Ultrasound-Assisted Enzyme Extraction, Physicochemical Properties and Antioxidant Activity of Polysaccharides from Cordyceps militaris Solid Medium
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Test
2.2. Extraction Process Optimization
2.2.1. Results of Response Surface
2.2.2. Interaction of Different Factors
2.3. Verification Test
2.4. Molecular Weight and Chemical Composition
2.5. Monosaccharide Composition Analysis
2.6. FT-IR Analysis
2.7. UV-Vis
2.8. Congo Red Test
2.9. SEM
2.10. AFM Analysis
2.11. TGA
2.12. Antioxidant Assay
3. Materials and Methods
3.1. Material and Chemicals
3.2. Extraction of CMMPs Using the Ultrasonic–Enzymatic Method
3.3. Single-Factor Test
3.4. RSM Design
| Variables | Level | |||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| X1 | Extraction time (min) | 50 | 60 | 70 |
| X2 | Extraction temperature (°C) | 55 | 60 | 65 |
| X3 | Liquid–solid ratio (v/m) | 30 | 40 | 50 |
| X4 | Enzyme contents (%) | 2 | 3 | 4 |
3.5. Purification and Characterization of CMMP
3.5.1. Preparation and Purification of CMMP
3.5.2. Molecular Weight Distribution Determination
3.5.3. Monosaccharide Composition Determination
3.5.4. FT-IR Spectroscopy
3.5.5. UV-Vis Spectroscopy
3.5.6. Congo Red Test
3.5.7. SEM Analysis
3.5.8. AFM
3.5.9. TGA
3.5.10. Comparison of the Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rupa, E.J.; Li, J.F.; Arif, M.H.; Yaxi, H.; Puja, A.M.; Chan, A.J.; Hoang, V.-A.; Kaliraj, L.; Yang, D.C.; Kang, S.C. Cordyceps militaris Fungus Extracts-Mediated Nanoemulsion for Improvement Antioxidant, Antimicrobial, and Anti-Inflammatory Activities. Molecules 2020, 25, 5733. [Google Scholar] [CrossRef] [PubMed]
- Prommaban, A.; Sriyab, S.; Marsup, P.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-Anun, C.; Chaiyana, W. Comparison of chemical profiles, antioxidation, inhibition of skin extracellular matrix degradation, and anti-tyrosinase activity between mycelium and fruiting body of Cordyceps militaris and Isaria tenuipes. Pharm. Biol. 2022, 60, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Jędrejko, K.; Kała, K.; Sułkowska-Ziaja, K.; Krakowska, A.; Zięba, P.; Marzec, K.; Szewczyk, A.; Sękara, A.; Pytko-Polończyk, J.; Muszyńska, B. Cordyceps