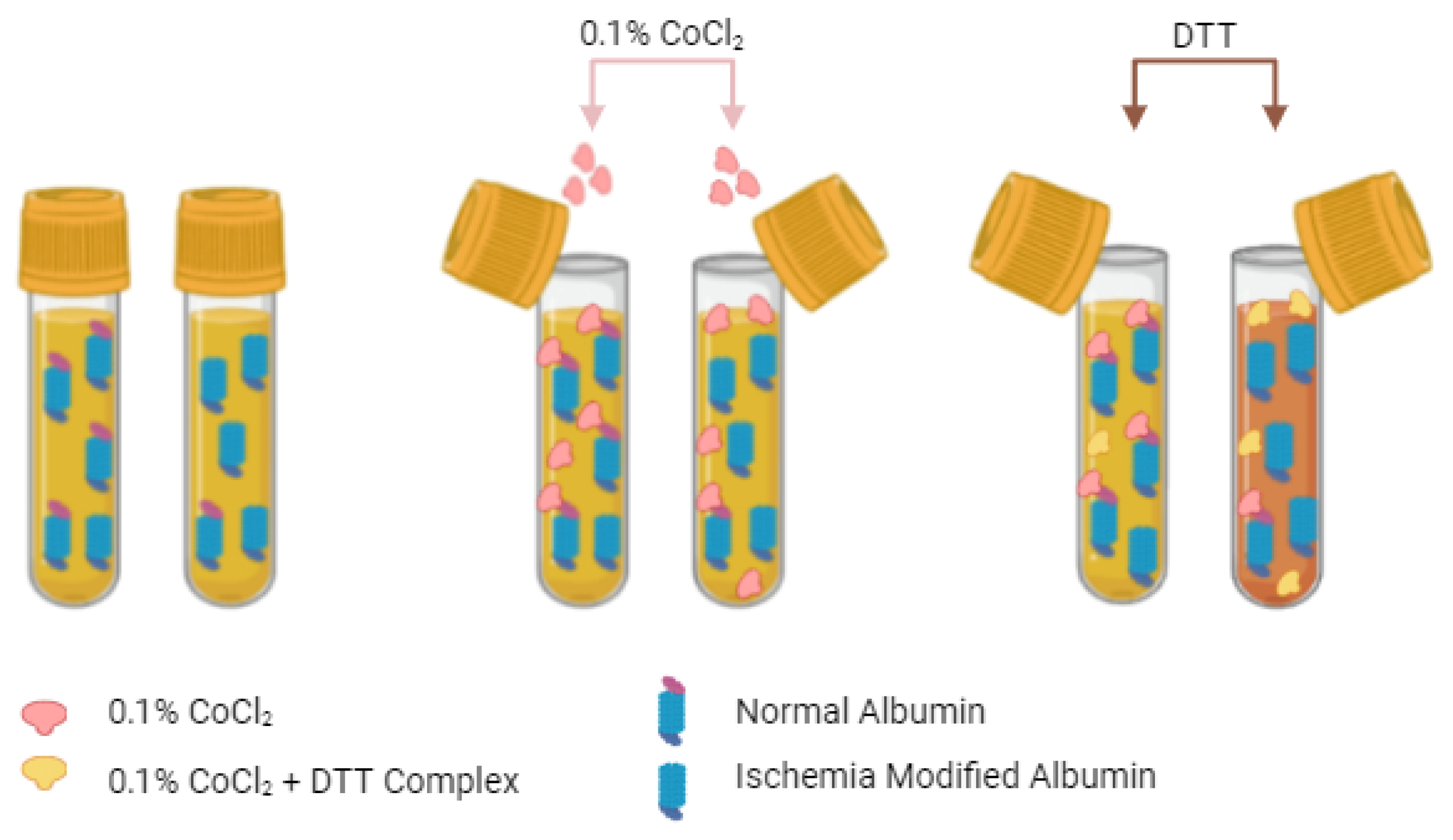
3.1. The Albumin Cobalt Binding (ACB) Test
3.2. Cobalt vs. Copper
3.3. Liquid Crystal Biosensor (LCB)
3.4. Quantum Dot-Coupled X-ray Fluorescence Spectroscopy (Q-XRF)
3.5. Mass Spectrometry (MS)
3.6. Immunoassays: ELISA and Gold Nanoparticles
3.7. Electron Paramagnetic Resonance (EPR) Spectroscopy
| Method. | Sensitivity | Specificity | Advantages | Litimations |
|---|---|---|---|---|
| Albumin Cobalt Binding (ACB) | 70–85 | 75–90 | Cost-effective, easy to use | Interference by bilirubin, fatty acids, moderate specificity |
| Albumin Copper Binding (ACuB) | 85–90 | 90–95 | Higher sensitivity and specificity than ACB, broader clinical use | More expensive, requires copper reagents |
| ELISA | 40–60 | 55–75 | Good for large-scale screening | Lower sensitivity compared to newer methods, variability |
| Liquid Crystal Biosensors (LCB) | Moderate | Moderate | Inexpensive and easy to use, potential for point of care | Moderate sensitivity, early in development |
| Quantum Dot Coupled X-Ray Fluorescence (Q-XRF) | High | High | Very high sensitivity, suitable for small sample volumes | High cost, complex equipment |
| Mass Spectrometry (MS) | Very high | Very high | Gold standard for specificity, highly accurate | Requires specialized equipment and expertise, high cost |
| Electron Paramagnetic Resonance (EPR) | High | High | High sensitivity for detecting transport and molecular changes | Limited availability, requires technical expertise |
4. Comparative Analysis in Different Biological Samples
4.1. Serum
- Preanalytical Variability: sample handling, particularly the time from collection to processing, can significantly impact IMA concentrations. Delays in processing or storage at temperatures different from those used during analysis may alter or degrade albumin, consequently affecting its metal-binding capacity and thus influencing IMA readings [52]. Specifically, IMA concentrations have been reported to remain stable for up to 24 h when serum is stored at 4 °C; however, significant degradation can occur with prolonged storage at room temperature [4].
- Storage Conditions: the temperature at which serum samples are stored is critical for preserving the integrity of IMA [52,53,54]. Samples should be stored at –20 °C or lower if not analyzed immediately. Research indicates that –80 °C is the optimal storage temperature for maintaining IMA concentrations over extended periods [52]. At this temperature, the risk of protein degradation or modification is minimized, thereby reducing assay interference to a minimum [55]. While –20 °C is adequate for short-term storage, it may not be sufficient for long-term preservation, whereas –80 °C provides maximum stability.
