Eco-Friendly Cellulose-Supported Nickel Complex as an Efficient and Recyclable Heterogeneous Catalyst for Suzuki Cross-Coupling Reaction
Abstract
1. Introduction
2. Results and Discussion
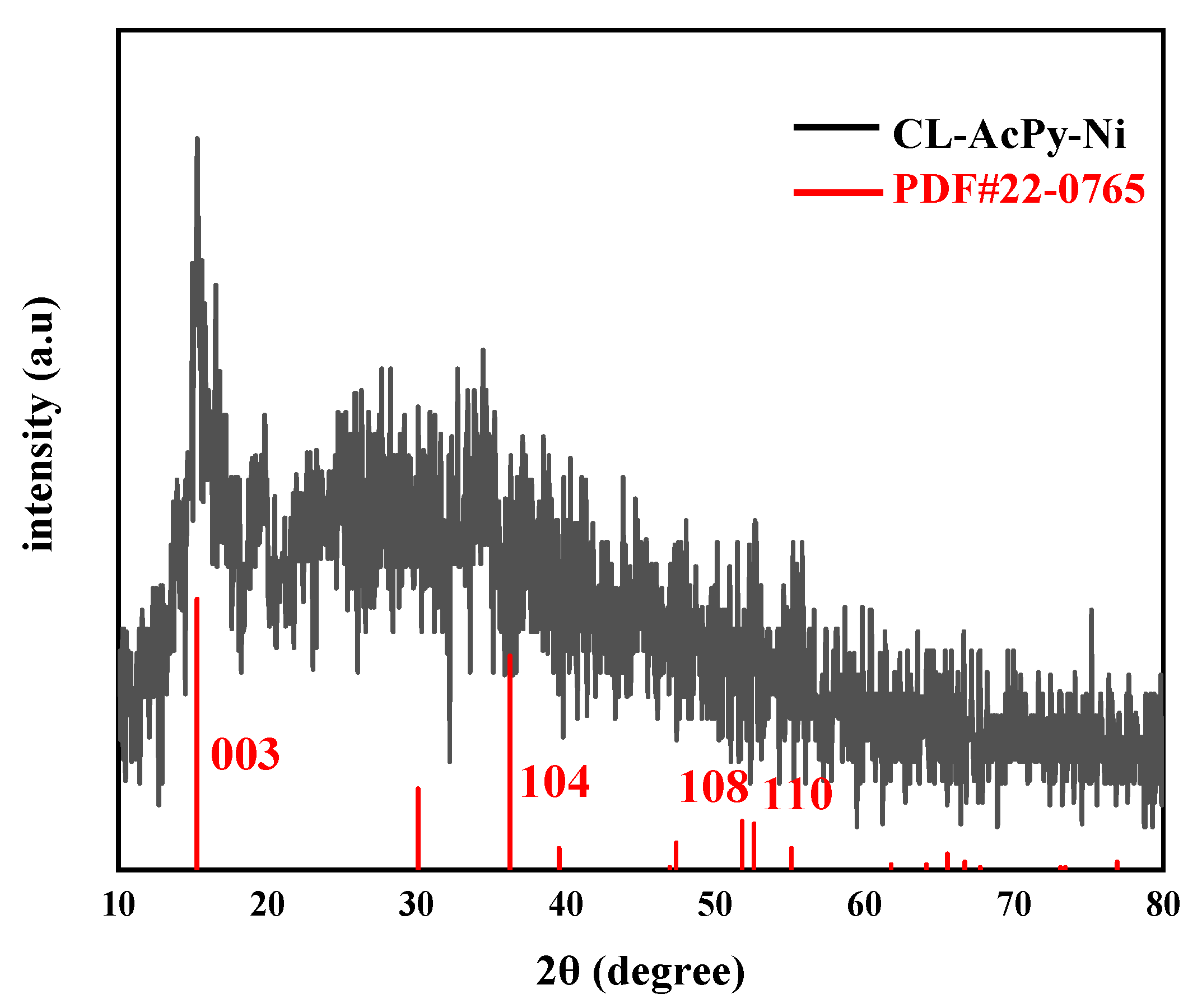
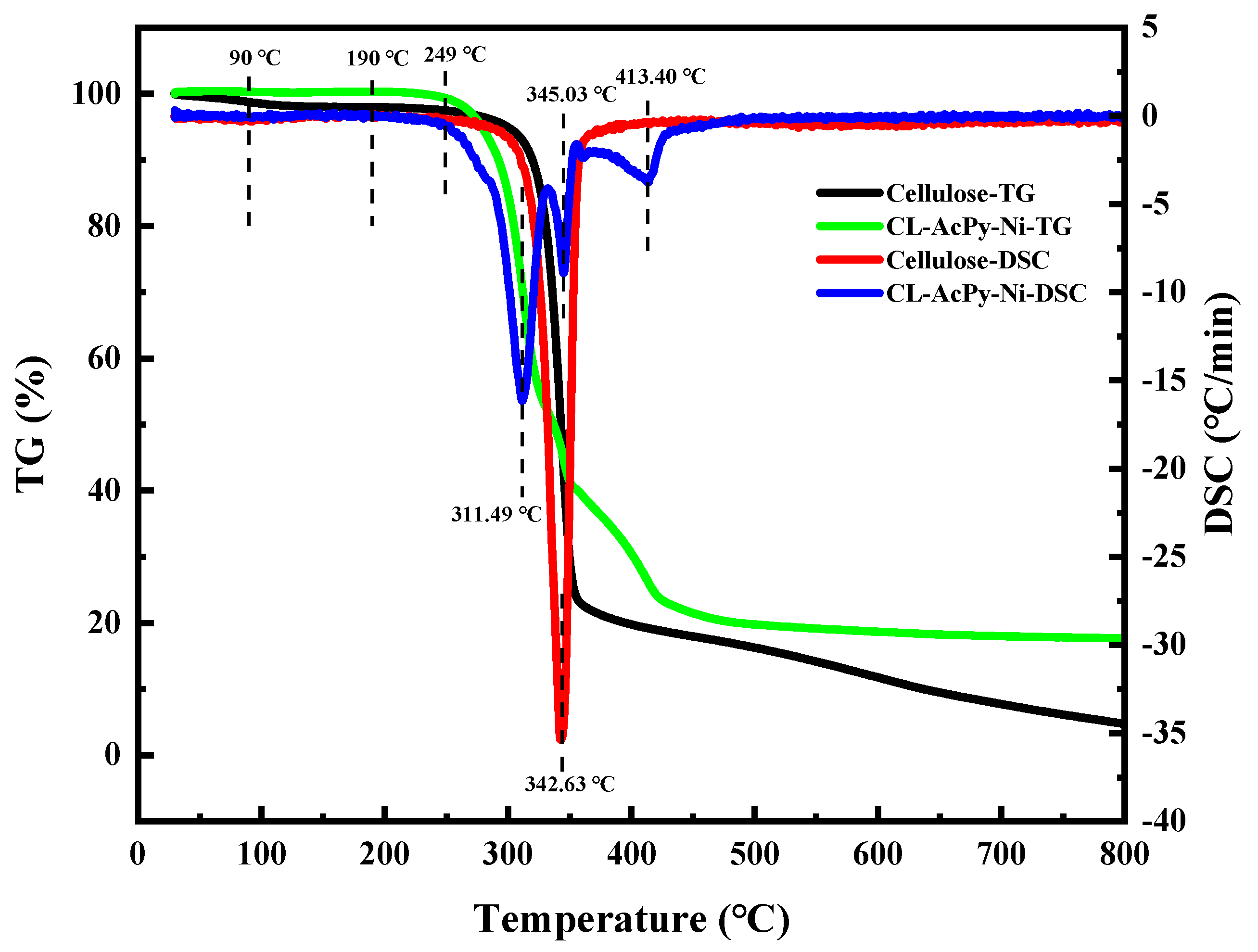
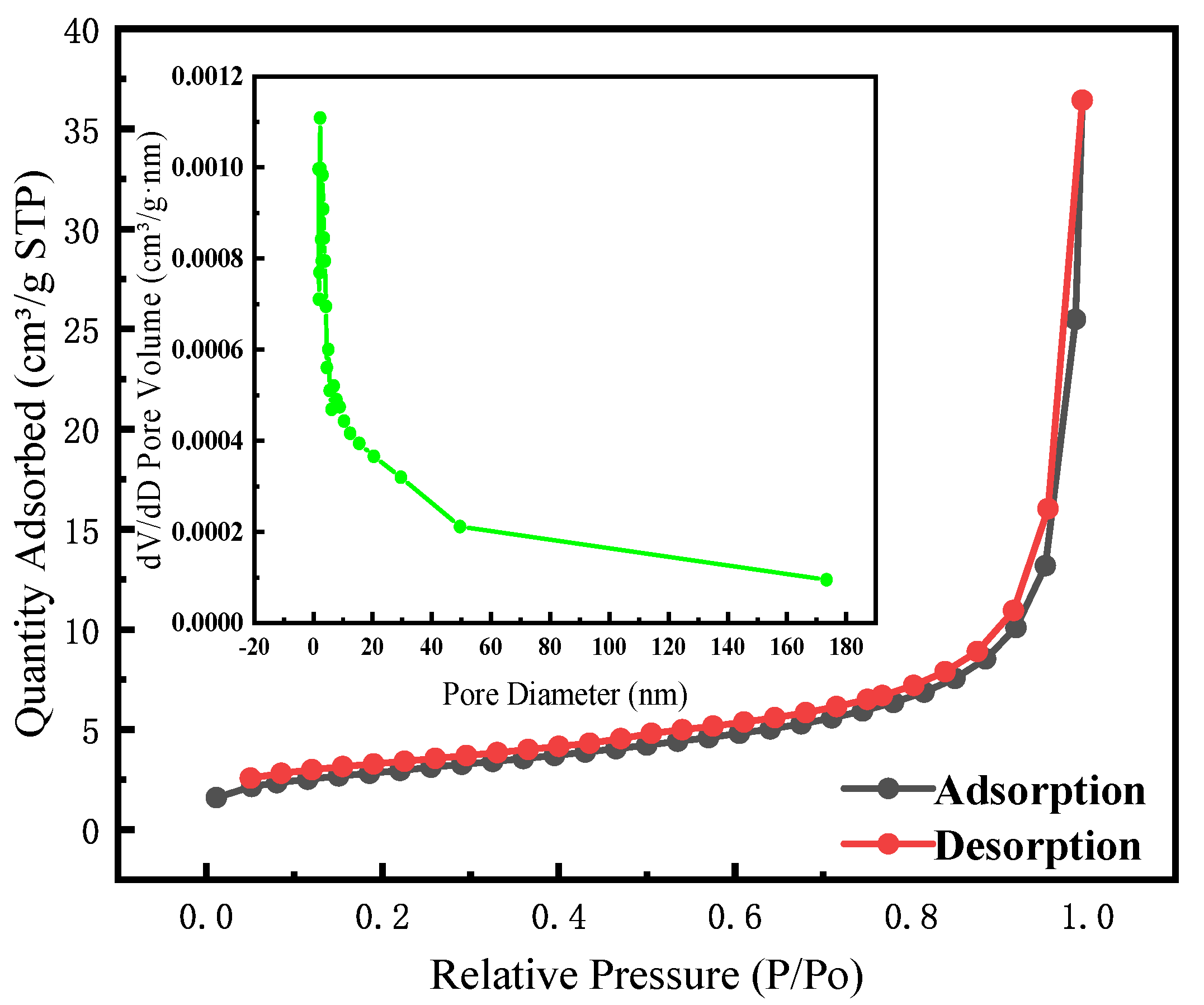
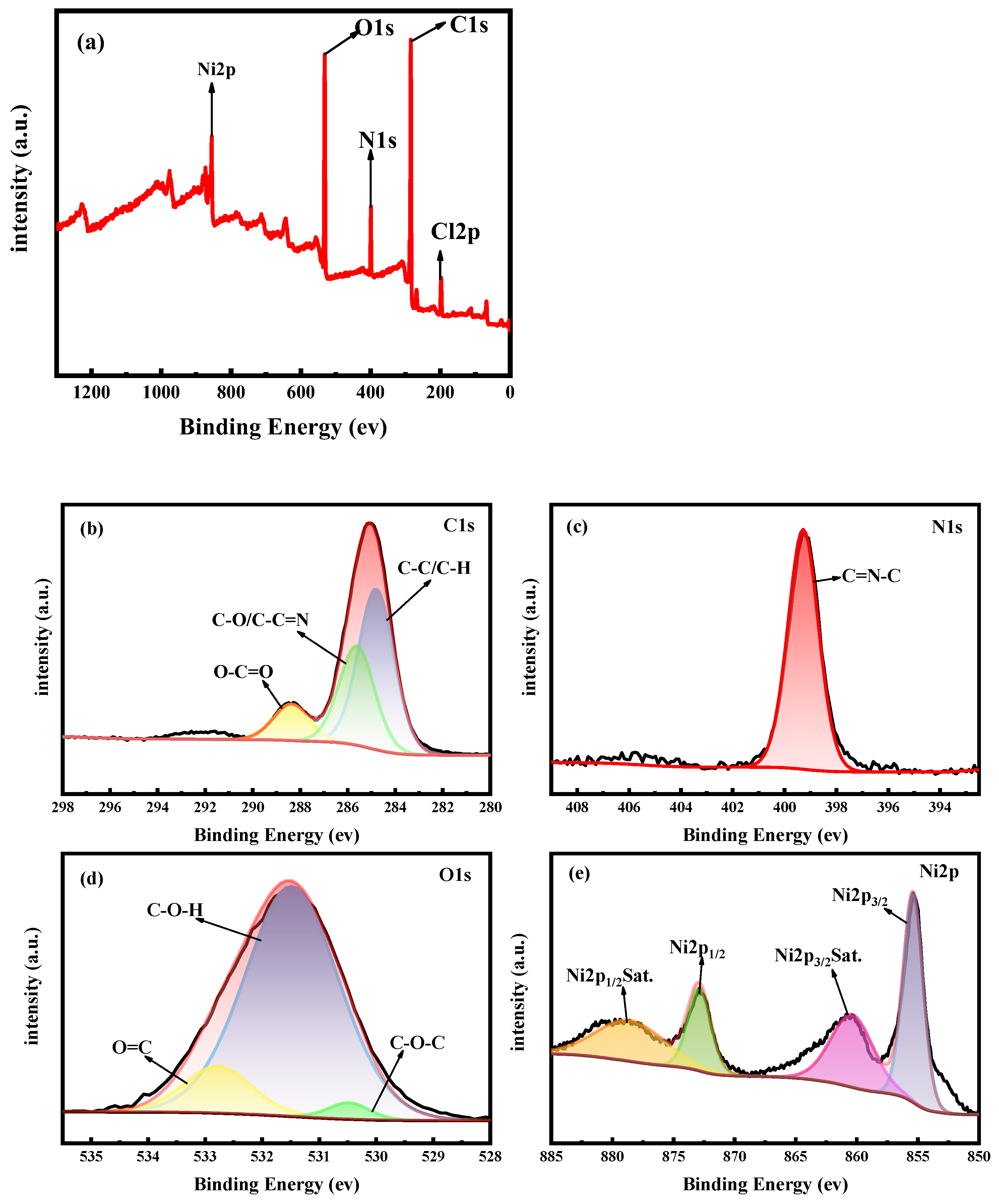
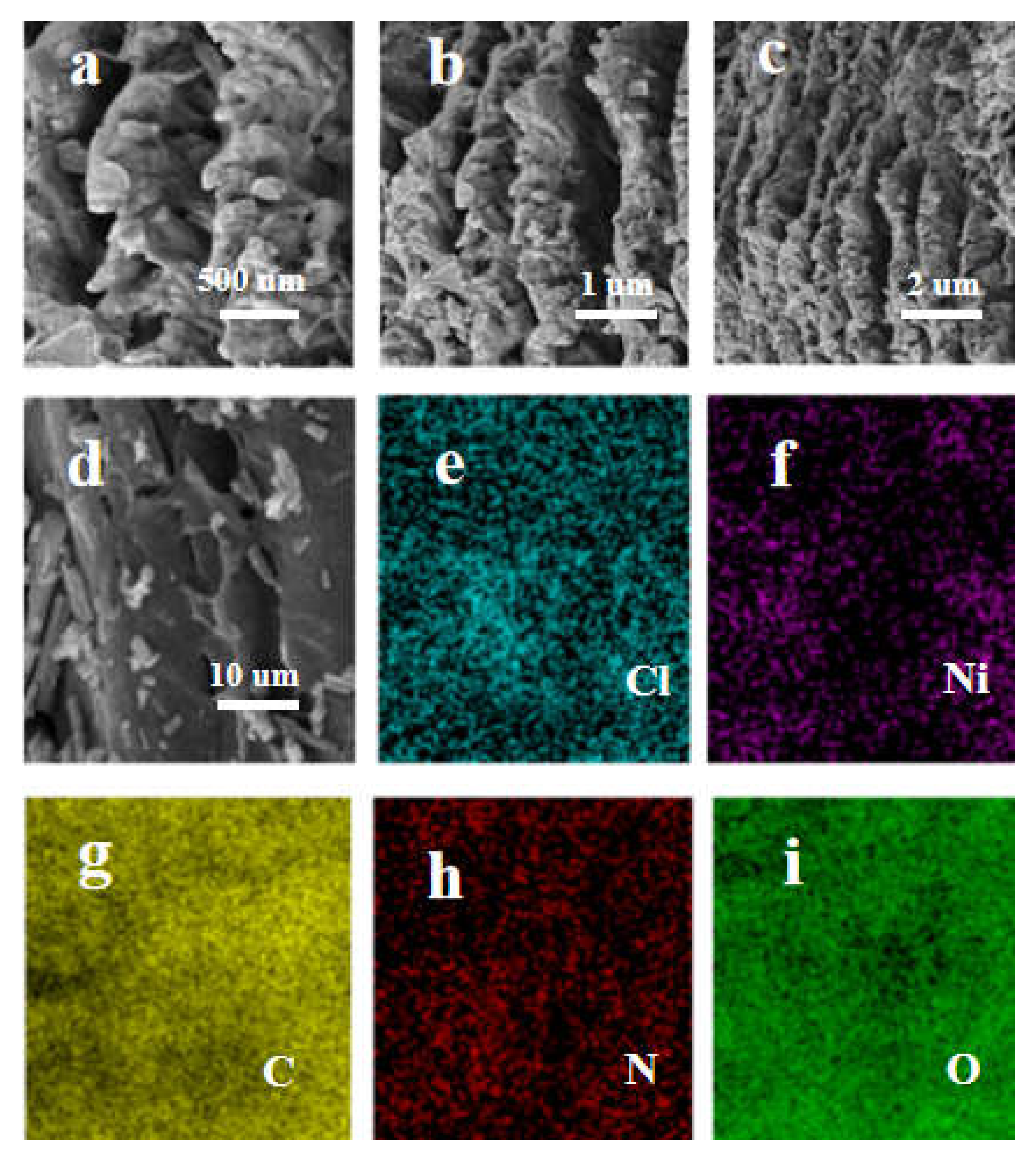
2.1. Catalyst Characterization
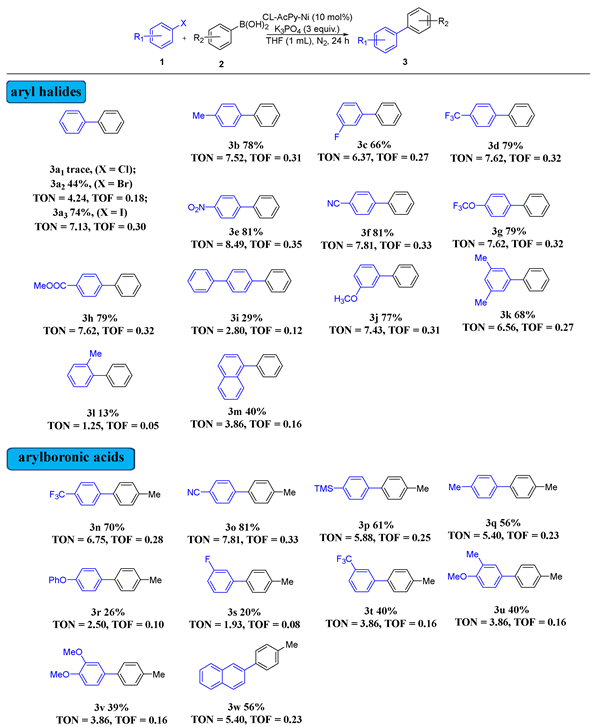
2.2. CL-AcPy-Ni Catalytic Performance in Suzuki Cross-Coupling
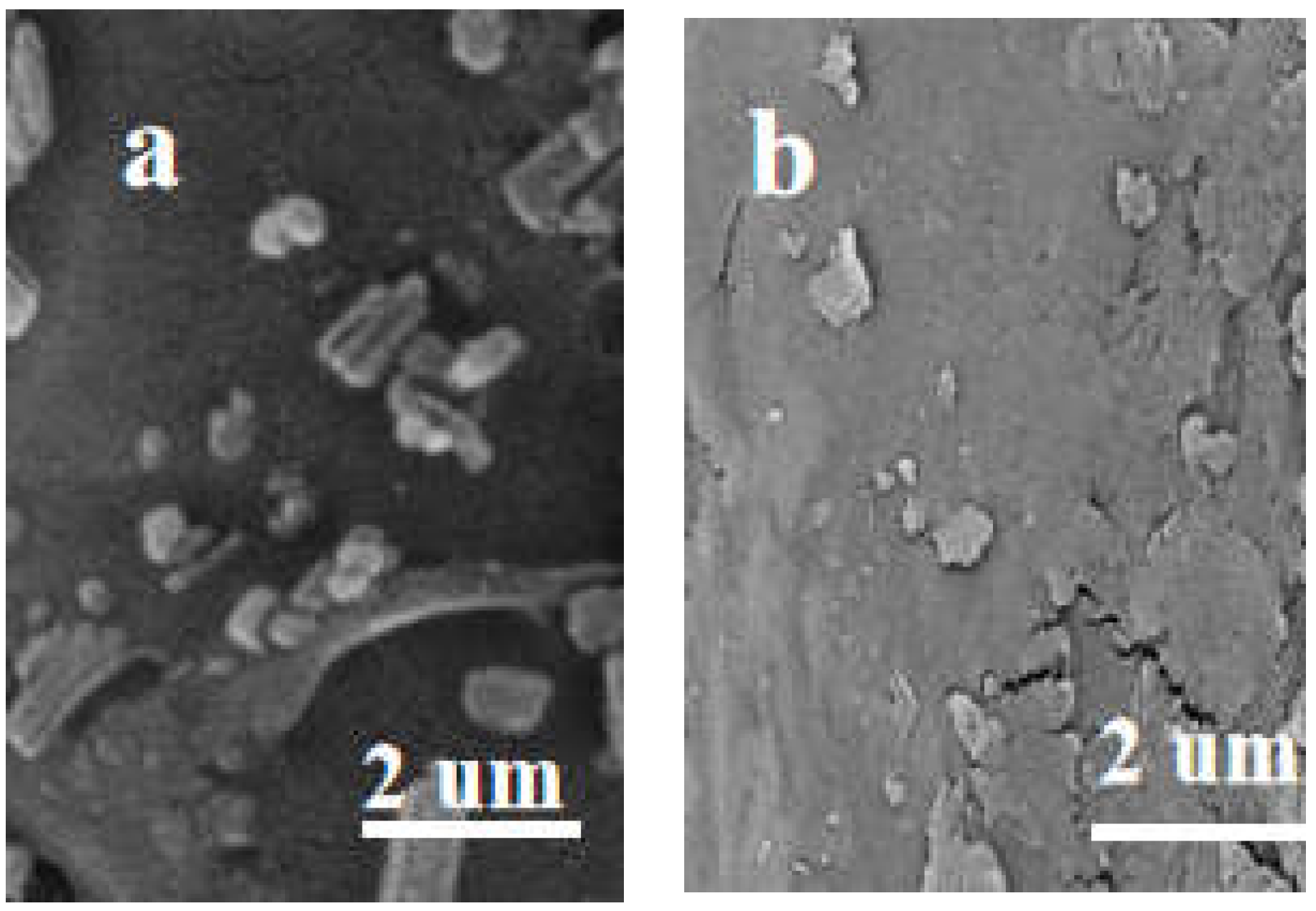
2.3. Recyclability of Heterogeneous CL-AcPy-Ni Catalyst
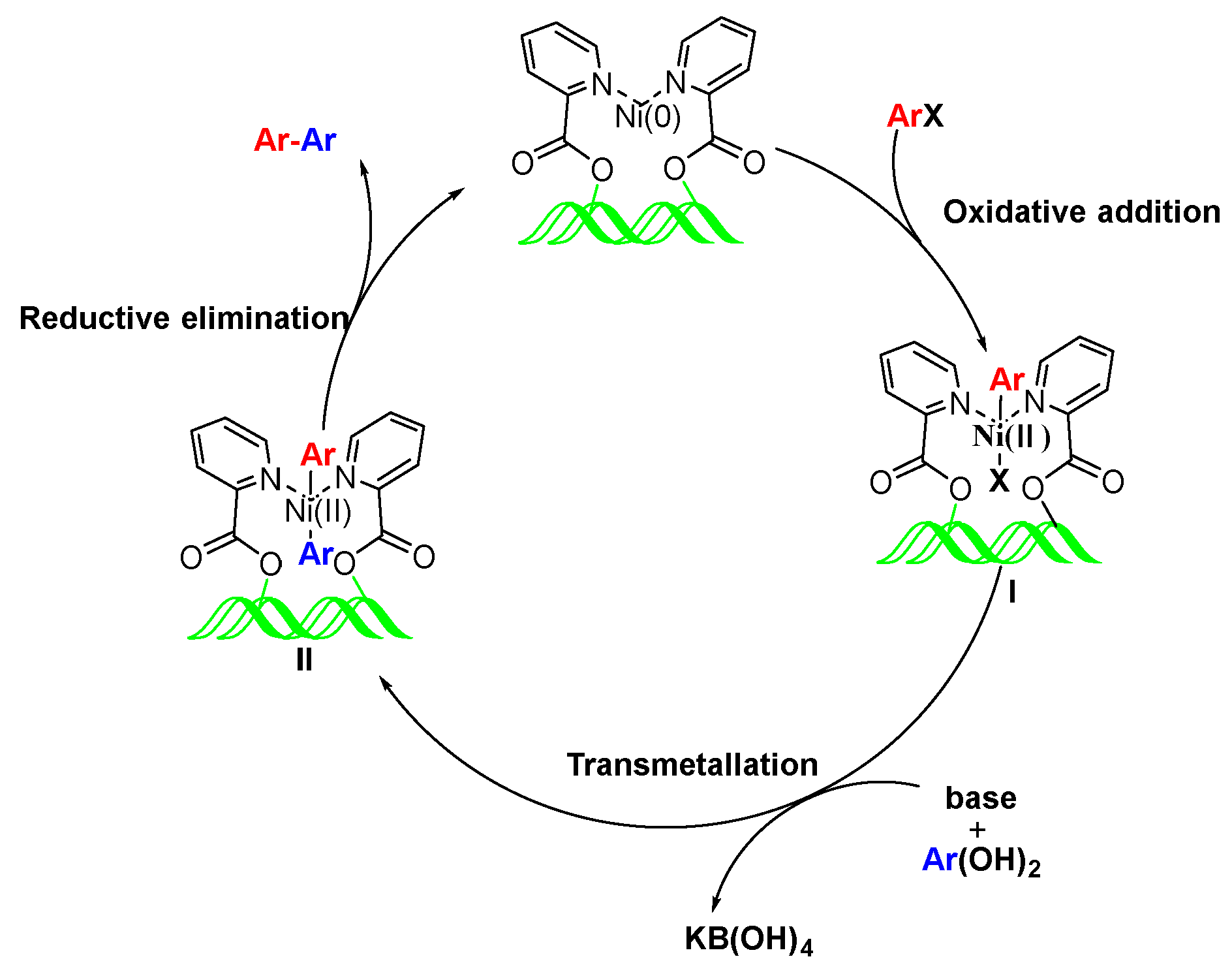
2.4. Possible Mechanism of CL-AcPy-Ni-Catalyzed Suzuki Cross-Coupling
3. Materials and Methods
3.1. Materials
3.2. General Remarks
3.3. General Procedure for the Preparation of CL-AcPy-Ni
3.3.1. Preparation of 2-Pyridinecarbonyl Chloride
3.3.2. Pre-Activation of Microcrystalline Cellulose
3.3.3. Preparation of CL-AcPy
3.3.4. Preparation of CL-AcPy-Ni
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, D.G.; Ojeda-Carralero, G.M.; Reguera, L. Peptide macrocyclization by transition metal catalysis. Chem. Soc. Rev. 2020, 49, 2039–2059. [Google Scholar] [CrossRef]
- Xiao, S.; Ai, L.; Liu, Q. Total Synthesis of Natural Terpenoids Enabled by Cobalt Catalysis. Front. Chem. 2022, 10, 941184. [Google Scholar] [CrossRef]
- Xia, Y.; Campbell, C.T.; Cuenya, B.R. Introduction: Advanced Materials and Methods for Catalysis and Electrocatalysis by Transition Metals. Chem. Rev. 2021, 121, 563–566. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3427. [Google Scholar] [CrossRef]
- He, A.; Falck, J.R. Stereospecific Suzuki Cross-Coupling of Alkyl α-Cyanohydrin Triflates. J. Am. Chem. Soc. 2010, 132, 2524–2525. [Google Scholar] [CrossRef]
- Zhang, M.; Lee, P.S.; Allais, C. Desymmetrization of Vicinal Bis(boronic) Esters by Enantioselective Suzuki-Miyaura Cross-Coupling Reaction. J. Am. Chem. Soc. 2023, 145, 8308–8313. [Google Scholar] [CrossRef]