militaris—Fruiting Bodies, Mycelium, and Supplements: Valuable Component of Daily Diet. Antioxidants 2022, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Israilides, C.; Philippoussis, A. Bio-technologies of recycling agro-industrial wastes for the production of commercially important fungal polysaccharides and mushrooms. Biotechnol. Genet. Eng. Rev. 2003, 20, 247–260. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, X.; Chen, S.; Liu, C.; Li, D. Synthesis extraction and separation of cordycepin, polysaccharide and polypeptide from Cordyceps pupa residue. Shaanxi Agric. Sci. 2021, 67, 32–37. [Google Scholar]
- Liu, X.-C.; Zhu, Z.-Y.; Liu, Y.-L.; Sun, H.-Q. Comparisons of the anti-tumor activity of polysaccharides from fermented mycelia and cultivated fruiting bodies of Cordyceps militaris in vitro. Int. J. Biol. Macromol. 2019, 130, 307–314. [Google Scholar] [CrossRef]
- Wang, L.; Xu, N.; Zhang, J.; Zhao, H.; Lin, L.; Jia, S.; Jia, L. Antihyperlipidemic and hepatoprotective activities of residue polysaccharide from Cordyceps militaris SU-12. Carbohydr. Polym. 2015, 131, 355–362. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Novel polysaccharide from Chaenomeles speciosa seeds: Structural characterization, α-amylase and α-glucosidase inhibitory activity evaluation. Int. J. Biol. Macromol. 2020, 153, 755–766. [Google Scholar] [CrossRef]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2016, 7, 530–539. [Google Scholar] [CrossRef]
- Sun, Y.; Bao, J.Z.; Liu, H.; Ma, H.; Zhang, T.; Chen, X.L. Research on Extraction and Antineoplasmic Activity of Polysaccharide inCulture Medium of Cordyceps militaris. J. Food Biotechnol. 2019, 38, 118–126. (In Chinese) [Google Scholar]
- Ren, S.-Y.; Zhao, C.-Y.; Song, H.-Y.; Zhao, H.-L.; Sun, J.-D. Optimum of Polysaccharide Distillation on Scrap Cordyceps militaris Medium. Tradit. Chin. Med. Mater. 2008, 31, 342–343. (In Chinese) [Google Scholar]
