Abstract
Polychlorinated naphthalenes (PCNs) are a new type of persistent organic pollutant (POP) characterized by persistence, bioaccumulation, dioxin-like toxicity, and long-range atmospheric transport. Focusing on one type of PCN, monochlorinated naphthalenes (CN-1, CN-2), this study aimed to examine their photodegradation in the environment. In this work, CN-1 and CN-2 were employed as the model pollutants to investigate their photodegradation process under UV-C irradiation. Factors like the pH, initial concentrations of CN-1, and inorganic anions were investigated. Next, the roles of hydroxyl radicals (•OH), superoxide anion radicals (O2•−), and singlet oxygen (1O2) in the photodegradation process were discussed and proposed via theory computation. The results show that the photodegradation of CN-1 and CN-2 follows pseudo-first-order kinetics. Acidic conditions promote the photodegradation of CN-1, while the effects of pH on the photodegradation of CN-2 are not remarkable. Cl−, NO3−, and SO32− accelerate the photodegradation of CN-1, whereas the effect of SO42− and CO32− is not significant. Additionally, the contributions of •OH and O2•− to the photodegradation of CN-1 are 20.47% and 38.80%, while, for CN-2, the contribution is 16.40% and 16.80%, respectively. Moreover, the contribution of 1O2 is 15.7%. Based on DFT calculations, C4 and C6 of the CN-1 benzene ring are prioritized attack sites for •OH, while C2 and C9 of CN-2 are prioritized attack sites.
1. Introduction
Polychlorinated naphthalenes (PCNs) are a group of 75 theoretically possible chlorinated naphthalenes, containing from one to eight chlorine atoms [1]. PCNs have been listed as new persistent organic pollutants (POPs) due to their persistence, long-range transport, bioaccumulation, and potential for causing adverse effects in organisms [2]. Although the manufacture of PCNs has been prohibited in the majority of nations since the 1980s, these persistent organic pollutants continue to be detected within different environmental mediums such as the atmosphere, soil, water, sediments, and biota [3,4,5,6,7]. Nowadays, unintentional releases from thermal treatment such as the incineration of waste and metallurgical processes, from which PCNs are directly released into the atmosphere, are likely the main source of PCNs in the environment [1]. The homologue’s distributed profile is dominated in the atmosphere by less chlorinated naphthalenes, in which trichlorinated naphthalenes (tri-CNs) and tetrachlorinated naphthalenes (tetra-CNs) are the main homologues [8,9,10,11,12,13]. In atmospheric samples from Barcelona (Spain), monochlorinated naphthalenes (mono-CNs) and dichlorinated naphthalenes (di-CNs) are found whose concentrations are several magnitudes higher than other more highly chlorinated naphthalenes, which indicates that mono-CN is also a primary homologue in the atmosphere [14]. Atmospheric dispersion is considered an important pathway for the global distribution of semi-volatile and persistent environmental pollutants due to their long-range atmospheric transport ability [15]. PCNs have also been found in the polar regions, where mono-CNs dominate the total mass of Arctic air samples [16]. Moreover, the long-range transport potentials of several PCN homologs were assessed using QSAR models: high mobility (mono-CNs), relatively high mobility (di-CNs to tetra-CNs), relatively low mobility (pentachlorinated naphthalenes to hexachlorinated naphthalenes), and low mobility (heptachlorinated naphthalenes and octachloronaphthalene). This assessment indicates that mono-CNs could be transported globally in the stratosphere [2], where UV-C irradiation can be an important source for the conversion of mono-CNs.
There are rare reports involving the photodegradation of PCNs. Early studies on PCNs were mainly carried out in organic solvents or mixed organic–water solutions under simulated sunlight. They investigated less chlorinated naphthalenes (mono-CNs to tetra-CNs), octachloronaphthalene, and industrial mixtures of PCNs in methanol, ethanol, n-hexane, cyclohexane, benzene, and 4:1 acetonitrile–water solutions (v/v) under simulated sunlight. They found photoconversion processes of PCNs involving dechlorination and dimerization. Furthermore, methoxynaphthalene and its dimer were identified in methanol, 1-phenyl naphthalene was identified in benzene, and 1-naphthol in acetonitrile–water solution, and these were also found as products of photoconversions. These processes of products’ formation refer to the direct photolysis of the C-Cl bond, and radicals attacking naphthalene or benzene [17,18,19,20,21]. In recent years, the photodegradation of technical PCNs, and Halowax, under solar irradiation was investigated, and it was found that the most highly chlorinated octachloronaphthalene is much more stable than PCNs with a low molecular mass [22]. Moreover, the photodegradation of PCNs including CN-1 in various organic solvents under simulated sunlight by using a 400 W high-pressure mercury lamp as a light source has been reported, and it was found that the photoconversion process of PCNs went through two stages of dechlorination and oxidative ring opening [23]. Also, the photodegradation of 1-chlorinated naphthalene (CN-1), 2-chlorinated naphthalene (CN-2), and 2,3-dichlorinated naphthalenes (CN-10) in water at λ > 280 nm was investigated and it was verifyied that singlet oxygen (1O2) and hydroxyl radicals (•OH) were produced during photodegradation and positively affected the photodegradation process. In addition, possible photodegradation pathways of CN-1, CN-2, and CN-10 in water were proposed [24,25]. However, these reports mainly focus on the photoconversion of PCNs under simulated sunlight, while the mechanisms and processes of PCNs’ photodegradation in water under UV-C irradiation are still unclear.
In this work, the photodegradation of CN-1 and CN-2 under UV-C irradiation was investigated. The roles of •OH and 1O2 in the photodegradation process were identified via radical quenching experiments and the second-order rate constants of CN-1/CN-2 with •OH were measured using a competition method. The effects of pH and common inorganic anions (Cl−, CO32−, SO42−, NO3−, SO32−) on the photodegradation of CN-1 and CN-2 were also considered. The primary photodegradation pathway was proposed using frontier electron density, transition-state theory, and intrinsic reaction coordinates calculations. The result of this study will help to further reveal the mechanism of the photodegradation of PCNs under UV-C irradiation.
2. Results and Discussion
2.1. Comparison of Dark and Light Reactions of CN-1 and CN-2
Figure 1a illustrates the contrast between the light and dark reactions of CN-1. The UV-Vis absorption spectra of CN-1 and CN-2 are presented in Figure S2. In the dark controls, approximately a 10% loss of CN-1 was observed after 60 min due to the volatility of CN-1, while the volatile loss of CN-2 was slightly higher than that of CN-1 due to the low water solubility of CN-2. The photodegradation efficiency of CN-1 after 60 min of irradiation reached 42% under the medium-pressure mercury lamp. The absorption of 0.1 mg L−1 of CN-2 is higher than that of CN-1 at the wavelength band of 224 nm, as shown in Figure S2. As a result, the photodegradation reaction is strong, leading to about the 75% photodegradation of CN-2 in 60 min. This photodegrading rate and efficiency are similar compared to other chlorinated aromatic pollutants, such as the observed reaction rate constant (kobs) of 2,4-dichlorophenol in UV-C irradiation, which was 0.076 ± 0.004 min−1 [26], and that of 1,4-dichlorobenzene which was degraded to 56% in UV-C irradiation [27].