- Monks, B.M.; Cook, S.P. Palladium-Catalyzed Alkyne Insertion/Suzuki Reaction of Alkyl Iodides. J. Am. Chem. Soc. 2012, 134, 15297–15300. [Google Scholar] [CrossRef]
- Li, B.X.; Le, D.N.; Mack, K.A. Highly Stereoselective Synthesis of Tetrasubstituted Acyclic All-Carbon Olefins via Enol Tosylation and Suzuki-Miyaura Coupling. J. Am. Chem. Soc. 2017, 139, 10777–10783. [Google Scholar] [CrossRef]
- Yokoyama, A.; Suzuki, H.; Kubota, Y. Chain-Growth Polymerization for the Synthesis of Polyfluorene via Suzuki-Miyaura Coupling Reaction from an Externally Added Initiator Unit. J. Am. Chem. Soc. 2007, 129, 7236–7237. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Singer, R.A.; Yang, B.H.; Buchwald, S.L. Highly Active Palladium Catalysts for Suzuki Coupling Reactions. J. Am. Chem. Soc. 1999, 121, 9550–9561. [Google Scholar] [CrossRef]
- Yadav, M.R.; Nagaoka, M.; Kashihara, M. The Suzuki-Miyaura Coupling of Nitroarenes. J. Am. Chem. Soc. 2017, 139, 9423–9426. [Google Scholar] [CrossRef]
- Trost, B.M. The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Trost, B.M. Selectivity: A Key to Synthetic Efficiency. Science 1983, 219, 245–250. [Google Scholar] [CrossRef]
- Trost, B.M.; Dong, G. Total synthesis of bryostatin 16 using atom-economical and chemoselective approaches. Nature 2008, 456, 485–488. [Google Scholar] [CrossRef]
- Veisi, H.; Neyestani, N.; Pirhayati, M. Copper nanoparticle anchored biguanidine-modified Zr-UiO-66 MOFs: A competent heterogeneous and reusable nanocatalyst in Buchwald–Hartwig and Ullmann type coupling reactions. RSC Adv. 2021, 11, 22278–22286. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Koga, H.; Qi, Z.-D. Nanofibrillar Chitin Aerogels as Renewable Base Catalysts. Biomacromolecules 2014, 15, 4314–4319. [Google Scholar] [CrossRef]
- Jadhava, S.; Jagdaleb, A.; Kamble, S. Palladium nanoparticles supported on a titanium dioxide cellulose composite (PdNPs@TiO2–Cell) for ligand-free carbon–carbon cross coupling reactions. RSC Adv. 2016, 6, 3406–3420. [Google Scholar] [CrossRef]