- Freeze–Thaw Cycles: IMA measurements in serum are significantly affected by repeated freeze–thaw cycles. Each cycle can lead to protein denaturation and a potential loss of albumin’s metal-binding sites, thereby reducing the accuracy of IMA detection [56]. To minimize this risk, it is recommended to aliquot serum samples before freezing, thereby avoiding multiple freeze–thaw cycles.
4.2. Saliva
- Assay Sensitivity: the concentration of albumin in saliva is significantly lower than in serum, presenting a challenge for detecting IMA with high sensitivity [58]. Existing assays require further optimization, potentially through the development of more advanced signal-detection methods or amplification techniques, to accurately measure IMA concentrations in saliva.
- Influence of pH and Composition: the pH of saliva can vary significantly under different conditions, such as food intake, oral hygiene, and time of day [59]. These pH fluctuations may affect the stability of IMA and, consequently, the efficiency of the detection test, leading to variability in results [60]. Additionally, interference from other proteins, enzymes, and contaminants in saliva could affect the measurement of IMA, necessitating the development of robust sample-preparation protocols to mitigate these effects [59,60].
- Temperature and Stability: although the stability of IMA in saliva under different storage conditions has not been studied as extensively as in serum, it is known that protein biomarkers in saliva are sensitive to storage temperature. Therefore, freezing at -80°C is recommended for optimal preservation, as suggested by practices used for other protein biomarkers in saliva [61]. However, further research is required to determine the most effective storage conditions for IMA in saliva to ensure its long-term stability.
4.3. Urine
- Albumin Concentration: the lower concentration of albumin in urine compared to serum or saliva makes detecting IMA more challenging [62]. Therefore, assays must be highly sensitive and specific, potentially requiring concentration steps or the development of enhanced detection methodologies to accurately quantify IMA in urine samples.
