Influence of Fe Ions on Anode Performance and the Mechanism of Action during Copper Electrowinning Process
Abstract
1. Introduction
2. Experiment Section
2.1. Electrolyte Composition
2.2. Anode Specimen Preparation and Electrochemical Testing
2.3. Physical Characterization
3. Results and Discussion
3.1. Changes in Electrochemical Behavior of Anode under the Effect of Fe3+
3.2. Changes in the Electrochemical Behavior of the Anode in the Presence of Fe2+ and Fe3+
3.3. Anode Phase Change
3.4. Microscopic Morphology of Surface Film Layer
3.5. Potential Mechanism of Reconstruction Engineering of Pb/Fe-PbO2
3.6. Changes in Cell Voltage and Current Efficiency under the Effect of Fe Ions
4. Conclusions
- (1)
- The addition of Fe3+ inhibits the formation of PbO2 and PbSO4 in the film layer, and excessively high concentrations of Fe3+ lead to a reduction in the corrosion resistance of anode and catalytic activity. When the Fe2+ concentration is controlled around 2 g/L, the oxygen evolution catalytic activity of the anodic film layer is enhanced.
- (2)
- When 2 g/L of Fe2+ is present in the electrolyte, the PbO2 content in the anode film layer increases, improving both the catalytic activity and corrosion resistance of the anode. However, as the Fe2+ concentration increases, the Fe3+ concentration in the electrolyte also rises, leading to a decline in the catalytic activity of anode and corrosion resistance.
- (3)
- When the concentration of iron ions (Fe3+ and Fe2+) is controlled at 2 g/L, the catalytic activity of the anode is enhanced. This enhanced catalytic activity is attributed to the doping of iron ions. Substitutional doping induces changes in the PbO2 crystal structure and increases the content of oxygen vacancies in the film, thereby improving the catalytic activity of the anode.