- Ma, T.; Sun, X.; Tian, C.; Luo, J.; Zheng, C.; Zhan, J. Polysaccharide extraction from Sphallerocarpus gracilis roots by response surface methodology. Int. J. Biol. Macromol. 2016, 88, 162–170. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Zhu, B.W. Optimization of Enzyme Extraction Conditions of Cordycepin Polysaccharide by Using Response Surface Methodology. Anhui Agric. Sci. 2012, 40, 12666–12668+12670. (In Chinese) [Google Scholar]
- Liang, C.; Shang, H.X. Extraction and Anti-Oxidation Performances of Polysaccharide from Culture Medium of Cordyceps Militaris. Food Res. Dev. 2010, 31, 26–29. (In Chinese) [Google Scholar]
- Wang, L.; Li, T.; Liu, F.; Liu, D.; Xu, Y.; Yang, Y.; Zhao, Y.; Wei, H. Ultrasonic-assisted enzymatic extraction and characterization of polysaccharides from dandelion (Taraxacum officinale) leaves. Int. J. Biol. Macromol. 2019, 126, 846–856. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic activity of Armillaria mellea polysaccharides: Joint ultrasonic and enzyme assisted extraction. Ultrason. Sonochem. 2023, 95, 106370. [Google Scholar] [CrossRef]
- Lin, B.; Wang, S.; Zhou, A.; Hu, Q.; Huang, G. Ultrasound-assisted enzyme extraction and properties of Shatian pomelo peel polysaccharide. Ultrason. Sonochem. 2023, 98, 106507. [Google Scholar] [CrossRef]
- Gao, J.; Hu, D.; Shen, Y.; Zheng, Y.; Liang, Y. Optimization of ultrasonic-assisted polysaccharide extraction from Hyperici Perforati Herba using response surface methodology and assessment of its antioxidant activity. Int. J. Biol. Macromol. 2023, 225, 255–265. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Cheng, L.; Shi, R.; Qayum, A.; Bilawal, A.; Gantumur, M.-A.; Hussain, M.A.; Jiang, Z.; Tian, B. Comparison in bioactivity and characteristics of Ginkgo biloba seed polysaccharides from four extract pathways. Int. J. Biol. Macromol. 2020, 159, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Ji, X.; Chen, Y.; Zhao, Z.; Gao, Y.; Gu, L.; She, D.; Ri, I.; Wang, W.; Wang, H. Ultrasound-assisted enzymatic extraction of Scutellaria baicalensis root polysaccharide and its hypoglycemic and immunomodulatory activities. Int. J. Biol. Macromol. 2023, 227, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cui, P.; Ge, S.; Cai, X.; Li, Q.; Xue, H. Ultrasound assisted aqueous two-phase extraction of polysaccharides from Cornus officinalis fruit: Modeling, optimization, purification, and characterization. Ultrason. Sonochem. 