- Influence of Urine pH and Composition: the pH and composition of urine can vary depending on factors such as hydration status, diet, and kidney function [63,64]. While these variations are known to affect the stability and binding properties of other proteins in urine, their specific impact on IMA detection remains unclear and warrants further investigation. Therefore, it is important to account for these variables when developing and standardizing urine collection and preparation protocols to minimize potential variability in assay results.
- Storage Conditions: similar to other biological fluids, urine samples may be susceptible to degradation when stored at room temperature [65]. The stability of IMA in urine under different storage conditions has not been extensively studied, requiring further research to establish optimal preservation methods. Although it is generally accepted that lower storage temperatures, such as −80 °C, may prevent the breakdown of proteins and other biological molecules, including IMA, this recommendation is based on general best practices for protein preservation rather than specific evidence for IMA in urine [65]. Therefore, further studies are needed to determine the most effective storage conditions for maintaining the integrity of IMA in urine samples, particularly for long-term storage.
| Biological Matrix | Advantages | Limitations |
|---|---|---|
| Serum | Gold standard, reliable, well-validated in clinical settings | May require rapid processing for accurate results |
| Saliva | Non-invasive, easy to collect, promising with technological advancements | Lower albumin concentration, assay sensitivity challenges, variable pH |
| Urine | Non-invasive, potential for continuous monitoring, easy to collect | Lower albumin concentration, assay sensitivity challenges, variable pH and composition |
4.4. Other Factors Affecting Sensitivity and Specificity
- Assay Calibration: the calibration of assays using appropriate standards is critical for maintaining accuracy. Variability in the calibration process between different laboratories or analyzer models can lead to variability in results [67].
- Timing of Sample Collection: the timing of sample collection relative to the onset of ischemia can significantly impact IMA concentrations. Since IMA concentrations peak within a few hours after ischemia onset and then decline, the timing of sample collection can affect detection sensitivity [68].
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinellu, A.; Zoroddu, S.; Fois, S.; Mellino, S.; Scala, C.; Virdis, E.; Zinellu, E.; Sotgia, S.; Paliogiannis, P.; Mangoni, A.A.; et al. Ischemia-Modified Albumin (IMA) Is Associated with Poor Survival in Patients with Newly Diagnosed Idiopathic Pulmonary Fibrosis (IPF): A Pilot Study. Antioxidants 2024, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Regulation of Coronary Blood Flow in Normal and Ischemic States: Myocardial Ischemia: Lack of Coronary Blood Flow, Myocardial Oxygen Supply-Demand Imbalance, or What? Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1439. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M. Pathobiology of Myocardial Ischemia and Reperfusion Injury: Models, Modes, Molecular Mechanisms, Modulation, and Clinical Applications. Cardiol. Rev. 2023, 31, 252. [Google Scholar] [CrossRef]
- Senadeera, N.N.; Ranaweera, C.B.; Perera, I.C.; Kottahachchi, D.U. Biochemical Insights and Clinical Applications of Ischemia-Modified Albumin in Ischemic Conditions. J. Vasc. Dis. 2024, 3, 245–266. [Google Scholar] [CrossRef]
- Albumin, an Interesting and Functionally Diverse Protein, Varies from ‘Native’ to ‘Effective’ (Review). Available online: https://www.spandidos-publications.com/10.3892/mmr.2023.13147 (accessed on 2 August 2024).