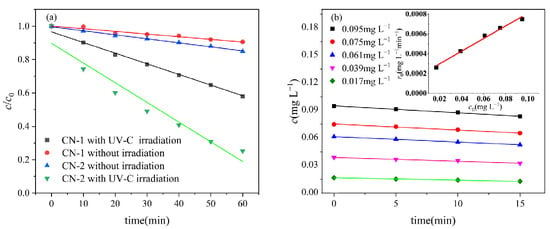
Figure 1.
(a) Comparison of light and dark reactions between CN-1 and CN-2; (b) effect of initial concentration of CN-1 on photodegradation rate. Conditions: [CN-1]0 = 0.615 μmol L−1, [CN-2]0 = 0.615 μmol L−1, 20 °C.
The kinetics of CN–1 photodegradation at different initial concentrations indicated that the photodegradation of CN-1 follows pseudo-first-order kinetics with a kinetic constant of 0.011 min−1, as shown in Figure 1b.
2.2. The pH Effect on the Photodegradation of CN-1 and CN-2
The pH value of a solution has varying effects on different systems and, at the same time, the presence of both acid and alkali also exhibits significant differences in the degradation of various substances [28,29]. Research has demonstrated that the pH value has a significant impact on the photodegradation of organic pollutants [30,31]. The effects of the pH were investigated by adjusting the initial pH with perchloric acid (HClO4) and sodium hydroxide (NaOH), shown in Figure 2. The findings indicate that the influence of pH on the photodegradation of CN-2 is not significant, which may be due to the fast reaction rate of CN-2; with pH 3, CN-1 has the fastest photodegradation rate, and the CN-1 photodegradation conversion was promoted under acidic conditions. Nevertheless, alkaline conditions had little effect on its photodegradation. It is reported that the aqueous solution could generate hydrating electrons under irradiation, and that these electrons react with O2 to produce superoxide anion radicals (O2•−) (Equation (1)) [25,32]. Usually at low pH levels, the photosensitization of O2•− to produce hydrogen peroxide (H2O2) is promoted due to a higher H+ abundance (Equation (2)) [33], and, subsequently, H2O2 photolysis produces more •OH (Equation (3)) [34]. The •OH is highly oxidizing and reacts quickly with the target pollutants. Under alkaline conditions, the self-scavenging effect can produce H2O2 (Equation (4)), which may reduce the removal efficiency [35]. Therefore, under acidic conditions, •OH can promote the photodegradation and transformation of CN-1. We reached a similar conclusion in regards to the photodegradation of polycyclic aromatic hydrocarbons, with •OH exhibiting a higher oxidation potential under low pH conditions, and we noted that, under acidic conditions, the degradation rate of phenanthrene (PHE) accelerates [33].
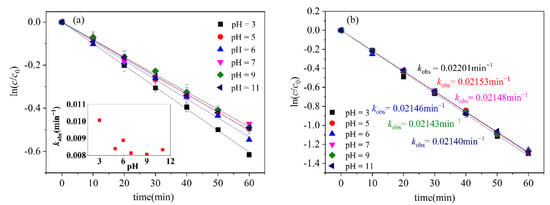
Figure 2.
Effect of different pH on photodegradation of (a) CN-1, (b) CN-2. Conditions: [CN-1]0 = 0.615 μmol L−1, [CN-2]0 = 0.615 μmol L−1, 20 °C.
2.3. Role of •OH, O2•− and 1O2
•OH is recognized as a highly oxidizing free radical that can participate in the non-selective degradation of many organic compounds at a very high reaction rate [36,37]. •OH was quenched by adding isopropanol (IPA) as a scavenger during the photodegradation of CN-1 and CN-2 [38]. IPA has strong reactivity towards •OH, with a second-order reaction rate of k (IPA, •OH) = 1.9 × 109 L mol−1s−1 [39,40]. It can be seen from Figure 3 that, with an increase in IPA concentration, the rate of the photodegradation of CN-1 and CN-2 decreased gradually. Also, the inhibition effect of IPA on the photodegradation of CN-1 and CN-2 remained stable and almost unchanged when the concentration of IPA was increased to 3074.65 μmol L−1. As a result, the contribution of •OH to CN-1 degradation was 20.47%, and that for CN-2 was 16.40%, respectively. However, the influence of IPA on the photodegradation of CN-1 and CN-2 was not obvious because of the lower amount of •OH produced during the photodegradation of CN-1 and CN-2.
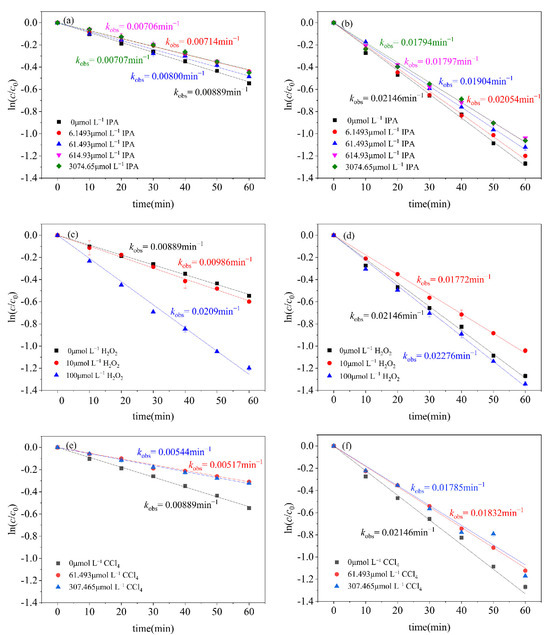
Figure 3.
Effects of IPA on photodegradation of (a) CN-1, (b) CN-2; effects of H2O2 on photodegradation reaction of (c) CN-1, (d) CN-2; effects of CCl4 on photodegradation of (e) CN-1, (f) CN-2; conditions: [CN-1]0 = 0.615 μmol L−1, [CN-2]0 = 0.615 μmol L−1, 20 °C.
To further determine the role of •OH in the photodegradation conversion system, H2O2 was added to the CN-1 and CN-2 photoreaction systems. H2O2 is a very simple and stable molecule that serves as a reservoir for •OH, which can be produced by the photolysis of H2O2, as shown in Equation (4) [41]. When the H2O2 concentration was 10 μmol L−1 and 100 μmol L−1, the photodegradation rate of CN-1 increased by 10.91% and 54.7%, respectively, as presented in Figure 3c. With an increase in the H2O2 concentration, the photolysis produced more •OH, which greatly promoted the degradation of CN-1. However, the photodegradation of CN-2 was inhibited by the addition of 10 μmol L−1 H2O2, exhibitng an inhibition rate of 17.42% (Figure 3d), because H2O2 has strong absorption in UV-C and can compete with CN-2 for UV-C irradiation [42]. The amount of H2O2 of 100 μmol L−1 promoted the photodegradation of CN-2 with an acceleration rate of 6.06%, which was determined by the amount of •OH produced by H2O2 photolysis.