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Li, B.; Wei, Y.; Wang, H. Effect of Zn2+ on the extraction of copper by cyclone electrowinning from simulated copper-containing electrolyte. Sep. Purif. Technol. 2022, 282, 120014. [Google Scholar] [CrossRef]
- Felder, A.; Prengaman, R.D. Lead alloys for permanent anodes in the nonferrous metals industry. JOM 2006, 58, 28–31. [Google Scholar] [CrossRef]
- Cachet, C.; Pape-rérolle, C.L.; Wiart, R. Influence of Co2+ and Mn2+ ions on the kinetics of lead anodes for zinc electrowinning. J. Appl. Electrochem. 1999, 29, 811–818. [Google Scholar] [CrossRef]
- Mureşan, L.; Maurin, G.; Oniciu, L.; Avram, S. Effects of additives on zinc electrowinning from industrial waste products. Hydrometallurgy 1996, 40, 335–342. [Google Scholar] [CrossRef]
- Ivanov, I. Increased current efficiency of zinc electrowinning in the presence of metal impurities by addition of organic inhibitors. Hydrometallurgy 2004, 72, 73–78. [Google Scholar] [CrossRef]
- Boyanov, B.S.; Konareva, V.V.; Kolev, N.K. Purification of zinc sulfate solutions from cobalt and nickel through activated cementation. Hydrometallurgy 2004, 73, 163–168. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Wang, J.; Hong, X. The influence of nickel ions on the long period electrowinning of zinc from sulfate electrolytes. Hydrometallurgy 2009, 99, 127–130. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Das, S.C.; Misra, V.N. Effect of antimony(III) on the electrocrystallisation of zinc from sulphate solutions containing SLS. Hydrometallurgy 2003, 69, 81–88. [Google Scholar] [CrossRef]