2022, 84, 105966. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, G. Preparation and Analysis of Pueraria lobata Polysaccharides. ACS Biomater. Sci. Eng. 2023, 9, 2329–2334. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Li, J.; Jiang, G.; Shen, G.; Li, S.; Luo, Q.; Wu, H.; Li, M.; Liu, X.; et al. Extraction Optimization and Evaluation of the Antioxidant and α-Glucosidase Inhibitory Activity of Polysaccharides from Chrysanthemum morifolium cv. Hangju. Antioxidants 2020, 9, 59. [Google Scholar] [CrossRef]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, G. Preparation and analysis of polysaccharide from Solanum tuberdsm. Ultrason. Sonochem. 2023, 98, 106520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, K.; Zhang, N.; Chitrakar, B.; Huang, P.; Wang, X.; Yang, B.; Sang, Y. Structural characterization and immunomodulatory effects of extracellular polysaccharide from Lactobacillus paracasei VL8 obtained by gradient ethanol precipitation. J. Food Sci. 2022, 87, 2034–2047. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Wang, F.; Xu, J.; Tang, X.; Li, N. Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 2018, 111, 862–869. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; Luo, A.; Chun, Z. Purification, composition analysis and antioxidant activity of the polysaccharides from Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 1014–1019. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Huang, W.-W.; Zhao, H.-T.; Wang, J.; Xu, R.-B.; Hu, X.-L.; Shen, S.-Y.; Qin, D. Characterization and Bioactivity of Polysaccharides Obtained from Pine Cones of Pinus koraiensis by Graded Ethanol Precipitation. Molecules 2013, 18, 9933–9948. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, W.; Huang, G.; Huang, H. Ultrasonic-assisted extraction, characteristics and activity of Ipomoea batatas polysaccharide. Ultrason. Sonochem. 2023, 96, 106420. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Chang, S.; Liu, J.; Li, H.; Xu, P.; Wang, P.; Wang, X.; Zhao, M.; Zhao, B.; Wang, L.; et al. Physicochemical Properties, Antioxidant and Antidiabetic Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Molecules 2020, 25, 3840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nie, S.; Guo, Q.; Wang, Q.; Cui, S.W.; Xie, M. Conformational properties of a bioactive polysaccharide from Ganoderma atrum by light scattering and molecular modeling. Food Hydrocoll. 2018, 84, 16–25. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, S.; Lai, S.; Chen, F.; Yang, H. Combined effects of ultrasound and calcium on the chelate-soluble pectin and quality of strawberries during storage. Carbohydr. Polym. 2018, 200, 427–435. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Wei, X.; Yang, Z.; Xiao, J.; Jin, Z. Sulfation of tea polysaccharides: Synthesis, characterization and hypoglycemic activity. Int. J. Biol. Macromol. 2010, 46, 270–274. [Google Scholar] [CrossRef]
- Xue, H.; Xu, J.; Zhang, J.; Wei, Y.; Cai, X.; Tan, J. Modeling, optimization, purification, and characterization of polysaccharides from Lilium lancifolium Thunb. LWT 2022, 162, 113491. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, Y.; Huang, Y.; Jiang, C.; Zhao, L.; Hu, J.; Pang, W. Physicochemical Characteristics and Antidiabetic Properties of the Polysaccharides from Pseudostellaria heterophylla. Molecules 2022, 27, 3719. [Google Scholar] [CrossRef]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical Composition and Antioxidant Activity of the Essential Oils of Citral-Rich Chemotype Cinnamomum camphora and Cinnamomum bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, Z.; Guo, Y.; Lu, S.; Du, H.; Cao, Y. Antioxidant capacity of flavonoids from Folium Artemisiae Argyi and the molecular mechanism in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 279, 114398. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chu, X.; Zhang, X.; Kan, Q.; Liu, R.; Fu, X. Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, X.; Ye, Q.; Dong, L. Ultrasound extraction optimization of Acanthopanax senticosus polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 2013, 59, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Lin, W.; Wang, Q.; Zhou, S. Extraction and radicals scavenging activity of polysaccharides with microwave extraction from mung bean hulls. Int. J. Biol. Macromol. 2012, 51, 612–617. [Google Scholar] [CrossRef]