As far as we know, there are no second-order rate constants of CN-1 and CN-2 with •OH in the literature. Therefore, we performed a competitive experiment to determine the rate constants. Rhodamine B (RhB) was selected as the competent, and the values of k (CN-1, •OH) and k (CN-2, •OH) were calculated as 1.15 × 1010 L mol−1 s−1 and 1.9 × 1010 L mol−1s−1, respectively (Figure S3).
O2•− has a good oxidizing ability for organic compounds [43] and, with UV irradiation, monochlorinated naphthalenes could elect electrons trapped by oxygen in an aerated medium to generate O2•−, which is similar to the photosensitive reaction of naphthalene [44]. Carbon tetrachloride (CCl4), an effective electron acceptor, can hinder the acceptance of electrons by dissolved oxygen to form O2•−. Therefore, CCl4 has been used as a scavenger for O2•− in our experiments. From Figure 3e,f, it can be seen that, as the concentration of CCl4 increases, the kobs gradually decrease. When the concentration increases to 307.465 μmol L−1, the kobs gradually stabilizes; the contribution of O2•− to CN-1 was 38.80%, and that on CN-2 was 16.80%, respectively.
An EPR analysis with DMPO as spin-trapping agents was further performed to recognize the •OH and O2•− produced in this system. The DMPO-•OH (1:2:2:1 with αN = 14.8 G, αH = 14.6 G) [45] values were detected and are shown in Figure 4a. Strong signals were found for DMPO-O2•− adducts (αN = 14.2 G, αH = 11.2 G, and αH = 1.3 G) [46] in the EPR spectrum (Figure 4b), suggesting that O2•− could stem from the addition of CN-1, while, without CN-1, the O2•− signals were not found. This confirms what was mentioned earlier with regard UV irradiation: monochlorinated naphthalenes will undergo photosensitization to produce O2•− (Figure S4).
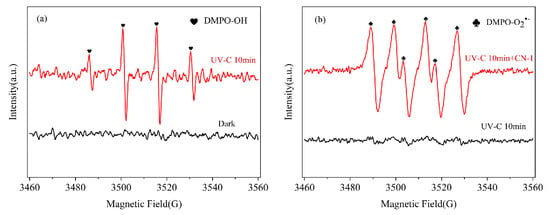
Figure 4.
EPR spectra for different systems with (a) DMPO-•OH; (b) DMPO-O2•−.
1O2 is a highly reactive form of molecular oxygen and is recognized as a strong oxidizing agent. To investigate its effects, 1O2 was quenched by adding furfuryl alcohol (FAA) and sodium azide (NaN3) as scavengers during the photodegradation of CN-1 (k (FFA-1O2) = 1.2 × 108 M−1s− 1, k (NaN3-1O2) = 2 × 109 M−1s−1) [47,48]. When the concentration of added FFA was from 6.15 to 307.465 μmol L−1, it had little effect on the reaction, with only 10% being inhibited (Figure S5a). Furthermore, when increasing the concentration of NaN3, the photodegradation rate of CN-1 decreases. The inhibition of the CN-1 photodegradation by NaN3 reached its limit when the concentration of NaN3 increased to 1229.86 μmol L−1 (Figure S5b), where the inhibition ratio was 15.7% for its addition to 1O2.
2.4. The Influence of Typical Inorganic Ions on CN-1 in Natural Water
Natural water contains common inorganic ions such as Cl−, CO32−, SO42−, and NO3− [49], some of which are photoactive and can produce reactive species to carry out the photodegradation of pollutants [50].
The impact of Cl− on the photodegradation process was examined, as illustrated in Figure 5a, with both 10 and 100 μmol L−1 of Cl− accelerating the photodegradation of CN-1. This could be caused by the generation of chlorine radicals (Cl•) which accelerate the degradation of CN-1. Specifically, Cl− can be converted into the free radical •HOCl−, which then forms Cl• radicals that promote the degradation of CN-1 (Equations (5) and (6)) [51]. Some studies show that Cl• radicals formed from Cl− can accelerate the degradation of PHE. Additionally, it was found that the photochemical degradation rate of PHE rises with the elevation of Cl− levels [51,52].
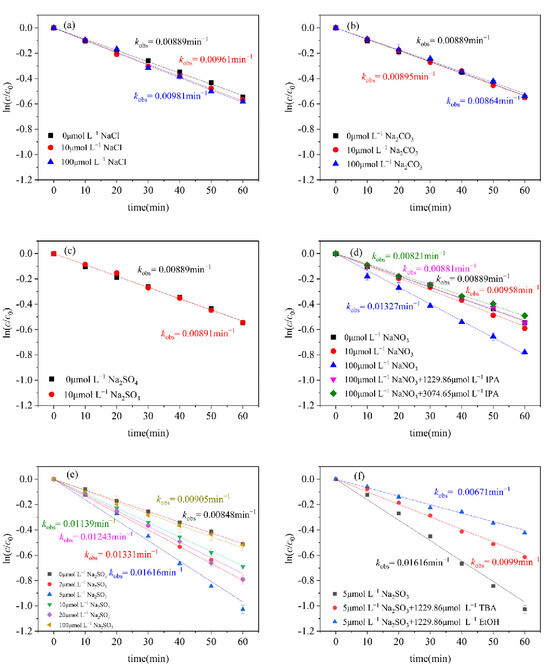
Figure 5.
Effect of different substances on the photodegradation of CN–. (a) NaCl; (b) Na2CO3; (c) NaSO4; (d) NaNO3; (e) Na2SO3; (f) effects of TBA and EtOH on the photodegradation of CN-1 with Na2SO3. Conditions: [CN-1]0 = 0.615 μmol L−1, 20 °C.
When adding different concentrations of Na2CO3 or NaSO4, the rate constant of CN-1 remains unchanged, indicating that the effect of CO32− and SO42− on the photocatalytic conversion of CN-1 is not significant (Figure 5b,c).
NO3−, as a photosensitizer, has a significant effect on the photodegradation of CN-1, as shown in Figure 5d. When elevating the NO3− concentration from 10 to 100 μmol L−1, the kobs of degradation was strengthened from 0.00958 min−1 to 0.01327 min−1. This intensification can be attributed to the amount of •OH generated through the photolysis of NO3−, which promotes the degradation of CN-1 (Equation (7)) [53]. NO3− can significantly promote the light conversion of CN-1, as demonstrated in a previous study [23]. Some reports have also confirmed that the production of •OH under sunlight has a positive correlation with the nitrate concentration [33,54]. To further attest the effects of •OH produced during the NO3− photolysis process, IPA was used as the scavenger, the results of which are shown in Figure 5d. By adding 1229.86 μmol L−1 and 3074.65 μmol L−1 of IPA, the promoting effects of NO3− were almost completely inhibited, which further proves that •OH produced by photosensitive NO3− promotes the photodegradation of CN-1.