- Kumbhar, A.; Jadhav, S.; Kamble, S. Palladium supported hybrid cellulose-aluminum oxide composite for Suzuki-Miyaura cross coupling reaction. Tetrahedron Lett. 2013, 54, 1331–1337. [Google Scholar] [CrossRef]
- Shafiei, N.; Nasrollahzadeh, M.; Baran, T. Pd nanoparticles loaded on modified chitosan-Unye bentonite microcapsules: A reusable nanocatalyst for Sonogashira coupling reaction. Carbohydr. Polym. 2021, 262, 117920. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Li, W. Chitosan derived efficient and stable Pd nano-catalyst for high efficiency hydrogenation. Int. J. Biol. Macromol. 2023, 241, 124615. [Google Scholar] [CrossRef]
- Mirhosseyni, M.S.; Ziarani, G.M.; Badiei, A. Chitosan-derived mesoporous N-doped carbon catalyst embedded with NiO for highly selective benzyl alcohol oxidation. Int. J. Biol. Macromol. 2024, 259, 129093. [Google Scholar] [CrossRef]
- Jadhav, S.N.; Kumbhar, A.S.; Mali, S.S. A Merrifield resin supported Pd–NHC complex with a spacer (Pd–NHC@SP–PS) for the Sonogashira coupling reaction under copper- and solvent-free conditions. New J. Chem. 2015, 39, 2333–2341. [Google Scholar] [CrossRef]
- Lee, M.; Chen, B.-Y.; Den, W. Chitosan as a Natural Polymer for Heterogeneous Catalysts Support: A Short Review on Its Applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef]
- Ahmad, H. Celluloses as Green Support of Palladium Nanoparticles for Application in Heterogeneous Catalysis: A Brief Review. J. Clust. Sci. 2022, 33, 421–438. [Google Scholar] [CrossRef]
- Baran, N.Y.; Baran, T.; Mentes, A. Fabrication and application of cellulose Schiff base supported Pd(II) catalyst for fast and simple synthesis of biaryls via Suzuki coupling reaction. Appl. Catal. A Gen. 2017, 531, 36–44. [Google Scholar] [CrossRef]
- Dong, B.H.; Hinestroza, J.P. Metal Nanoparticles on Natural Cellulose Fibers: Electrostatic Assembly and In Situ Synthesis. ACS Appl. Mater. Interfaces 2009, 1, 797–803. [Google Scholar] [CrossRef]