- Mirza, A. Corrosion of lead anodes in base metals electrowinning. J. S. Afr. Inst. Min. Metall. 2016, 116, 533–538. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Barmi, M.J. Novel lead–cobalt composite anodes for copper electrowinning. Hydrometallurgy 2013, 137, 45–52. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, F.; Nie, H.; Wang, R.; Xu, Z. Reducing the energy consumption and cell sludge of the zinc electrowinning process by using a pyramid-shaped 3D-Pb anode. Hydrometallurgy 2019, 190, 105188. [Google Scholar] [CrossRef]
- Yu, P.; O’Keefe, T.J. Evaluation of lead anode reactions in acid sulfate electrolytes: II. Manganese reactions. J. Electrochem. Soc. 2002, 149, A558. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, T.; Zou, L.; Li, H.; Li, L.; Li, R. Effects of Co2+ in diaphragm electrolysis on the electrochemical and corrosion behaviors of Pb Ag and Pb anodes for zinc electrowinning. Hydrometallurgy 2020, 195, 105412. [Google Scholar] [CrossRef]
- McGinnity, J.J.; Nicol, M.J. The role of silver in enhancing the electrochemical activity of lead and lead–silver alloy anodes. Hydrometallurgy 2014, 144–145, 133–139. [Google Scholar] [CrossRef]
- Chahmana, N.; Matrakova, M.; Zerroual, L.; Pavlov, D. Influence of some metal ions on the structure and properties of doped β-PbO2. J. Power Sources 2009, 191, 51–57. [Google Scholar] [CrossRef]
- Chahmana, N.; Zerroual, L.; Matrakova, M. Influence of Mg2+, Al3+, Co2+, Sn2+ and Sb3+ on the electrical performance of doped β-lead dioxide. J. Power Sources 2009, 191, 144–148. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, P.; Li, Y.; Zhai, X.; Lu, K.; Chen, X.; Yang, J.; Wang, Z.; Zhang, H.; Ge, G. Prussian blue analogues derived Fe-NiCoP reveals the cooperation of Fe doping and phosphating for enhancing OER activity. Appl. Surf. Sci. 2023, 615, 156378. [Google Scholar] [CrossRef]