Additionally, SO32− can be present in atmospheric droplets during acid rain processes [55,56] and, thus, could contribute to the photodegradation of CN-1. Because of this, Na2SO3 was added to investigate the effect of SO32− on the photodegradation of CN-1. The result (Figure 5e) shows that, when the presence of Na2SO3 increases from 0 μmol L−1 to 5 μmol L−1, the kobs gradually increases, with the CN-1 degradation efficiency able to rise to 90.56% at its best performance, which is attributed to the increased reactivity of the sulfur oxygen species. SO32− activated by UV light will produce SO3•− and hydrated electrons (eaq−) (Equation (8)), and eaq− can react quickly with O2 in water, forming O2•− (Equation (9)) [57]. Ultimately, the eaq− will transform into H2O2 (Equations (10)-(12)) [58]. When SO3•− reacts with O2 to generate SO5•−, it triggers a series of chain reactions in which SO4•− and •OH as the oxidative radicals are formed therein and play a promoting role in the photodegradation process (Equations (13)–(16)) [58]. When further elevating the SO32− concentration from 5 to 100 μmol L−1, the efficiency is lower, which is due to excess SO32− reducing the concentrations of SO4•− through Equations (17)–(19) [59].
Ethanol (EtOH) can react rapidly with SO4•− (k = 1.6 × 107–7.7 × 107 M−1s−1) and •OH (k = 1.2 × 109 − 2.8 × 109 M−1s−1), while tert-butanol (TBA) mainly reacts with •OH, with a rate constant (k = 3.8 × 108 − 7.6 × 108 M−1s−1) that is significantly higher than that of the reaction with SO4•− (k = 4 × 105 − 9.1 × 105 M−1s−1) [60]. Hence, to ascertain the roles of SO4•− and •OH, EtOH and TBA were used as radical scavengers in the photodegradation of CN–1 during the photolysis process of SO32−. As a result, it was found that TBA selectively captures only •OH, while EtOH captures both SO4•−and •OH. As shown in Figure S6a, as the concentration of TBA in the system increases from 61.493 to 307.465 μmol L−1, the kobs gradually decreases from 0.01616 to 0.01036 min−1. When the concentration of TBA increases from 307.465 μmol L−1 to 1229.86 μmol L−1, there is no significant change in the kobs, indicating that sufficient TBA completely inhibits •OH. After increasing the concentration of TBA from 1229.86 to 4949.14 μmol L−1, the kobs slightly changes, indicating that excessive TBA inhibits the effects of SO4•−. As shown in Figure S6b, it is evident that, as the concentration of EtOH in the system ascends, the kobs descends remarkably, down to 0.00671 min−1 with 1299.86 μmol L−1 EtOH. This is attributed to the fact that •OH and SO4•− are completely inhibited by EtOH during the photolysis of SO32−. As shown in Figure 5f, the contributions of SO4•− and •OH can be further calculated to show that the contribution of •OH to the photodegradation of CN-1 was 38.74%, while the contribution of SO4•− to the photodegradation of CN-1 was 19.74%.
2.5. Photodgrdation of 1-Naphthol and Naphthalene
It is known from previous studies that the two products of CN-1 photodegradation are 1-naphthol and naphthalene [24,25]. However, the photodegradation products of CN-1 were not detected by gas chromatography-mass spectrometry (GC-MS) in this experiment. The photodegradation experiments for naphthalene and 1-naphthol were carried out for 0, 10, 20, 30, 40, 50, and 60 min, with the 1.5 mL photodegradation samples being determined by liquid chromatography. The kobs of the photodegradation of naphthalene is 0.01667 min−1, and that of 1-naphthol is 0.06646 min−1 (Figure S7). Given identical circumstances, the degradation rates of naphthalene and 1-naphthol are much faster than that of CN-1. When processing the photodegradation of CN-1, the productions of naphthalene and 1-naphthol were quickly converted into smaller substances, so the productions of naphthalene and 1-naphthol were not detected in gas chromatography-mass spectrometry.
2.6. Density Functional Theory (DFT)
DFT has a wide range of applications and is an invaluable tool in scientific research, providing information on the structure and properties of compounds while further exploring their reaction behavior and intrinsic mechanisms [61,62]. DFT calculations were used to further determine the relevant reaction mechanisms of CN-1 and CN-2 and to explore the products and degradation pathways.
According to the frontier electron density (FED) the chemistry of the molecule is closely related between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), with electrophilic reactions being most likely to occur at 2FED2HOMO and atoms with larger charge distributions, while free radicals are prone to attacking the higher positions of FED2HOMO + FED2LUMO [63,64]. As can be seen from Table 1, the highest value of FED2HOMO + FED2LUMO among the unsaturated carbons occurs at C4 (0.26946), followed by the C1 (0.25793), C9 (0.23643), and C6 (0.2356) atoms. This result suggests that these carbons are susceptible to free radical attack. In CN-2, the FED2HOMO + FED2LUMO values of the C1, C9, C4, and C6 sites are higher, as shown in Table 2, which indicates that the free radicals will preferentially attack the above sites during the reaction process. In CN-1, the values of 2FED2HOMO at C4, C1, and C9 are the highest, which means that electrons at these positions are more vulnerable to electron extraction, while, in CN-2, the C1, C9, and C6 sites are more vulnerable to electron extraction [65]. The changes in energy of CN-1 and CN-2 during their degradation by •OH were additionally assessed using TS theory, with the results depicted in Figure 6. CN-1 has eight reaction sites in the benzene ring, namely C1–C4 and C6–C9 [66]. The lower the reaction barriers (ΔrG), the easier it is to attack [67], and the energies of TS4 and TS6 are 0.12 kcal mol−1 and 0.14 kcal mol−1, which are very close in proximity. Therefore, the reaction between •OH and CN-1 will prioritize attacking C4 and C6 connected to the benzene ring. In the reaction of •OH with CN-2, the energies of TS2 and TS9 are 0.0081 kcal mol−1 and 0.0082 kcal mol−1, respectively. Therefore, C2 and C9 are attacked first, with the substitution reaction between •OH and Cl occurring at the C2 site, while those at all other C sites are addition reactions. Research indicates that the •OH addition route possesses a lower energy barrier and is more likely to take place compared to the H abstraction route [68], while •OH with C-Cl prefers substitution in its reactions [69,70].

Table 1.
Results of frontier electron density for CN-1.

Table 2.
Results of frontier electron density for CN-2.
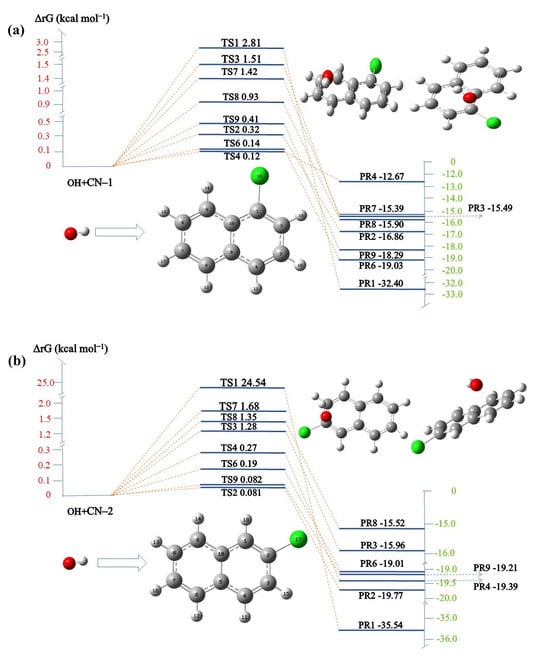
Figure 6.