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Amuzescu, A.; Matei, C.; Georgescu, S.R. Ischemia-Modified Albumin—A Potential New Marker of Oxidative Stress in Dermatological Diseases. Medicina 2022, 58, 669. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Kumar, M.; Sayyar, M.; Sapna, F.; John, C.; Memon, S.; Qureshi, K.; Agbo, E.C.; Ariri, H.I.; Chukwu, E.J.; et al. Revolutionizing Cardiac Care: A Comprehensive Narrative Review of Cardiac Rehabilitation and the Evolution of Cardiovascular Medicine. Cureus 2023, 15, e46469. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Zinellu, A. Ischemia-modified Albumin in Rheumatic Diseases: A Systematic Review and Meta-analysis. Immun. Inflamm. Dis. 2024, 12, e1324. [Google Scholar] [CrossRef] [PubMed]
- Bsat, S.; Halaoui, A.; Kobeissy, F.; Moussalem, C.; El Houshiemy, M.N.; Kawtharani, S.; Omeis, I. Acute Ischemic Stroke Biomarkers: A New Era with Diagnostic Promise? Acute Med. Surg. 2021, 8, e696. [Google Scholar] [CrossRef]
- Cepoi, M.R.; Duca, S.T.; Chetran, A.; Costache, A.D.; Spiridon, M.R.; Afrăsânie, I.; Leancă, S.A.; Dmour, B.A.; Matei, I.T.; Miftode, R.S.; et al. Chronic Kidney Disease Associated with Ischemic Heart Disease: To What Extent Do Biomarkers Help? Life 2024, 14, 34. [Google Scholar] [CrossRef]
- Saeed, D.; Reza, T.; Shahzad, M.W.; Mandokhail, A.K.; Bakht, D.; Qizilbash, F.H.; Silloca-Cabana, E.O.; Ramadhan, A.; Bokhari, S.F.H. Navigating the Crossroads: Understanding the Link Between Chronic Kidney Disease and Cardiovascular Health. Cureus 2023, 15, e51362. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and Septic Shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood–Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 594672. [Google Scholar] [CrossRef] [PubMed]
- Nian, K.; Harding, I.C.; Herman, I.M.; Ebong, E.E. Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Front. Physiol. 2020, 11, 605398. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Zinellu, A. Serum Concentrations of Ischaemia-Modified Albumin in Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4205. [Google Scholar] [CrossRef]
- Bonds, B.W.; Yang, S.; Hu, P.F.; Kalpakis, K.; Stansbury, L.G.; Scalea, T.M.; Stein, D.M. Predicting Secondary Insults after Severe Traumatic Brain Injury. J. Trauma. Acute Care Surg. 2015, 79, 85–90. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A Novel Assay for Cobalt-Albumin Binding and Its Potential as a Marker for Myocardial Ischemia-a Preliminary Report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Lai, E.M.; Rios, P.A.; Yang, J.; Ortega-Lopez, A.M.; Shinoda, H.; Honda, S.A.A.; Rios, C.N.; Sugiyama, C.E.; Ha, C.E. Evaluation of Human Serum Albumin Cobalt Binding Assay for the Assessment of Myocardial Ischemia and Myocardial Infarction. Clin. Chem. 2003, 49, 581–585. [Google Scholar] [CrossRef]
- Wu, A.; Wu, A.H.B.; Morris, D.L.; Fletcher, D.R.; Apple, F.S.; Christenson, R.H.; Painter, P.C. Albumin Cobalt Binding Test for Acute MI Analysis of the Albumin Cobalt Binding (ACBTM) Test as an Adjunct to Cardiac Troponin I for the Early Detection of Acute Myocardial Infarction. Cardiovasc. Toxicol. Humana Press. 2001, 1, 1. [Google Scholar]
- Barbosa, S.; Taboada, P.; Mosquera, V. Fibrillation and Polymorphism of Human Serum Albumin. Bio-Nanoimaging Protein Misfolding Aggreg. 2014, 32, 345–362. [Google Scholar] [CrossRef]
- Mishra, V.; Heath, R.J. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef] [PubMed]
- Kibel, A.; Lukinac, A.M.; Dambic, V.; Juric, I.; Relatic, K.S. Oxidative Stress in Ischemic Heart Disease. Oxid. Med. Cell Longev. 2020, 2020, 6627144. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, J.P.C.; Katundu, K.G.H.; Sobczak, A.I.S.; Arya, S.; Blindauer, C.A.; Stewart, A.J. Ischemia-Modified Albumin: Crosstalk between Fatty Acid and Cobalt Binding. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 147. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Novel Invasive and Noninvasive Cardiac-Specific Biomarkers in Obesity and Cardiovascular Diseases. Metab. Syndr. Relat. Disord. 2020, 18, 10. [Google Scholar] [CrossRef]
- Christenson, R.H.; Hong Duh, S.; Sanhai, W.R.; Wu, A.H.; Holtman, V.; Painter, P.; Branham, E.; Apple, F.S.; Murakami, M.; Morris, D.L. Characteristics of an Albumin Cobalt Binding Test for Assessment of Acute Coronary Syndrome Patients: A Multicenter Study. Clin. Chem. 2001, 47, 464–470. [Google Scholar] [CrossRef]
- Maguire, O.C.; O’sullivan, J.; Ryan, J.; Cunningham, S.K. Evaluation of the Albumin Cobalt Binding (ACB) Assay for Measurement of Ischaemia-Modified Albumin (IMA) on the Beckman Coulter LX-20. Ann. Clin. Biochem. 2006, 43, 494–499. [Google Scholar] [CrossRef]
- Gidenne, S.; Ceppa, F.; Fontan, E.; Perrier, F.; Burnat, P. Analytical Performance of the Albumin Cobalt Binding (ACB®) Test on the Cobas MIRA® Plus Analyzer. Clin. Chem. Lab. Med. 2004, 42, 455–461. [Google Scholar] [CrossRef]
- Zapico-Muñiz, E.; Santaló-Bel, M.; Mercé-Muntañola, J.; Montiel, J.A.; Martínez-Rubio, A.; Ordóñez-Llanos, J. Ischemia-Modified Albumin during Skeletal Muscle Ischemia. Clin. Chem. 2004, 50, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Albumin Cobalt Binding Assay to Rule Out Acute Coronary Syndrome. Available online: https://www.annclinlabsci.org/content/35/1/66.long (accessed on 26 August 2024).