- Shaw, D.R.; Dreisinger, D.B.; Lancaster, T.; Richmond, G.D.; Tomlinson, M. The commercialization of the FENIX iron control system for purifying copper electrowinning electrolytes. JOM 2004, 56, 38–41. [Google Scholar] [CrossRef]
- Huang, Z.; Li, P.; Feng, M.; Zhu, W.; Woldu, A.R.; Tong, Q.-X.; Hu, L. Unlocking Fe(III) Ions Improving Oxygen Evolution Reaction Activity and Dynamic Stability of Anodized Nickel Foam. Inorg. Chem. 2024, 63, 15493–15502. [Google Scholar] [CrossRef]
- Sathiyan, K.; Mondal, T.; Mukherjee, P.; Patra, S.G.; Pitussi, I.; Kornweitz, H.; Bar-Ziv, R.; Zidki, T. Enhancing the catalytic OER performance of MoS2 via Fe and Co doping. Nanoscale 2022, 14, 16148–16155. [Google Scholar] [CrossRef]
- Ye, J.; Chen, B.; Guo, J.; Huang, H.; He, Y.; Wang, S. Fabrication of a highly active β-PbO2-Co3O4 electrode for zinc electrowinning by pulse electrodeposition: Characterization and catalytic performance analysis. Appl. Surf. Sci. 2024, 651, 159296. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Gao, Y.; Chong, K.; Liu, C.; Cao, Y.; Wu, D. Zou Electrochemical corrosion and high temperature hot corrosion behavior of NbTaTiV and CrNbTaTiV high entropy alloy. J. Mater. Res. Technol. 2024, 28, 216–234. [Google Scholar] [CrossRef]
- Luo, H.; Zou, S.; Chen, Y.-H.; Li, Z.; Du, C.; Li, X. Influence of carbon on the corrosion behaviour of interstitial equiatomic CoCrFeMnNi high-entropy alloys in a chlorinated concrete solution. Corros. Sci. 2020, 163, 108287. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Wang, J.; Zheng, W.; Cao, Y. Doping and transformation mechanisms of Fe3+ ions in Fe-doped TiO2. CrystEngComm 2017, 19, 1100–1105. [Google Scholar] [CrossRef]
- Bao, H.; Qian, K.; Fang, J. Huang Fe-doped CeO2 solid solutions: Substituting-site doping versus interstitial-site doping, bulk doping versus surface doping. Appl. Surf. Sci. 2017, 414, 131–139. [Google Scholar] [CrossRef]