- Zhang, W.; Duan, W.; Huang, G.; Huang, H. Ultrasonic-assisted extraction, analysis and properties of mung bean peel polysaccharide. Ultrason. Sonochem. 2023, 98, 106487. [Google Scholar] [CrossRef]
- Cowieson, A.; Kluenter, A. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim. Feed. Sci. Technol. 2019, 250, 81–92. [Google Scholar] [CrossRef]
- Li, P.; Yan, Z.; Chen, Y.; He, P.; Yang, W. Analysis of monosaccharide composition of water-soluble polysaccharides from Codium fragile by ultra-performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2021, 44, 1452–1460. [Google Scholar] [CrossRef]
- Tao, W.; Liu, W.; Wang, M.; Zhou, W.; Xing, J.; Xu, J.; Pi, X.; Wang, X.; Lu, S.; Yang, Y. Dendrobium officinale Polysaccharides Better Regulate the Microbiota of Women Than Men. Foods 2022, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, Q.; Ren, J.; Jian, L.; Peng, F.; Sun, R.; Liu, G. Effect of structural characteristics of corncob hemicelluloses fractionated by graded ethanol precipitation on furfural production. Carbohydr. Polym. 2016, 136, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. Int. J. Biol. Macromol. 2020, 163, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, Y.; Huang, G.; Huang, H. Ultrasound-assisted extraction, analysis and antioxidant activity of polysaccharide from the rinds of Garcinia mangostana L. Ultrason. Sonochem. 2023, 97, 106474. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Y.; Zhang, R.; Pan, J.; Qi, D.; Wang, J.; Yang, X. The protective effects of walnut green husk polysaccharide on liver injury, vascular endothelial dysfunction and disorder of gut microbiota in high fructose-induced mice. Int. J. Biol. Macromol. 2020, 162, 92–106. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Li, J.; Xu, J.; Zeng, J.; Gao, W.; Chen, K. Comparative study on the extraction efficiency, characterization, and bioactivities of Bletilla striata polysaccharides using response surface methodology (RSM) and genetic algorithm-artificial neural network (GA-ANN). Int. J. Biol. Macromol. 2023, 226, 982–995. [Google Scholar] [CrossRef]