Calculated reaction barriers (ΔrG) for the •OH; (a) CN-1; (b) CN-2.
Based on the FED results and TS theory, it can be surmised that free radicals will show a propensity for attacking the benzene ring moieties of CN-1 and CN-2. This preferential attack induces the benzene rings to open. Nevertheless, the abundance of potential attack sites leads to an overly rapid and extensive fragmentation during the ring opening of CN-1 and CN-2, resulting in fragmentation patterns that are elusive to detection by techniques such as gas chromatography-mass spectrometry (GC-MS) [71]. Thus, it is crucial to comprehensively examine the reaction mechanisms of CN-1 and CN-2 with the •OH group.
3. Materials and Methods
3.1. Reagents
In this study, CN-1 (98% purity), CN-2 (98% purity), 1-naphthol (98% purity), naphthalene (98% purity), IPA, NaN3, FFA, H2O2 (30%, v/v), HClO4, NaOH, NaCl, NaSO4, Na2CO3, NaNO3, Na2SO3, EtOH, and ferrous sulfate heptahydrate were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The methanol and acetonitrile used are all chromatographically pure, being purchased from Thermo Fisher Scientific Inc. (Fair Lawn, NJ, USA). The RhB and TBA were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). All reagents with no specification are analytical grades. Ultrapure water (≥18.2 MΩ cm) was used throughout the entire experiment. The detailed usage information of the reagents is listed in the Supplementary Materials (Text S1).
3.2. Photodegradation Experiments
The photochemical degradation experiments were carried out in a 500 mL self-designed photodegradation device, which was previously used for the photodegradation of hexabromocyclododecane (HBCD) [42], as shown in Figure S1. A 14 W UV-C lamp immersed directly in the 500 mL reaction solution was used as the light source, with the part of the lamp above the liquid level wrapped with tin foil. Reactions were initiated by adding 0.615 μmol L−1 CN-1/CN-2 into solutions containing acetonitrile–water (1‰, v/v). Except when otherwise mentioned, experiments were performed at room temperature (20 ± 2 °C) under open atmospheric conditions. At specific time intervals, every 10 min, a 2 mL sample was then taken out and analyzed with HPLC. Each set of experiments was repeated at least in triplicate, with the mean values with error bars being calculated and reported.
3.3. Competitive Reaction
A competitive reaction was designed to determine the second-order rate constants of CN-1 and CN-2 with •OH. RhB was employed as the competitive chemical to react with •OH. The detailed experimental methods are described in our previous study [42].
3.4. Samples Analysis
High-performance liquid chromatography (HPLC) was utilized to analyze the content of CN-1 and CN-2 in the sample. The sample was separated with a reversed-phase C18 column (250 mm × 4.6 mm, SHIMADZU (China) Co., Ltd., Shanghai, PRC) at 25 °C column temperature. The mobile phase consisted of 90% methanol and 10% water, with a flow rate of 1 mL min−1 and detection at a wavelength of 225 nm. The UV absorption spectra of CN-1 and CN-2, as well as the concentration of Rhodamine B, were determined using an ultraviolet spectrophotometer (Varian Technology China Co., Ltd., Beijing, China).
3.5. Density Functional Theory (DFT) Calculation
Optimization of the CN-1 and CN-2 molecular structure was performed with density functional theory (DFT)/B3LYP/6-311G(2d, p) level via Gaussian 09 software [72]. After optimization, Multiwfn Version 3.8 software was employed to calculate the contribution of each atomic orbital for frontier electron density (FED) analyses (HOMO and LUMO) [73] and to predict the most reactive part for degradation of CN-1 and CN-2, and the results were visualized and analyzed with the help of the Gaussview 6.0 program.
Transition states (TSs) were searched by the pseudo-reaction coordinate method and further confirmed by intrinsic reaction coordinate (IRC) analysis. Vibrational frequency calculations were performed to ensure a true energy minimum, and IRC analyses were to confirm a first-order saddle point [70]. From IRC analysis, the initial guess structures of reactant complexes (REs) and product complexes (PRs) were also obtained, which were subsequently optimized with the same method and basis set of CN-1 and CN-2. The plausibility of the reaction and the prior reaction channel were determined by the reaction energy barriers (ΔrG, ΔrG = GTS-GRE) [74].
4. Conclusions
Monochlorinated naphthalene (CN-1, CN-2) can be photodegraded under UV-C irradiation, with this photodegradation following the pseudo-first-order kinetics. In acidic conditions, the photodegradation of CN-1 accelerates while, in alkaline conditions, the photodegradation is almost unaffected. The second-order rate constant between CN-1 and •OH (k (CN-1, •OH)) was determined to be 1.15 × 1010 L mol−1s−1, while the k (CN-2, •OH) was determined to be 1.9 × 1010 L mol−1s−1. The contributions of •OH and O2•− to CN-1 degradation were 20.47% and 38.80%, those for CN-2 were 16.40% and 16.80%, respectively, and the contribution to 1O2 was 15.7%. Additionally, Cl−, NO3−, and SO32− can enhance the photodegradation efficiency of CN-1 and CN-2, while the effects of SO42− and CO32− were not significant. Through DFT calculation, it can be determined that •OH reacts the most favorably with the C4 and C6 sites in CN-1, whereas •OH reacts the most favorably with the C2 and C9 sites in CN-2.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29194535/s1, Figure S1 Photodegradation device. Figure S2 UV-C lamp emission spectrum, UV absorption spectra of CN-1 and CN-2. Figure S3 Concentration variation in a competitive reaction (a) CN-1 and RhB; (b) CN-2 and RhB. Figure S4 Scheme for generating O2•− by monochlorinated naphthalene under UV irradiation. Figure S5 Effects of 1O2 scavengers on photodegradation of CN-1 (a) FFA (b) NaN3. Figure S6 Effects of scavengers on the photodegradation of CN-1 with Na2SO3(a) TBA; (b) EtOH. Figure S7 photodegradation experiment (a) naphthalene (b) 1-naphthol. Text S1. Specific use of reagents.