- Rezaei, R.; Jafarzadeh, K.; Mirali, S.M.; Abbasi, H.M. A new Co-doped PbO2 anode for copper electrowinning: Electrochemical and morphological characterization. J. Energy Storage 2024, 85, 111053. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q.; Wang, P.; Ding, J.; Cheng, W.; Huang, W. Fe doping regulates the surface reconstruction and activates lattice oxygen of NiCr LDH for water oxidation. Chem. Eng. J. 2024, 483, 149383. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mohammadi, F.; Alfantazi, F. Electrochemical Reactions on Metal-Matrix Composite Anodes for Metal Electrowinning. J. Electrochem. Soc. 2013, 160, E35–E43. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Sun, Y.; Chen, Y.; Chen, H.; Han, S. Lin Fe2O3 nanocatalysts on N-doped carbon nanomaterial for highly efficient electrochemical hydrogen evolution in alkaline. J. Power Sources 2019, 426, 74–83. [Google Scholar] [CrossRef]
- Ye, P.; Fang, K.; Wang, H.; Wang, Y.; Huang, H.; Mo, C.; Ning, J. Hu Lattice oxygen activation and local electric field enhancement by co-doping Fe and F in CoO nanoneedle arrays for industrial electrocatalytic water oxidation. Nat. Commun. 2024, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Duan, N.; Zuo, J.; Jiang, L.; Li, J.; Zhuang, S.; Liu, Y.; Xu, F. Fe doped γ-MnO2 of anode for lead release inhibition in zinc electrowinning. Chem. Eng. J. 2023, 476, 146475. [Google Scholar] [CrossRef]
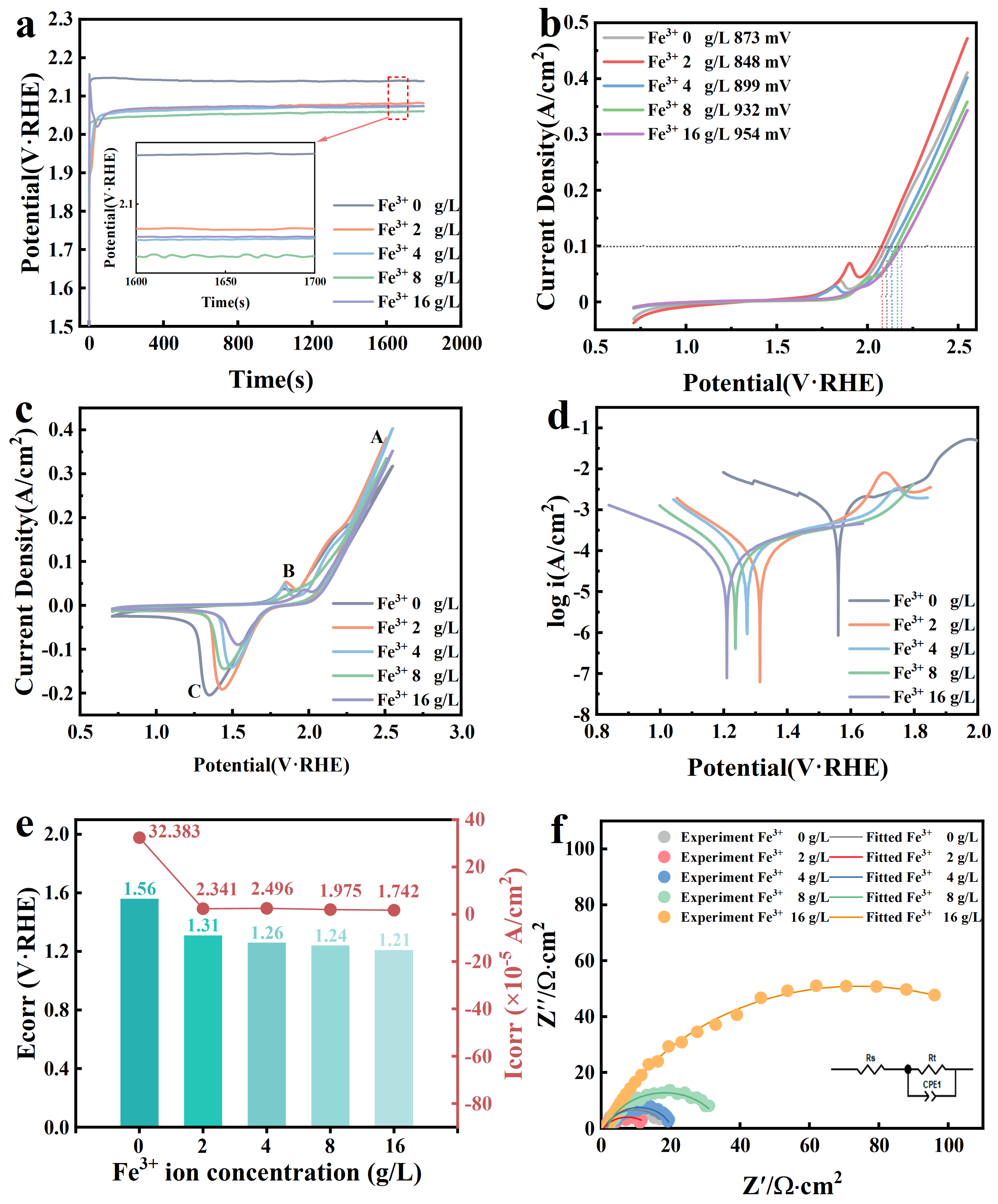

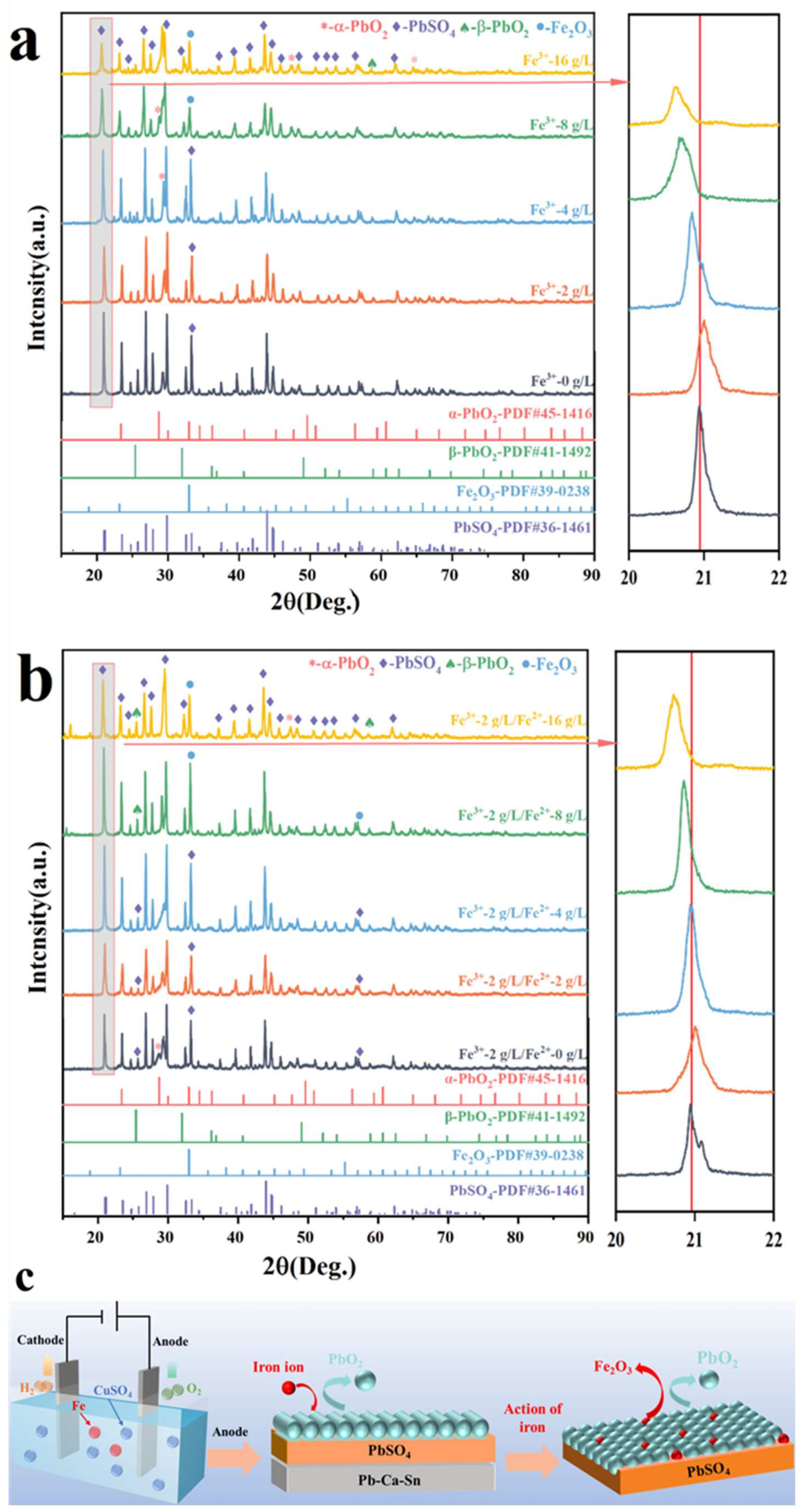

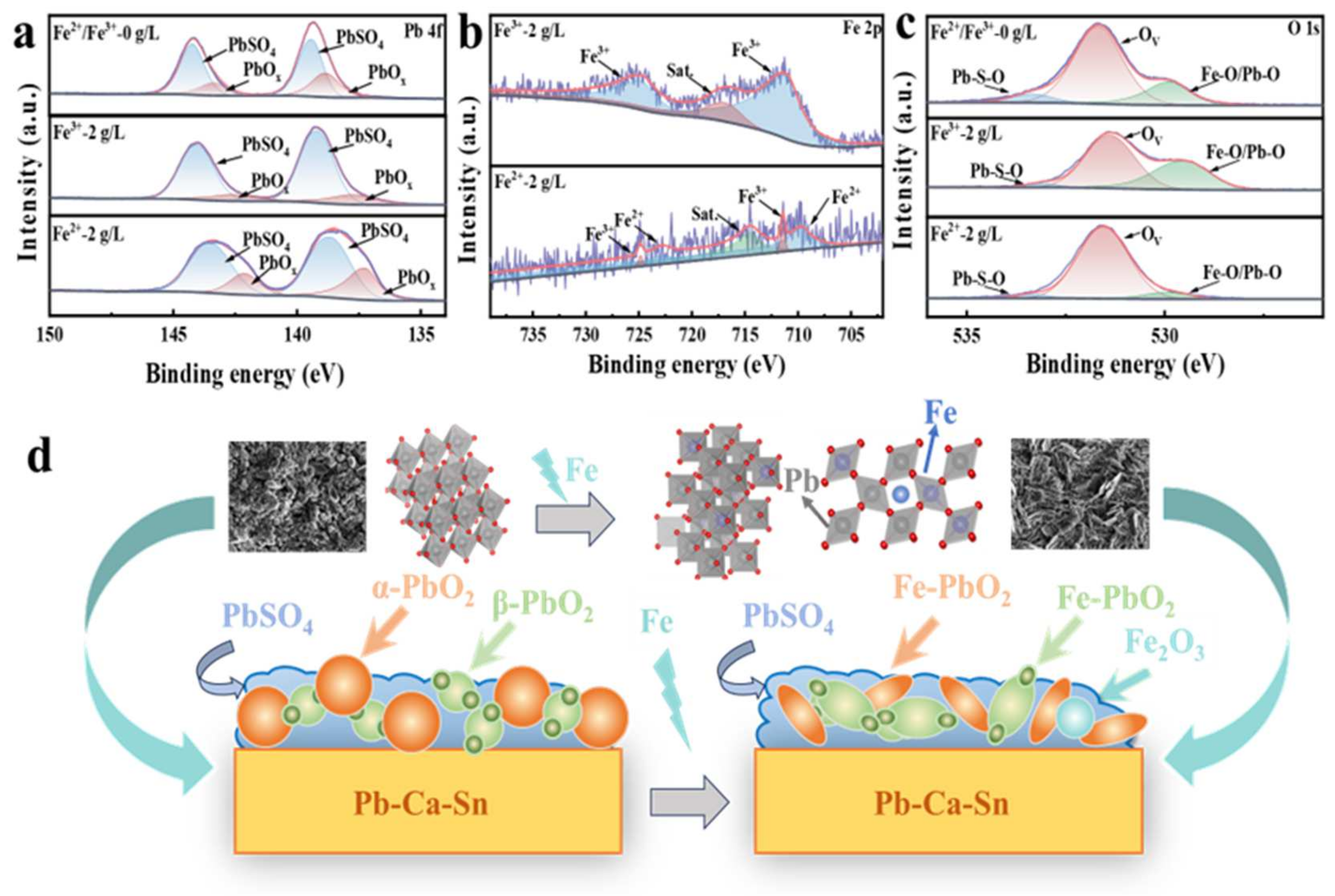

| Fe3+ Concentration (g/L) | Rs Ω·cm2 | Rct Ω·cm2 | Qdl Ω−1·cm−2·sn | Cdl μF·cm−2 | n | RF |
|---|---|---|---|---|---|---|