- Anwaruddin, S.; Januzzi, J.L.; Baggish, A.L.; Lewandrowski, E.L.; Lewandrowski, K.B. Ischemia-Modified Albumin Improves the Usefulness of Standard Cardiac Biomarkers for the Diagnosis of Myocardial Ischemia in the Emergency Department Setting. Am. J. Clin. Pathol. 2005, 123, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.T.; Blindauer, C.A. Allosteric Inhibition of Cobalt Binding to Albumin by Fatty Acids: Implications for the Detection of Myocardial Ischemia. J. Med. Chem. 2012, 55, 4425–4430. [Google Scholar] [CrossRef]
- Van Der Zee, P.M.; Verberne, H.J.; Van Straalen, J.P.; Sanders, G.T.B.; Van Eck-Smit, B.L.F.; De Winter, R.J.; Fischer, J.C. Ischemia-Modified Albumin Measurements in Symptom-Limited Exercise Myocardial Perfusion Scintigraphy Reflect Serum Albumin Concentrations but Not Myocardial Ischemia. Clin. Chem. 2005, 51, 1744–1746. [Google Scholar] [CrossRef] [PubMed]
- Yücel, D. Ischemia—Modified Albumin by Albumin Cobalt Binding Test: A False Myth or Reality. Turk. J. Biochem. 2023, 48, 1–4. [Google Scholar] [CrossRef]
- FDA. Updates--January-February 2003 FDA Consumer. Available online: https://permanent.access.gpo.gov/lps1609/www.fda.gov/fdac/departs/2003/303_upd.html#heart (accessed on 3 May 2024).
- Eom, J.E.; Lee, E.; Jeon, K.H.; Sim, J.; Suh, M.; Jhon, G.J.; Kwon, Y. Development of an Albumin Copper Binding (ACuB) Assay to Detect Ischemia Modified Albumin. Anal. Sci. 2014, 30, 985–990. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lei, H.; Luo, S.; Tang, P.; Peng, X.; Wang, X. Liquid Crystal Biosensor for Detecting Ischemia Modified Albumin. Res. Chem. Intermed. 2017, 43, 353–360. [Google Scholar] [CrossRef][Green Version]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Seshadri Reddy, V.; Hemadri, V.; Pasupuleti, P. Comment on “Interference-Free Determination of Ischemia-Modified Albumin Using Quantum Dot Coupled X-Ray Fluorescence Spectroscopy”. Biosens. Bioelectron. 2015, 65, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Jiang, T.; Zhang, B.; Huang, J.; Liao, P.; Fu, W. Interference-Free Determination of Ischemia-Modified Albumin Using Quantum Dot Coupled X-Ray Fluorescence Spectroscopy. Biosens. Bioelectron. 2014, 51, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Yoon, T.; Song, J.J.; Park, Y.B.; Lee, S.W. Serum Albumin, Prealbumin, and Ischemia-Modified Albumin Levels in Patients with ANCA-Associated Vasculitis: A Prospective Cohort Study. PLoS ONE 2022, 17, e0271055. [Google Scholar] [CrossRef]