Author Contributions
S.W., C.Z. and X.Z. performed the photodegradation experiments; Y.Y. and S.X. conceived and designed the experiments; Y.Y. and M.L. analyzed the data; Y.Y. and L.L. provided funding support; Y.Y. and M.L. participated in drafting the article; all authors revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number No. 41703110, Basic Scientific Research Projects of Liaoning Province Education Department, grant number LJKMZ20220440, Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, grant number No. ES202309, and the Fundamental Research Funds for Public Universities in Liaoning, grant number LJKLJ202403.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. The materials used in the study are commercially available and can be purchased from the relevant firms.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 41703110), Basic Scientific Research Projects of Liaoning Province Education Department (LJKMZ20220440), Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No. ES202309) and the Fundamental Research Funds for Public Universities in Liaoning (LJKLJ202403). Comments from anonymous reviewers are also appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Die, Q.; Nie, Z.; Fang, Y.; Yang, Y.; Gao, X.; Tian, Y.; He, J.; Liu, F.; Huang, Q.; Tian, S. Seasonal and Spatial Distributions of Atmospheric Polychlorinated Naphthalenes in Shanghai, China. Chemosphere 2016, 144, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, W.; Guo, E.; Cui, C.; Li, Y. Assessment of Long-Range Transport Potential of Polychlorinated Naphthalenes Based on Three-Dimensional QSAR Models. Environ. Sci. Pollut. Res. 2017, 24, 14802–14818. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, M.; Adali, M. Determination of Temperature Dependent Henry’s Law Constants of Polychlorinated Naphthalenes: Application to Air-Sea Exchange in Izmir Bay, Turkey. Atmos. Environ. 2016, 147, 200–208. [Google Scholar] [CrossRef]
- Schuhmacher, M.; Nadal, M.; Domingo, J.L. Levels of PCDD/Fs, PCBs, and PCNs in Soils and Vegetation in an Area with Chemical and Petrochemical Industries. Environ. Sci. Technol. 2004, 38, 1960–1969. [Google Scholar] [CrossRef]
- Guo, W.; He, M.; Yang, Z.; Lin, C.; Quan, X.; Wang, H. Distribution of Polycyclic Aromatic Hydrocarbons in Water, Suspended Particulate Matter and Sediment from Daliao River Watershed, China. Chemosphere 2007, 68, 93–104. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Mortimer, D.; Holmes, M.; Rose, M.; Zhihua, L.; Huang, X.; Smith, F.; Panton, S.; Marshall, L. Occurrence and Spatial Distribution of Chemical Contaminants in Edible Fish Species Collected from UK and Proximate Marine Waters. Environ. Int. 2018, 114, 219–230. [Google Scholar] [CrossRef]
- Lega, R.; Megson, D.; Hartley, C.; Crozier, P.; MacPherson, K.; Kolic, T.; Helm, P.A.; Myers, A.; Bhavsar, S.P.; Reiner, E.J. Congener Specific Determination of Polychlorinated Naphthalenes in Sediment and Biota by Gas Chromatography High Resolution Mass Spectrometry. J. Chromatogr. A 2017, 1479, 169–176. [Google Scholar] [CrossRef]
- Lee, S.C.; Harner, T.; Pozo, K.; Shoeib, M.; Wania, F.; Muir, D.C.G.; Barrie, L.A.; Jones, K.C. Polychlorinated Naphthalenes in the Global Atmospheric Passive Sampling (GAPS) Study. Environ. Sci. Technol. 2007, 41, 2680–2687. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Chakraborty, P.; Syed, J.H.; Malik, R.N.; Wang, Y.; Tian, C.; Luo, C.; Zhang, G.; Jones, K.C. Atmospheric Polychlorinated Naphthalenes (PCNs) in India and Pakistan. Sci. Total Environ. 2014, 466–467, 1030–1036. [Google Scholar] [CrossRef]
- Hogarh, J.N.; Seike, N.; Kobara, Y.; Masunaga, S. Atmospheric Polychlorinated Naphthalenes in Ghana. Environ. Sci. Technol. 2012, 46, 2600–2606. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, L.; Yan, Y.; Dong, L.; Huang, Y.; Li, X. Concentrations and Patterns of Polychlorinated Naphthalenes in Urban Air in Beijing, China. Chemosphere 2016, 162, 199–207. [Google Scholar] [CrossRef]
- Herbert, B.M.J.; Halsall, C.J.; Villa, S.; Fitzpatrick, L.; Jones, K.C.; Lee, R.G.M.; Kallenborn, R. Polychlorinated Naphthalenes in Air and Snow in the Norwegian Arctic: A Local Source or an Eastern Arctic Phenomenon? Sci. Total Environ. 2005, 342, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Helm, P.A.; Bidleman, T.F.; Li, H.H.; Fellin, P. Seasonal and Spatial Variation of Polychlorinated Naphthalenes and Non-/Mono-Ortho-Substituted Polychlorinated Biphenyls in Arctic Air. Environ. Sci. Technol. 2004, 38, 5514–5521. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Schuhmacher, M.; Feliubadaló, J.; Domingo, J.L. Air Concentrations of PCDD/Fs, PCBs and PCNs Using Active and Passive Air Samplers. Chemosphere 2008, 70, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Orlikowska, A.; Hanari, N.; Wyrzykowska, B.; Bochentin, I.; Horii, Y.; Yamashita, N.; Falandysz, J. Airborne Chloronaphthalenes in Scots Pine Needles of Poland. Chemosphere 2009, 75, 1196–1205. [Google Scholar] [CrossRef]
- Gebru, T.B.; Li, Y.; Dong, C.; Yang, Y.; Yang, R.; Pei, Z.; Zhang, Q.; Jiang, G. Spatial and Temporal Trends of Polychlorinated Naphthalenes in the Arctic Atmosphere at Ny-Ålesund and London Island, Svalbard. Sci. Total Environ. 2023, 878, 163023. [Google Scholar] [CrossRef] [PubMed]
- Gulan, M.P.; Bills, D.D.; Putnam, T.B. Analysis of Polychlorinated Naphthalenes by Gas Chromatography and Ultraviolet Irradiation. Bull. Environ. Contam. Toxicol. 1974, 11, 438–441. [Google Scholar] [CrossRef]
- Ruzo, L.O.; Bunce, N.J.; Safe, S. Photoreactions of Simple Halonaphthalenes in Solution. Can. J. Chem. 1975, 53, 688–693. [Google Scholar] [CrossRef]
- Ruzo, L.O.; Bunce, N.J.; Safe, S.; Hutzinger, O. Photodegradation of Polychloronaphthalenes in Methanol Solution. Bull. Environ. Contam. Toxicol. 1975, 14, 341–345. [Google Scholar] [CrossRef]
- Järnberg, U.G.; Asplund, L.T.; Egebäck, A.-L.; Jansson, B.; Unger, M.; Wideqvist, U. Polychlorinated Naphthalene Congener Profiles in Background Sediments Compared to a Degraded Halowax 1014 Technical Mixture. Environ. Sci. Technol. 1999, 33, 1–6. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Li, Q.X. Photolysis of Octachloronaphthalene in Hexane. Bull. Environ. Contam. Toxicol. 2004, 72, 999. [Google Scholar] [CrossRef] [PubMed]