- Jamwal, N.; Sodhi, R.K.; Gupta, P. Nano Pd(0) supported on cellulose: A highly efficient and recyclable heterogeneous catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols under liquid phase catalysis. Int. J. Biol. Macromol. 2011, 49, 930–935. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Lu, L. Highly Dispersed Pd Clusters Anchored on Nanoporous Cellulose Microspheres as a Highly Efficient Catalyst for the Suzuki Coupling Reaction. ACS Appl. Mater. Interfaces 2021, 13, 44418–44426. [Google Scholar] [CrossRef]
- Rezayat, M.; Blundell, R.K.; Camp, J.E. Green One-Step Synthesis of Catalytically Active Palladium Nanoparticles Supported on Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2014, 2, 1241–1250. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, Z.; Li, Y. Carboxymethylcellulose-Supported Palladium Nanoparticles Generated in Situ from Palladium(II) Carboxymethylcellulose: An Efficient and Reusable Catalyst for Suzuki-Miyaura and Mizoroki–Heck Reactions. Ind. Eng. Chem. Res. 2015, 54, 790–797. [Google Scholar] [CrossRef]
- Baruah, D.; Das, R.N.; Hazarika, S. Biogenic synthesis of cellulose supported Pd(0) nanoparticles using hearth wood extract of Artocarpus lakoocha Roxb—A green, efficient and versatile catalyst for Suzuki and Heck coupling in water under microwave heating. Catal. Commun. 2015, 72, 73–80. [Google Scholar] [CrossRef]
- Dewan, A.; Sarmah, M.; Bhattacharjee, P. Sustainable nano fibrillated cellulose supported in situ biogenic Pd nanoparticles as heterogeneous catalyst for C-C cross coupling reactions. Sustain. Chem. Pharm. 2021, 23, 100502. [Google Scholar] [CrossRef]
- Salama, A. Novel cellulose derivative containing aminophenylacetic acid as sustainable adsorbent for removal of cationic and anionic dyes. Int. J. Biol. Macromol. 2023, 253, 126687. [Google Scholar] [CrossRef]
- Jebali, Z.; Granados, A.; Nabili, A. Cationic cellulose nanofibrils as a green support of palladium nanoparticles: Catalyst evaluation in Suzuki reactions. Cellulose 2018, 25, 6963–6975. [Google Scholar] [CrossRef]