- Shaker, O.G.; Abdelaleem, O.O.; Fouad, N.A.; Ali, A.M.E.A.; Ahmed, T.I.; Ibrahem, E.G.; Abdelghaffar, N.K. Association Between MiR-155, Its Polymorphism and Ischemia-Modified Albumin in Patients with Rheumatoid Arthritis. J. Interferon Cytokine Res. 2019, 39, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, X.; Yang, M.; Chen, M.-M.; Chen, L.-C.; Xiong, X.-L. A Gold Nanoparticles Enhanced Surface Plasmon Resonance Immunosensor for Highly Sensitive Detection of Ischemia-Modified Albumin. Sensors 2013, 13, 12794–12803. [Google Scholar] [CrossRef] [PubMed]
- Klinkmann, G.; Waterstradt, K.; Klammt, S.; Schnurr, K.; Schewe, J.C.; Wasserkort, R.; Mitzner, S. Exploring Albumin Functionality Assays: A Pilot Study on Sepsis Evaluation in Intensive Care Medicine. Int. J. Mol. Sci. 2023, 24, 12551. [Google Scholar] [CrossRef]
- Kazmierczak, S.C.; Gurachevsky, A.; Matthes, G.; Muravsky, V. Electron Spin Resonance Spectroscopy of Serum Albumin: A Novel New Test for Cancer Diagnosis and Monitoring. Clin. Chem. 2006, 52, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Gurachevsky, A.; Muravskaya, E.; Gurachevskaya, T.; Smirnova, L.; Muravsky, V. Cancer-Associated Alteration in Fatty Acid Binding to Albumin Studied by Spin-Label Electron Spin Resonance. Cancer Investig. 2007, 25, 378–383. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.A.; Mahmoud, A.M.; Khalifa, A.M.; Ali, S.S. Physiological and Pathophysiological Reactive Oxygen Species as Probed by EPR Spectroscopy: The Underutilized Research Window on Muscle Ageing. J. Physiol. 2016, 594, 4591. [Google Scholar] [CrossRef] [PubMed]
- Klare, J.P. Electron Paramagnetic Resonance of Membrane Proteins. Encycl. Spectrosc. Spectrom. 2017, 3, 442–446. [Google Scholar] [CrossRef]
- Shin, H.; Kim, J.G.; Jang, B.H.; Lim, T.H.; Kim, W.; Cho, Y.; Choi, K.S.; Na, M.K.; Ahn, C.; Lee, J. Diagnostic Accuracy of Ischemia-Modified Albumin for Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 614. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Sobecka, L.; Sołkiewicz, K.; Kokot, I.; Kiraga, A.; Płaczkowska, S.; Schlichtinger, A.M.; Kratz, E.M. The Influence of Serum Sample Storage Conditions on Selected Laboratory Parameters Related to Oxidative Stress: A Preliminary Study. Diagnostics 2020, 10, 51. [Google Scholar] [CrossRef]
- Sample Stability of Ischemia Modified Albumin (IMA (TM)) as Assessed by the Albumin Cobalt Binding (ACB (R)) Test.|Request PDF. Available online: https://www.researchgate.net/publication/295425536_Sample_stability_of_Ischemia_Modified_Albumin_IMA_TM_as_assessed_by_the_Albumin_Cobalt_Binding_ACB_R_test (accessed on 20 August 2024).
- The Effect of Sample Storage Conditions and Time to Assay Performance on the Measurement of Ischemia Modified Albumin.|Request PDF. Available online: https://www.researchgate.net/publication/296143389_The_Effect_of_Sample_Storage_Conditions_and_Time_to_Assay_Performance_on_the_Measurement_of_Ischemia_Modified_Albumin (accessed on 20 August 2024).