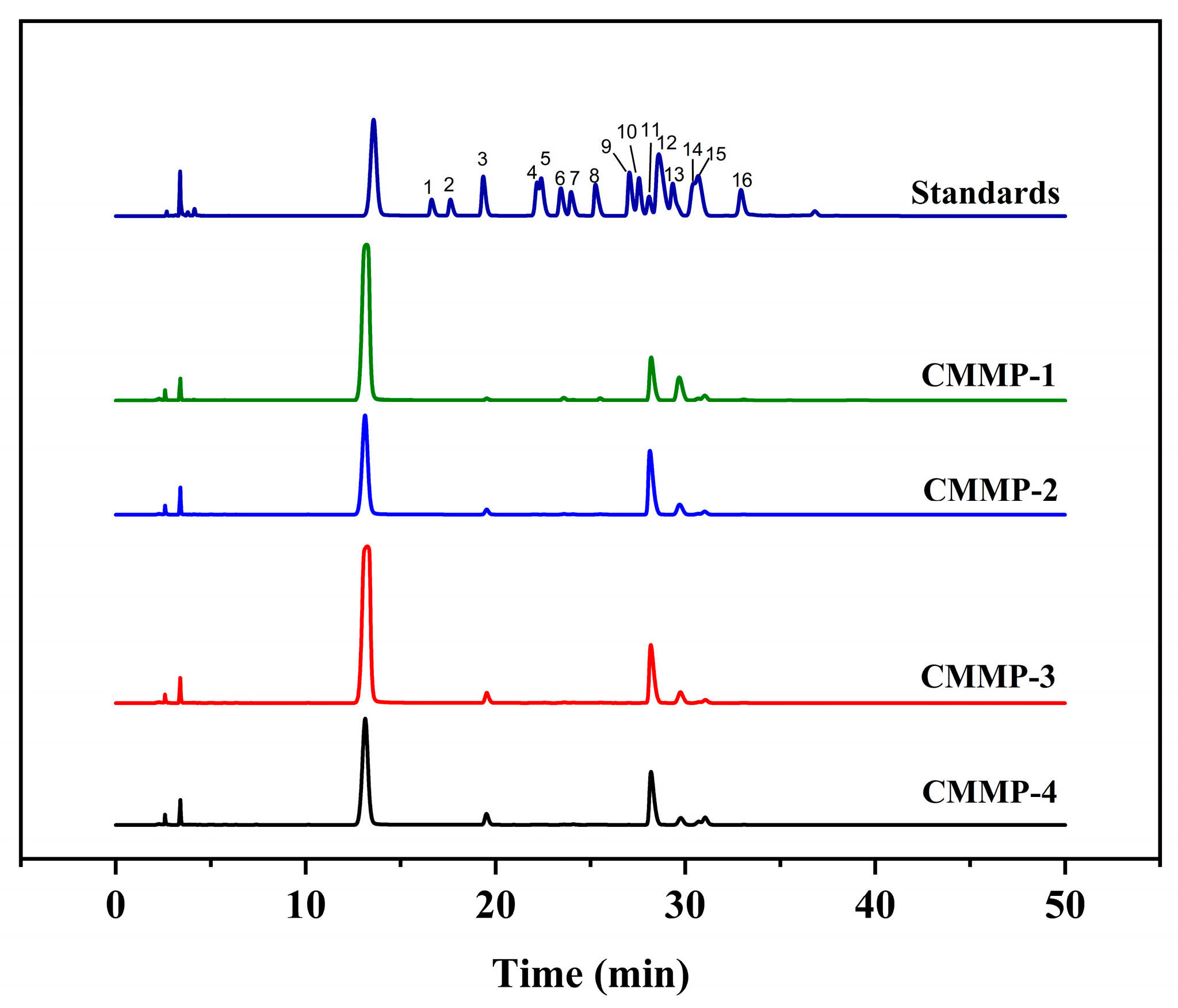

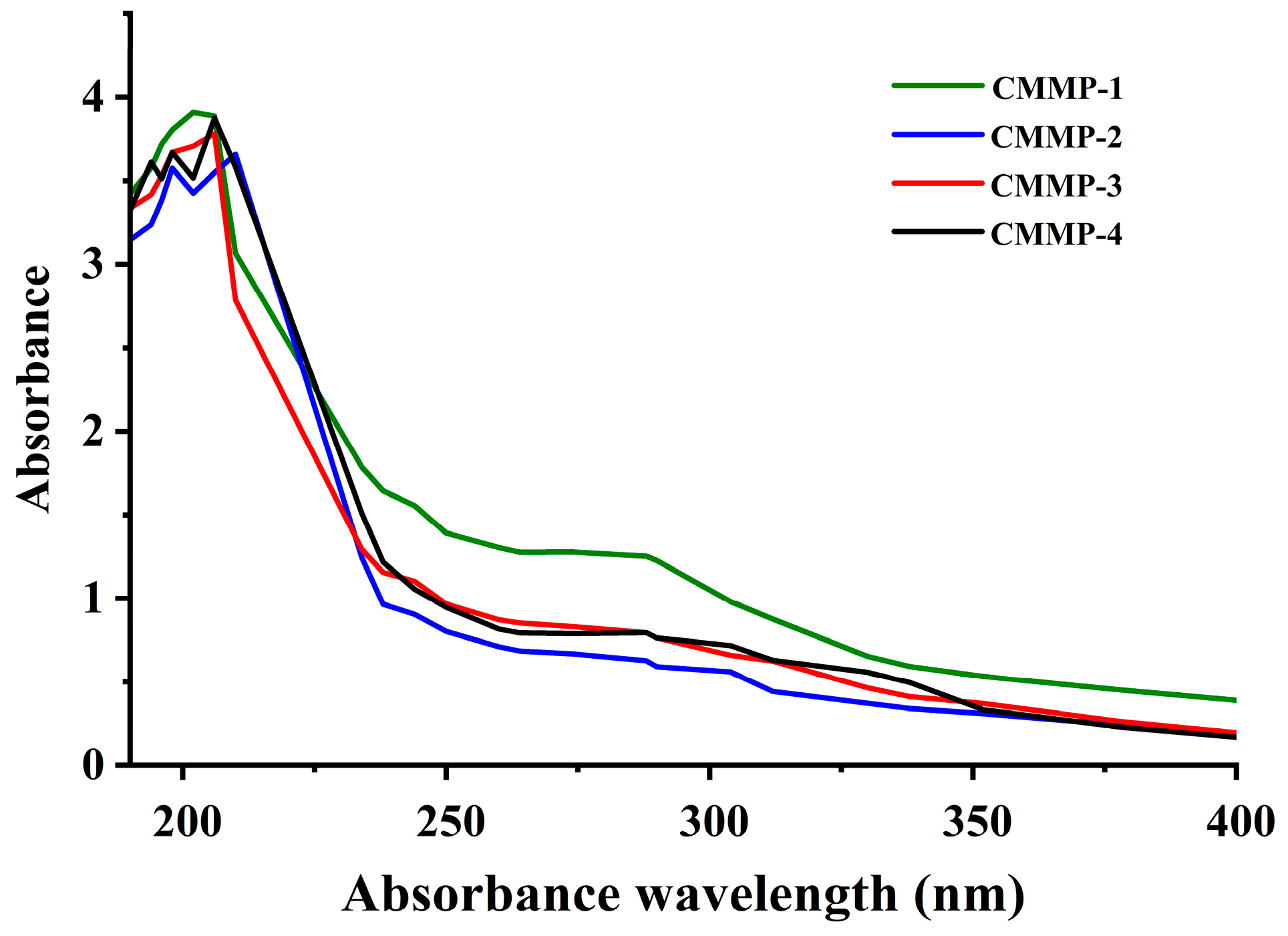
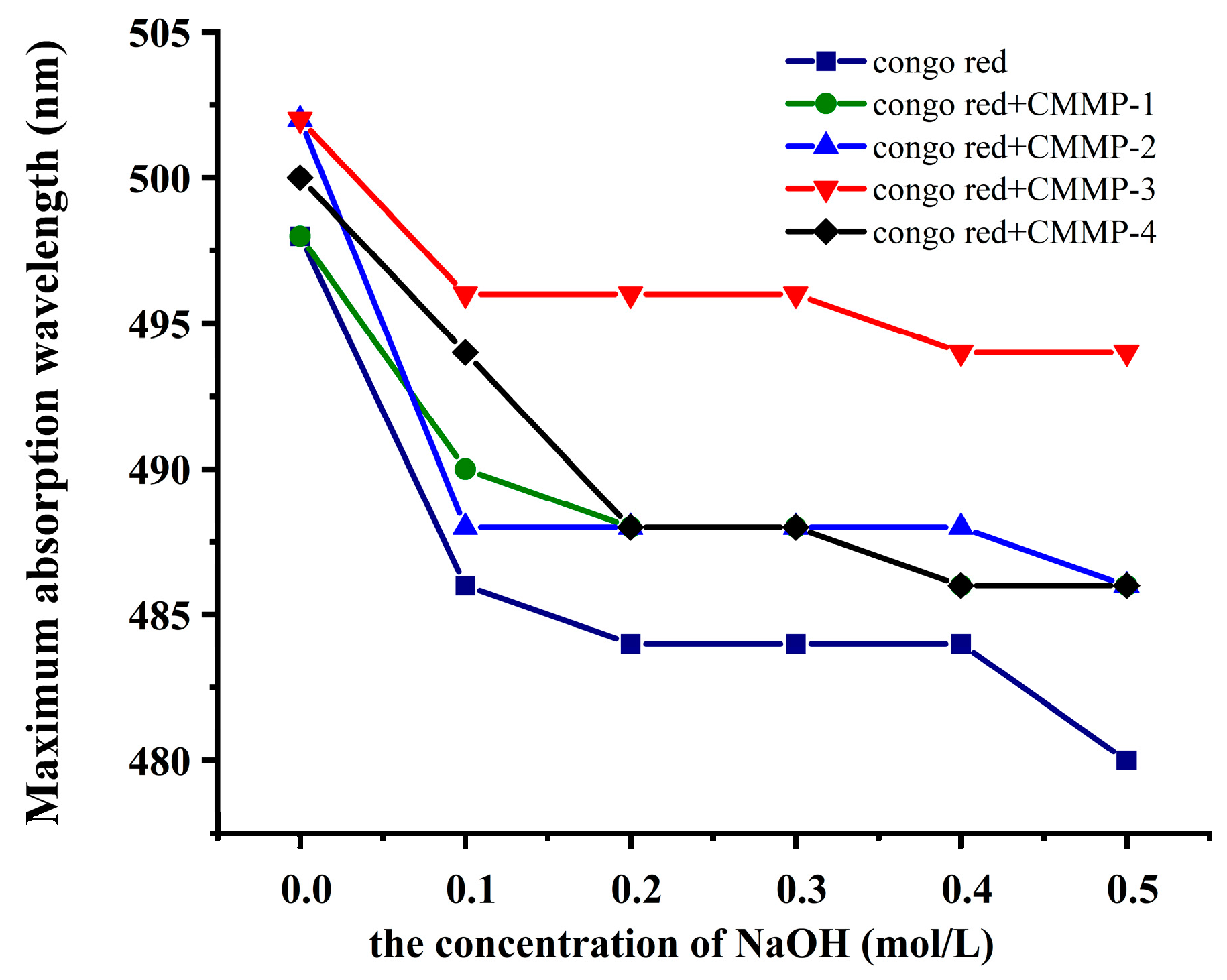

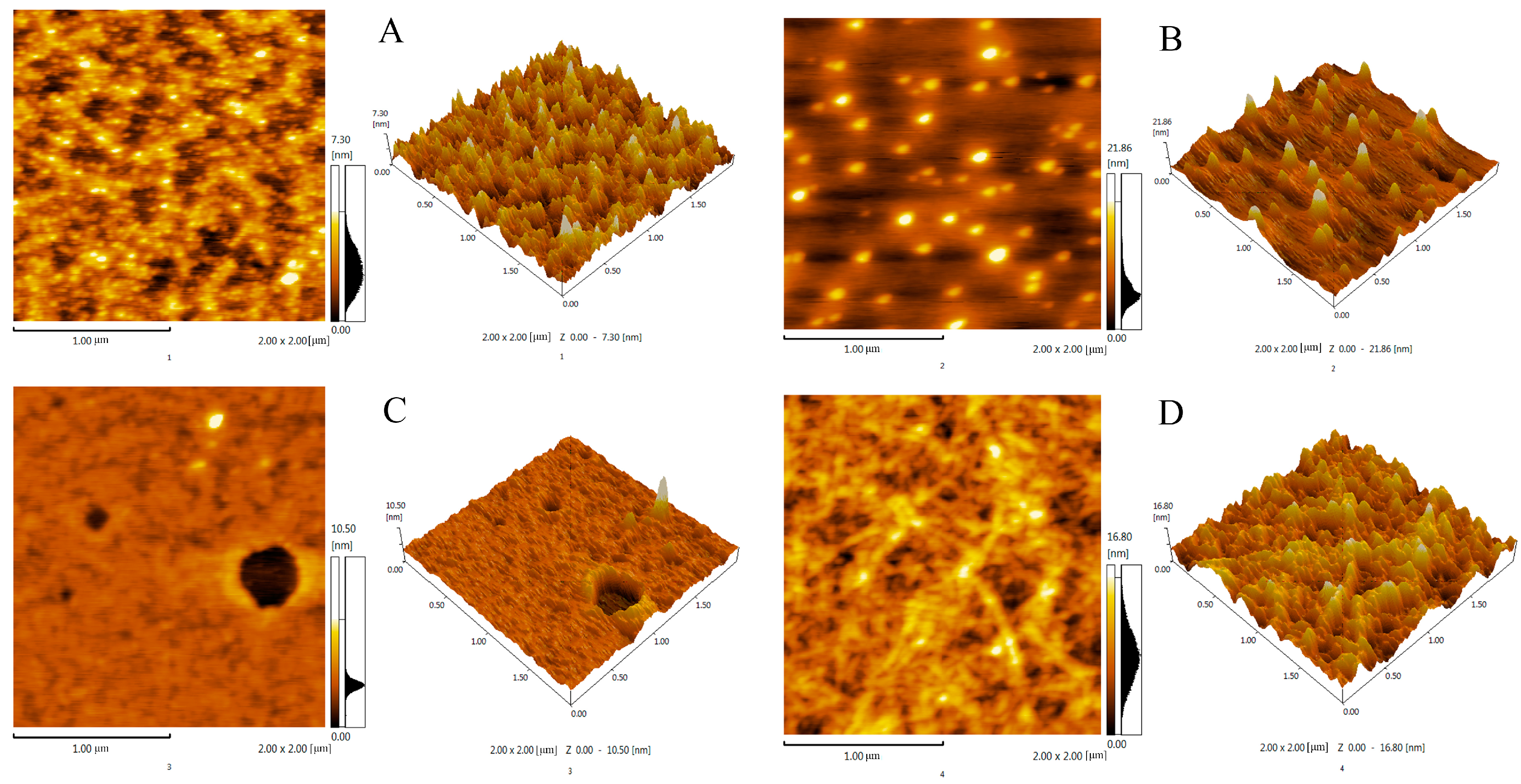


| Run | X1 (min) | X2 (°C) | X3 (mL/g) | X4 (%) | Actual Yield (%) | Predicted Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 70 | 60 | 40:1 | 2 | 10.05 | 9.92 |
| 2 | 60 | 65 | 50:1 | 3 | 14.28 | 14.22 |
| 3 | 60 | 65 | 40:1 | 4 | 13.58 | 13.58 |
| 4 | 70 | 60 | 30:1 | 3 | 11.61 | 11.61 |
| 5 | 60 | 55 | 40:1 | 4 | 13.29 | 13.29 |
| 6 | 50 | 60 | 30:1 | 3 | 11.75 | 11.75 |
| 7 | 70 | 60 | 50:1 | 3 | 12.67 | 12.66 |
| 8 | 60 | 60 | 30:1 | 2 | 10.77 | 10.79 |
| 9 | 70 | 65 | 40:1 | 3 | 12.56 | 12.66 |
| 10 | 60 | 60 | 50:1 | 2 | 12.85 | 12.73 |
| 11 | 60 | 60 | 40:1 | 3 | 15.13 | 15.12 |
| 12 | 60 | 55 | 40:1 | 2 | 12.46 | 12.46 |
| 13 | 50 | 55 | 40:1 | 3 | 12.49 | 12.47 |
| 14 | 60 | 65 | 30:1 | 3 | 13.38 | 13.26 |
| 15 | 60 | 60 | 40:1 | 3 | 15.02 | 15.12 |
| 16 | 60 | 55 | 50:1 | 3 | 13.94 | 14.03 |
| 17 | 60 | 60 | 30:1 | 4 | 12.56 | 12.75 |
| 18 | 60 | 60 | 40:1 | 3 | 15.21 | 15.12 |
| 19 | 70 | 60 | 40:1 | 4 | 12.57 | 12.54 |
| 20 | 50 | 65 | 40:1 | 3 | 12.88 | 12.79 |
| 21 | 60 | 60 | 50:1 | 4 | 12.58 | 12.63 |
| 22 | 60 | 55 | 30:1 | 3 | 13.13 | 13.16 |
| 23 | 50 | 60 | 40:1 | 2 | 11.57 | 11.57 |
| 24 | 50 | 60 | 50:1 | 3 | 12.33 | 12.38 |
| 25 | 50 | 60 | 40:1 | 4 | 10.71 | 10.81 |
| 26 | 60 | 65 | 40:1 | 2 | 12.02 | 12.24 |
| 27 | 70 | 55 | 40:1 | 3 | 12.52 | 12.69 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 41.98 | 14 | 3 | 117.37 | <0.0001 ** |
| X1—extraction time | 0.0052 | 1 | 0.0052 | 0.2039 | 0.6597 |
| X2—extraction temperature | 0.0631 | 1 | 0.0631 | 2.47 | 0.1421 |
| X3—solid–liquid ratio | 2.48 | 1 | 2.48 | 96.88 | <0.0001 ** |
| X4—enzyme addition | 2.59 | 1 | 2.59 | 101.2 | <0.0001 ** |
| X1X2 | 0.0306 | 1 | 0.0306 | 1.2 | 0.2951 |
| X1X3 | 0.0576 | 1 | 0.0576 | 2.25 | 0.1591 |
| X1X4 | 2.86 | 1 | 2.86 | 111.79 | <0.0001 ** |
| X2X3 | 0.002 | 1 | 0.002 | 0.0793 | 0.7831 |
| X2X4 | 0.1332 | 1 | 0.1332 | 5.21 | 0.0414 * |
| X3X4 | 1.06 | 1 | 1.06 | 41.52 | <0.0001 ** |
| X12 | 22.1 | 1 | 22.1 | 865.2 | <0.0001 ** |
| X22 | 1 | 1 | 1 | 39.2 | <0.0001 ** |