- Patil, S.; Rajmane, A.; Jadhav, S. CuNPs@Al2O3-cellulose composite for the ligand-free Suzuki cross-coupling reactions in batch and continuous flow process. J. Organomet. Chem. 2024, 1004, 122954. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Xu, B. Cellulose Sponge Supported Palladium Nanoparticles as Recyclable Cross-Coupling Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 17155–17162. [Google Scholar] [CrossRef]
- Chen, L.; Cao, W.; Quinlan, P.J. Sustainable Catalysts from Gold-Loaded Polyamidoamine Dendrimer-Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 978–985. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Huang, X. Regenerated cellulose supported palladium composite superfine fibers as an efficient and recyclable catalyst for Suzuki reaction. Cellulose 2023, 30, 10243–10255. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Fu, Q. Cellulose/β-cyclodextrin hydrogel supported metal nanoparticles as recyclable catalysts in the 4-nitrophenol reduction, Suzuk-Miyaura coupling and click reactions. Cellulose 2023, 30, 953–971. [Google Scholar] [CrossRef]
- Barana, N.Y.; Baranb, T.; Menteş, A. Production of novel palladium nanocatalyst stabilized with sustainable chitosan/cellulose composite and its catalytic performance in Suzuki-Miyaura coupling reactions. Carbohydr. Polym. 2018, 181, 596–604. [Google Scholar] [CrossRef]
- Wanga, X.; Hua, P.; Xue, F. Cellulose-supported N-heterocyclic carbene-palladium catalyst: Synthesis and its applications in the Suzuki cross-coupling reaction. Carbohydr. Polym. 2014, 114, 476–483. [Google Scholar] [CrossRef]
- Kale, D.; Rashinkar, G.; Kumbhar, A. Facile Suzuki-Miyaura cross coupling using ferrocene tethered N-heterocyclic carbene-Pd complex anchored on cellulose. React. Funct. Polym. 2017, 116, 9–16. [Google Scholar] [CrossRef]