- Resano-Barrio, P.; Alfaro, E.; Solano-Pérez, E.; Coso, C.; Cubillos-Zapata, C.; Díaz-García, E.; Romero-Peralta, S.; Luis Izquierdo-Alonso, J.; Barbé, F.; García-Rio, F.; et al. Analysis of the Ischemia-Modified Albumin as a Potential Biomarker for Cardiovascular Damage in Obstructive Sleep Apnea Patients with Acute Coronary Syndrome. Int. J. Mol. Sci. 2023, 24, 9019. [Google Scholar] [CrossRef]
- Cuhadar, S.; Koseoglu, M.; Atay, A.; Dirican, A. The Effect of Storage Time and Freeze-Thaw Cycles on the Stability of Serum Samples. Biochem. Med. 2013, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a Potential Non-Invasive Liquid Biopsy for Early and Easy Diagnosis/Prognosis of Head and Neck Cancer. Transl. Oncol. 2024, 40, 101827. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Aribas, A.; Yerlikaya, F.H.; Tasyurek, E.; Akbuǧa, K. Serum and Saliva Levels of Ischemia-Modified Albumin in Patients with Acute Myocardial Infarction. J. Clin. Lab. Anal. 2013, 27, 99. [Google Scholar] [CrossRef] [PubMed]
- Malamud, D.; Rodriguez-Chavez, I.R. Saliva as a Diagnostic Fluid. Dent. Clin. N. Am. 2011, 55, 159. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L. Saliva as a Diagnostic Fluid. Int. Dent. J. 2007, 57, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kaczor-Urbanowicz, K.E.; Wong, D.T.W. Salivary Biomarkers in Cancer Detection. Med. Oncol. 2017, 34, 7. [Google Scholar] [CrossRef]
- Buckalew, V.M.; Freedman, B.I. Effects of Race on Albuminuria and Risk of Cardiovascular and Kidney Disease. Expert. Rev. Cardiovasc. Ther. 2011, 9, 245. [Google Scholar] [CrossRef]
- Physical Characteristics of Urine|Anatomy and Physiology II. Available online: https://courses.lumenlearning.com/suny-ap2/chapter/physical-characteristics-of-urine/ (accessed on 20 August 2024).
- Chang, C.; Obeid, W.; Thiessen-Philbrook, H.; Parikh, C.R. Sample Processing and Stability for Urine Biomarker Studies. J. Appl. Lab. Med. 2021, 6, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Montenegro-Bethancourt, G.; Shi, L. Long-Term Urine Biobanking: Storage Stability of Clinical Chemical Parameters under Moderate Freezing Conditions without Use of Preservatives. Clin. Biochem. 2014, 47, 307–311. [Google Scholar] [CrossRef]
- Albumin Cobalt Binding (ACB) Test: Its Role as a Novel Marker of Acute Coronary Syndrome. Available online: https://www.researchgate.net/publication/6985756_Albumin_cobalt_binding_ACB_test_Its_role_as_a_novel_marker_of_acute_coronary_syndrome (accessed on 20 August 2024).
- O’Callaghan, K.M.; Roth, D.E. Standardization of Laboratory Practices and Reporting of Biomarker Data in Clinical Nutrition Research. Am. J. Clin. Nutr. 2020, 112, 453S. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, A.; Turkmen, S.; Turedi, S.; Mentese, A.; Yulug, E.; Ulusoy, H.; Karahan, S.C.; Topbas, M. Time-Dependent Variations in Ischemia-Modified Albumin Levels in Mesenteric Ischemia. Acad. Emerg. Med. 2009, 16, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, P.; Borra, S.K.; Yeruva, R.K.R.; Victor, D.; Babu, S.; Cherian, K.M. Estimation of Ischemia Modified Albumin (IMA) Levels in Patients with Acute Coronary Syndrome. Indian. J. Clin. Biochem. 2014, 29, 367. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoroddu, S.; Zinellu, A.; Carru, C.; Sotgia, S. Analytical Insights into Methods for Measuring Ischemia-Modified Albumin. Molecules 2024, 29, 4636. https://doi.org/10.3390/molecules29194636
Zoroddu S, Zinellu A, Carru C, Sotgia S. Analytical Insights into Methods for Measuring Ischemia-Modified Albumin. Molecules. 2024; 29(19):4636. https://doi.org/10.3390/molecules29194636
Chicago/Turabian StyleZoroddu, Stefano, Angelo Zinellu, Ciriaco Carru, and Salvatore Sotgia. 2024. "Analytical Insights into Methods for Measuring Ischemia-Modified Albumin" Molecules 29, no. 19: 4636. https://doi.org/10.3390/molecules29194636
APA StyleZoroddu, S., Zinellu, A., Carru, C., & Sotgia, S. (2024). Analytical Insights into Methods for Measuring Ischemia-Modified Albumin. Molecules, 29(19), 4636. https://doi.org/10.3390/molecules29194636