| 0 | 0.70 | 13.75 | 149,150 | 3278.928 | 0.67 | 163.9 |
| 2 | 0.89 | 17.93 | 104,500 | 11,314.369 | 0.80 | 565.7 |
| 4 | 1.60 | 18.63 | 63,221 | 9951.288 | 0.84 | 497.1 |
| 8 | 1.73 | 33.11 | 69,344 | 9959.024 | 0.80 | 497.9 |
| 16 | 1.51 | 144.8 | 33,972 | 3161.026 | 0.78 | 158.1 |
| Fe2+ Concentration (g/L) | Rs Ω·cm2 | Rct Ω·cm2 | Qdl Ω−1·cm−2·sn | Cdl μF·cm−2 | n | RF |
|---|---|---|---|---|---|---|
| 0 | 2.20 | 36.03 | 65,204 | 8820.071 | 0.80 | 441.0 |
| 2 | 3.87 | 29.76 | 35,765 | 10,368.597 | 0.89 | 518.4 |
| 4 | 2.33 | 53.07 | 60,587 | 8001.658 | 0.83 | 400.1 |
| 8 | 2.58 | 95.2 | 54,936 | 7548.613 | 0.83 | 377.4 |
| 16 | 5.59 | 125.6 | 31,470 | 6243.303 | 0.87 | 312.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Chen, Y.; Zhou, Y.; Chen, B.; Huang, H.; Guo, J.; Xu, R.; Guo, Z. Influence of Fe Ions on Anode Performance and the Mechanism of Action during Copper Electrowinning Process. Molecules 2024, 29, 4578. https://doi.org/10.3390/molecules29194578
Jiang C, Chen Y, Zhou Y, Chen B, Huang H, Guo J, Xu R, Guo Z. Influence of Fe Ions on Anode Performance and the Mechanism of Action during Copper Electrowinning Process. Molecules. 2024; 29(19):4578. https://doi.org/10.3390/molecules29194578
Chicago/Turabian StyleJiang, Cheng, Yiwen Chen, Yingping Zhou, Buming Chen, Hui Huang, Jun Guo, Ruidong Xu, and Zhongcheng Guo. 2024. "Influence of Fe Ions on Anode Performance and the Mechanism of Action during Copper Electrowinning Process" Molecules 29, no. 19: 4578. https://doi.org/10.3390/molecules29194578
APA StyleJiang, C., Chen, Y., Zhou, Y., Chen, B., Huang, H., Guo, J., Xu, R., & Guo, Z. (2024). Influence of Fe Ions on Anode Performance and the Mechanism of Action during Copper Electrowinning Process. Molecules, 29(19), 4578. https://doi.org/10.3390/molecules29194578