- Dong, Y.; Bi, J.; Zhang, S. Palladium supported on N-Heterocyclic carbene functionalized hydroxyethyl cellulose as a novel and efficient catalyst for the Suzuki reaction in aqueous media. Appl. Surf. Sci. 2020, 531, 147392. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Wang, F. Functionalized cellulose-supported triphenylphosphine and its application in Suzuki cross-coupling reactions. J. Appl. Polym. Sci. 2015, 132, 41427. [Google Scholar] [CrossRef]
- Sabaqiana, S.; Nematia, F.; Nahzomib, H.T. Palladium acetate supported on amidoxime-functionalized magnetic cellulose: Synthesis, DFT study and application in Suzuki reaction. Carbohydr. Polym. 2017, 177, 165–177. [Google Scholar] [CrossRef]
- Dong, Y.; Bi, J.; Zhu, D. Functionalized cellulose with multiple binding sites for a palladium complex catalyst: Synthesis and catalyst evaluation in Suzuki-Miyaura reactions. Cellulose 2019, 26, 7355–7370. [Google Scholar] [CrossRef]
- Mhaldar, P.; Vibhute, S.; Rashinkar, G. Highly effective cellulose supported 2-aminopyridine palladium complex (Pd(II)-AMP-Cell@Al2O3) for Suzuki-Miyaura and Mizoroki-Heck cross-coupling. React. Funct. Polym. 2020, 152, 104586. [Google Scholar] [CrossRef]
- Pharande, P.S.; Rashinkar, G.S.; Pore, D.M. Cellulose Schiff base-supported Pd(II): An efficient heterogeneous catalyst for Suzuki Miyaura cross-coupling. Res. Chem. Intermed. 2021, 47, 4457–4476. [Google Scholar] [CrossRef]
- Alazemi, A.M.; Dawood, K.M.; Al-Matar, H.A. Efficient and Recyclable Solid-Supported Pd(II) Catalyst for Microwave-Assisted Suzuki Cross-Coupling in Aqueous Medium. ACS Omega 2022, 7, 28831–28848. [Google Scholar] [CrossRef]
- Mirhosseyni, M.S.; Nemati, F. Fe/N co-doped mesoporous carbon derived from cellulose-based ionic liquid as an efficient heterogeneous catalyst toward nitro aromatic compound reduction reaction. Int. J. Biol. Macromol. 2021, 175, 432–442. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, M.-J.; Zhang, X.-Q. Per-O-acetylation of Cellulose in Dimethyl Sulfoxide with Catalyzed Transesterification. J. Agric. Food Chem. 2014, 62, 3446–3452. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Wang, X. Surface modification of cellulose nanocrystal using succinic anhydride and its effects on poly(butylene succinate) based composites. Cellulose 2019, 26, 3167–3181. [Google Scholar] [CrossRef]