| X32 | 5.53 | 1 | 5.53 | 216.48 | <0.0001 ** |
| X42 | 18.72 | 1 | 18.72 | 732.59 | <0.0001 ** |
| Residual | 0.3066 | 12 | 0.0255 | ||
| Lack of fit | 0.2884 | 10 | 0.0288 | 3.17 | 0.2636 |
| Pure error | 0.0182 | 2 | 0.0091 | ||
| Cor total | 42.29 | 26 | |||
| R2 = 0.9928 | R2Adj = 0.9843 | C.V. = 1.25 |
| CMMP−1 | CMMP−2 | CMMP−3 | CMMP−4 | |
|---|---|---|---|---|
| Mn (Da) | 173,376 | 60,355 | 85,571 | 64,793 |
| Mw (Da) | 600,804 | 65,753 | 91,513 | 80,570 |
| Dispersion coefficient | 3.465 | 1.089 | 1.069 | 1.243 |
| Single Factor | Fixed Factors |
|---|---|
| Enzyme addition (0, 0.5%, 1%, 2%, 3%, 4%) | 30:1 v/m, 50 min, 55 °C, 600 W |
| Liquid–solid ratio (10:1, 20:1, 30:1, 40:1, 50:1, 60:1 v/m) | 3%, 50 min, 55 °C, 600 W |
| Extraction time (30, 40, 50, 60, 70, 80 min) | 3%, 40:1 v/m, 55 °C, 600 W |
| Extraction temperature (40, 45, 50, 55, 60, 65 °C) | 3%, 40:1 v/m, 60 min, 600 W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, J.; Zhang, K.; Guo, Z.; Xu, G.; Huang, L.; Wang, L.; Li, J. Ultrasound-Assisted Enzyme Extraction, Physicochemical Properties and Antioxidant Activity of Polysaccharides from Cordyceps militaris Solid Medium. Molecules 2024, 29, 4560. https://doi.org/10.3390/molecules29194560
Wang X, Zhang J, Zhang K, Guo Z, Xu G, Huang L, Wang L, Li J. Ultrasound-Assisted Enzyme Extraction, Physicochemical Properties and Antioxidant Activity of Polysaccharides from Cordyceps militaris Solid Medium. Molecules. 2024; 29(19):4560. https://doi.org/10.3390/molecules29194560
Chicago/Turabian StyleWang, Xiaoya, Jingyan Zhang, Kang Zhang, Zhiting Guo, Guowei Xu, Liping Huang, Lei Wang, and Jianxi Li. 2024. "Ultrasound-Assisted Enzyme Extraction, Physicochemical Properties and Antioxidant Activity of Polysaccharides from Cordyceps militaris Solid Medium" Molecules 29, no. 19: 4560. https://doi.org/10.3390/molecules29194560
APA StyleWang, X., Zhang, J., Zhang, K., Guo, Z., Xu, G., Huang, L., Wang, L., & Li, J. (2024). Ultrasound-Assisted Enzyme Extraction, Physicochemical Properties and Antioxidant Activity of Polysaccharides from Cordyceps militaris Solid Medium. Molecules, 29(19), 4560. https://doi.org/10.3390/molecules29194560