- Hanari, N.; Falandysz, J.; Yamazaki, E.; Yamashita, N. Photodegradation of Polychlorinated Naphthalene in Mixtures. Environ. Pollut. 2020, 263, 114672. [Google Scholar] [CrossRef]
- Kang, Q.; Bao, S.; Chen, B. Photoconversion of Polychlorinated Naphthalenes in Organic Solvents under Simulated Sunlight: Solvent Effect and Mechanism. Chemosphere 2021, 272, 129887. [Google Scholar] [CrossRef]
- Kang, C.; Bao, S.; Wang, Y.; Xiao, K.; Zhu, L.; Liu, F.; Tian, T. Comparison of the Photoconversion of 1-Chloronaphthalene and 2,3-Dichlornaphthalene in Water. Water Sci. Technol. 2018, 78, 1946–1955. [Google Scholar] [CrossRef]
- Kang, C.; Bao, S.; Chen, B.; Zhong, Y.; Huang, D.; Wang, Y.; Xue, H.; Tian, T. Photoconversion of 2-Chloronaphthalene in Water. Bull. Environ. Contam. Toxicol. 2017, 99, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Karci, A.; Arslan-Alaton, I.; Olmez-Hanci, T.; Bekbolet, M. Degradation and Detoxification of Industrially Important Phenol Derivatives in Water by Direct UV-C Photolysis and H2O2/UV-C Process: A Comparative Study. Chem. Eng. J. 2013, 224, 4–9. [Google Scholar] [CrossRef]
- Real, F.J.; Benitez, F.J.; Rodríguez, C. Elimination of Benzene and Chlorobenzenes by Photodegradation and Ozonation Processes. Chem. Eng. Commun. 2007, 194, 811–827. [Google Scholar] [CrossRef]
- Tian, C.; Huang, W.; Wei, Z.; Liang, C.; Dong, Y.; Shi, J.; Zhang, X.; Nong, G.; Wang, S.; Xu, J. Photocatalytic Degradation of Different Antibiotics Using TiO2 –Carbon Composites: A Case Study of Tetracycline and Ciprofloxacin. New J. Chem. 2023, 47, 19646–19656. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, W.; Wang, H.; Pan, C.; Xu, J.; Pozdnyakov, I.P.; Wu, F.; Li, J. Interaction between Graphene Oxide and Acetaminophen in Water under Simulated Sunlight: Implications for Environmental Photochemistry of PPCPs. Water Res. 2023, 228, 119364. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, Y.; Liu, T.; Li, R.; Li, R.; Zhu, Y.; Fang, F. Zn-Doped MnCO3/CS Composite Photocatalyst for Visible-Light-Driven Decomposition of Organic Pollutants. Molecules 2024, 29, 1094. [Google Scholar] [CrossRef]
- Wang, M.; Shi, H.; Shao, S.; Lu, K.; Wang, H.; Yang, Y.; Gong, Z.; Zuo, Y.; Gao, S. Montmorillonite Promoted Photodegradation of Amlodipine in Natural Water via Formation of Surface Complexes. Chemosphere 2022, 286, 131641. [Google Scholar] [CrossRef] [PubMed]
- Novikov, M.V.; Snytnikova, O.A.; Fedunov, R.G.; Yanshole, V.V.; Grivin, V.P.; Plyusnin, V.F.; Xu, J.; Pozdnyakov, I.P. A New View on the Mechanism of UV Photodegradation of the Tricyclic Antidepressant Carbamazepine in Aqueous Solutions. Chemosphere 2023, 329, 138652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, X.; Fu, J.; Liu, W.; Cai, Z. Elevated Nitrate Promoted Photodegradation of PAHs in Aqueous Phase: Implications for the Increased Nutrient Discharge. J. Hazard. Mater. 2023, 443, 130143. [Google Scholar] [CrossRef]
- Zuo, Y.; Hoigné, J. Evidence for Photochemical Formation of H 2 O 2 and Oxidation of SO2 in Authentic Fog Water. Science 1993, 260, 71–73. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Javed, F.; Sohail, R.; Kazmi, M.; Rehman, A.; Qi, F. Solar Photo-Catalytic Ozonation on γ-Alumina for the Removal of Dyes in Wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 1967–1974. [Google Scholar] [CrossRef]
- Tyutereva, Y.E.; Sherin, P.S.; Polyakova, E.V.; Koscheeva, O.S.; Grivin, V.P.; Plyusnin, V.F.; Shuvaeva, O.V.; Pozdnyakov, I.P. Photodegradation of Para-Arsanilic Acid Mediated by Photolysis of Iron(III) Oxalate Complexes. Chemosphere 2020, 261, 127770. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Li, J.; Wu, F. Natural Montmorillonite Induced Photooxidation of As(III) in Aqueous Suspensions: Roles and Sources of Hydroxyl and Hydroperoxyl/Superoxide Radicals. J. Hazard. Mater. 2013, 260, 255–262. [Google Scholar] [CrossRef]
- Bouddouch, A.; Akhsassi, B.; Amaterz, E.; Bakiz, B.; Taoufyq, A.; Villain, S.; Guinneton, F.; El Aamrani, A.; Gavarri, J.-R.; Benlhachemi, A. Photodegradation under UV Light Irradiation of Various Types and Systems of Organic Pollutants in the Presence of a Performant BiPO4 Photocatalyst. Catalysts 2022, 12, 691. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the Hazardous Applications and Photodegradation Mechanisms of Chlorophenols over Different Photocatalysts. Environ. Res. 2021, 195, 110742. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (•OH/•O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Uzelac, M.M.; Srđenović Čonić, B.; Kladar, N.; Armaković, S.; Armaković, S.J. Removal of Hydrochlorothiazide from Drinking and Environmental Water: Hydrolysis, Direct and Indirect Photolysis. Energy Environ. 2023, 34, 1243–1257. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, D.; Wu, F. Mechanism and Products of the Photolysis of Hexabromocyclododecane in Acetonitrile–Water Solutions under a UV-C Lamp. Chem. Eng. J. 2015, 281, 892–899. [Google Scholar] [CrossRef]
- Xiang, R.; Zhou, C.; Liu, Y.; Qin, T.; Li, D.; Dong, X.; Muddassir, M.; Zhong, A. A New Type Co(II)-Based Photocatalyst for the Nitrofurantoin Antibiotic Degradation. J. Mol. Struct. 2024, 1312, 138501. [Google Scholar] [CrossRef]
- Vialaton, D.; Richard, C.; Baglio, D.; Paya-Perez, A.-B. Mechanism of the Photochemical Transformation of Naphthalene in Water. J. Photochem. Photobiol. Chem. 1999, 123, 15–19. [Google Scholar] [CrossRef]
- Wang, X.; Wei, J.; Zhang, H.; Zhou, P.; Yao, G.; Liu, Y.; Lai, B.; Song, Y. CoFe2O4@BC as a Heterogeneous Catalyst to Sustainably Activate Peroxymonosulfate for Boosted Degradation of Enrofloxacin: Properties, Efficiency and Mechanism. Sep. Purif. Technol. 2024, 345, 127349. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhou, W. Cu-Doped Ni-LDH with Abundant Oxygen Vacancies for Enhanced Methyl 4-Hydroxybenzoate Degradation via Peroxymonosulfate Activation: Key Role of Superoxide Radicals. J. Colloid Interface Sci. 2022, 610, 504–517. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Hao, M.; Yu, F.; Houda, C. Persistent Degradation of 2,4-Dichlorophenol in Groundwater by Persulfate Synergize with Fe(III)/CaSO3 System: Role of Fe(IV) and 1O2 Oxidation. Sep. Purif. Technol. 2024, 334, 125979. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Duan, X.; Wang, Y.; Wu, D.; Pang, J.; Wang, X.; Wang, S. Catalytic Oxidation of Sulfachloropyridazine by MnO2: Effects of Crystalline Phase and Peroxide Oxidants. Chemosphere 2021, 267, 129287. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water—Water Supply Paper, 3rd ed.; US Geological survey: Reston, VA, USA, 1985; pp. 16–17. [Google Scholar]