- Wang, W.; Liang, T.; Bai, H. All cellulose composites based on cellulose diacetate and nanofibrillated cellulose prepared by alkali treatment. Carbohydr. Polym. 2018, 179, 297–304. [Google Scholar] [CrossRef]
- Nam, S.; Hillyer, M.B.; Condon, B.D. Method for identifying the triple transition (glass transition-dehydration-crystallization) of amorphous cellulose in cotton. Carbohydr. Polym. 2020, 228, 115374. [Google Scholar] [CrossRef]
- Hirata, T.; Nishimoto, T. DSC, DTA, and TG of cellulose untreated and treated with flame-retardants. Thermochim. Acta 1991, 193, 99–106. [Google Scholar] [CrossRef]
- Abu-Dansoa, E.; Srivastava, V.; Sillanpää, M.; Bhatnagar, A. Pretreatment assisted synthesis and characterization of cellulose nanocrystals and cellulose nanofibers from absorbent cotton. Int. J. Biol. Macromol. 2017, 102, 248–257. [Google Scholar] [CrossRef]
- Reyes, A.; Calleja, A.; Gil-Guill, I. Optimization and characterization of reinforced biodegradable cellulose-based aerogels via polylactic acid/polyhydroxybutyrate coating. Int. J. Biol. Macromol. 2023, 253, 127224. [Google Scholar] [CrossRef]
- Khorasani, A.C.; Satvati, P.R. Reusable cellulose-based biosorbents for efficient iodine adsorption by economic microcrystalline cellulose production from walnut shell. Int. J. Biol. Macromol. 2024, 256, 128432. [Google Scholar] [CrossRef]
- Geng, T.-M.; Fang, X.-C.; Wang, F.-Q. Azine- and azo-based flexible covalent organic frameworks for fluorescence sensing nitro-aromatic compounds and iodine and adsorbing iodine. React. Funct. Polym. 2022, 176, 105309. [Google Scholar] [CrossRef]
- Ma, Z.; Duan, Y.; Liu, Y.; Han, Y.; Wang, X.; Sun, G.; Li, Y. Synergistic effects of hierarchical porous structures and ultra-high pyridine nitrogen doping enhance the oxygen reduction reaction electrocatalytic performance of metal-free laminated lignin-based carbon. Int. J. Biol. Macromol. 2024, 256, 128292. [Google Scholar] [CrossRef]