- Fazli, A.; Brigante, M.; Khataee, A.; Mailhot, G. Fe2.5Co0.3Zn0.2O4/CuCr-LDH as a Visible-Light-Responsive Photocatalyst for the Degradation of Caffeine, Bisphenol A, and Simazine in Pure Water and Real Wastewater under Photo-Fenton-like Degradation Process. Chemosphere 2022, 291, 132920. [Google Scholar] [CrossRef]
- Feng, Y.; Tao, Y.; Qu, J.; Zhang, Y. S-Scheme P-Doped g-C3N4/BiOBr Heterojunction with Oxygen Vacancy for Efficient Photocatalytic Degradation of Phenanthrene: Enhance Molecular Oxygen Activation and Mechanism Insights. Chem. Eng. J. 2023, 472, 145053. [Google Scholar] [CrossRef]
- Qiao, M.; Fu, L.; Barcelo, D. Removal of Polycyclic Aromatic Hydrocarbons by G-C3N4 Nanosheets under Visible Light Irradiation and Effect of Typical Co-Existence Substances in River Water. Process Saf. Environ. Prot. 2022, 159, 376–381. [Google Scholar] [CrossRef]
- Jacobi, H.-W.; Annor, T.; Quansah, E. Investigation of the Photochemical Decomposition of Nitrate, Hydrogen Peroxide, and Formaldehyde in Artificial Snow. J. Photochem. Photobiol. Chem. 2006, 179, 330–338. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Wang, S.; Lin, H.; Mailhot, G. Degradation of 2,4-Dichlorophenol by Ethylenediamine-N,N′-Disuccinic Acid-Modified Photo-Fenton System: Effects of Chemical Compounds Present in Natural Waters. Processes 2020, 9, 29. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Wang, S.; Li, H.; Francisco, J.S.; Zeng, X.C.; Gao, Y. Unraveling a New Chemical Mechanism of Missing Sulfate Formation in Aerosol Haze: Gaseous NO2 with Aqueous HSO3−/SO32−. J. Am. Chem. Soc. 2019, 141, 19312–19320. [Google Scholar] [CrossRef]
- Debnath, B.; Hussain, M.; Li, M.; Lu, X.; Sun, Y.; Qiu, D. Exogenous Melatonin Improves Fruit Quality Features, Health Promoting Antioxidant Compounds and Yield Traits in Tomato Fruits under Acid Rain Stress. Molecules 2018, 23, 1868. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, L.; Deng, F.; Zhu, W.; Guo, L.; Xu, C. Efficient Degradation of Orange IV by an Ultraviolet/Sulfite System: Influencing Factors, Degradation Mechanisms and Response Surface Analysis. J. Ind. Eng. Chem. 2024, 138, 270–281. [Google Scholar] [CrossRef]
- Cao, Y.; Qiu, W.; Li, J.; Jiang, J.; Pang, S. Review on UV/Sulfite Process for Water and Wastewater Treatments in the Presence or Absence of O2. Sci. Total Environ. 2021, 765, 142762. [Google Scholar] [CrossRef]
- Yu, Y.; Lyu, Y.; Zhang, T.; Liu, L.; Fan, B.; Wang, J.; Zhang, C. Efficient Degradation of Iopromide by Using Sulfite Activated with Mackinawite. Molecules 2021, 26, 6527. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, B.; Kumari, S.; Bux, F. Mechanistic Insight into SO4•−/•OH Radical for Enhancing Stability and Activity of LaMO3 Perovskite toward Detoxification of Bulk Pharmaceutical Wastewater: Stoichiometric Efficiency and Controlled Leaching Study. Sep. Purif. Technol. 2023, 319, 123967. [Google Scholar] [CrossRef]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the Origin and Nature of Internal Methyl Rotation Barriers: An Information-Theoretic Approach Study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Gong, S.; Yang, J.; Pan, Q.; Liu, X.; Zhang, Q.; Wang, D. Simultaneous Oxidation of Roxarsone and Adsorption of Released Arsenic by FeS-Activated Sulfite. Water Res. 2023, 237, 119979. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Nagata, C.; Shingu, H. Molecular Orbital Theory of Orientation in Aromatic, Heteroaromatic, and Other Conjugated Molecules. J. Chem. Phys. 2014, 22, 1433–1442. [Google Scholar] [CrossRef]
- Tu, Z.; Qi, Y.; Tang, X.; Wang, Z.; Qu, R. Photochemical Transformation of Anthracene (ANT) in Surface Soil: Chlorination and Hydroxylation. J. Hazard. Mater. 2023, 452, 131252. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, S.; Lu, Y.; Chen, K.; Luo, L.; Hao, C. Computational Study of Photodegradation Process and Conversion Products of the Antidepressant Citalopram in Water. Molecules 2023, 28, 4620. [Google Scholar] [CrossRef]
- Cao, W.; Wu, N.; Zhang, S.; Qi, Y.; Guo, R.; Wang, Z.; Qu, R. Photodegradation of Polychlorinated Biphenyls in Water/Nitrogen-Doped Silica and Air/Nitrogen-Doped Silica Systems: Kinetics, Mechanism and Quantitative Structure Activity Relationship (QSAR) Analysis. Sci. Total Environ. 2024, 924, 171586. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Zhang, M.; Wang, L.; Kang, G.; Wu, S.; She, Y. Ultrasonically Activated Persulfate Process for the Degradation of Phenanthrene in Soil-Washing Effluent: Experimental, DFT Calculation and Toxicity Evaluation. J. Environ. Chem. Eng. 2024, 12, 113035. [Google Scholar] [CrossRef]
- Dang, J.; Shi, X.; Zhang, Q.; Wang, W. Theoretical Perspectives on the Mechanism and Kinetics of the OH Radical-Initiated Gas-Phase Oxidation of PCB126 in the Atmosphere. Sci. Total Environ. 2015, 517, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Wu, N.; Pan, X.; Feng, J.; Al-Basher, G.; Allam, A.A.; Qu, R.; Wang, Z. Photochemical Formation of Hydroxylated Polychlorinated Biphenyls (OH-PCBs) from Decachlorobiphenyl (PCB-209) on Solids/Air Interface. J. Hazard. Mater. 2019, 378, 120758. [Google Scholar] [CrossRef]
- Mishra, B.K.; Lily, M.; Chakrabartty, A.K.; Deka, R.C.; Chandra, A.K. A DFT Study on Kinetics of the Gas Phase Reactions of CH3CH2OCF3 with OH Radicals and Cl Atoms. J. Fluor. Chem. 2014, 159, 57–64. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision A.02; Gaussian. Inv.: Wallingford, CT, USA, 2009; pp. 270–271. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, S.; Cao, W.; Wu, N.; Wei, Z.; Wang, Z.; Qu, R. Photocatalytic Degradation of Decabromodiphenyl Ethane (DBDPE) by Wide Solar Spectrum-Responsive Nitrogen Doped Silica. J. Clean. Prod. 2024, 458, 142556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).