- Dai, F.; Lan, K.; Wang, S. Adsorbents prepared from epoxy-based porous materials of microcrystalline cellulose for excellent adsorption of anionic and cationic dyes. Int. J. Biol. Macromol. 2024, 260, 129477. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Lv, C. Fabrication and characterization of flexible natural cellulosic fiber composites through collaborative modification strategy of sodium hydroxide and γ-Aminopropyl triethoxysilane. Int. J. Biol. Macromol. 2024, 261, 129831. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, X.; Hu, R.; Sun, G.; Zhao, H.; Liu, W.; Bai, Z.; Jiang, X.; Cui, Y. Cellulose phosphonate/polyethyleneimine nano-porous composite remove toxic Pb(II) and Cu(II) from water in a short time. Int. J. Biol. Macromol. 2023, 253, 127110. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, R.; Kumari, S. Enhancing wheat crop production with eco-friendly chitosan encapsulated nickel oxide nanocomposites: A safe and sustainable solution for higher yield. Int. J. Biol. Macromol. 2023, 253, 127413. [Google Scholar] [CrossRef]
- Kujur, J.P.; Moon, P.R.; Pathak, D.D. Surface modification of chitosan with Ni(II) Schiff base complex: A new heterogeneous catalyst for the synthesis of xanthones. Int. J. Biol. Macromol. 2023, 252, 126497. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Sclafani, J.A.; Blomgren, P.A. Biaryls via Suzuki Cross-Couplings Catalyzed by Nickel on Charcoal. Tetrahedron 2000, 56, 2139–2144. [Google Scholar] [CrossRef]
- Payamifar, S.; Kazemi, F.; Kaboudin, B. Nickel/β-CD-catalyzed Suzuki–Miyaura cross-coupling of aryl boronic acids with aryl halides in water. Appl. Organomet. Chem. 2021, 35, 11. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Abolfathi, P. Nickel stabilized by triazole-functionalized carbon nanotubes as a novel reusable and efficient heterogeneous nanocatalyst for the Suzuki–Miyaura coupling reaction. RSC Adv. 2016, 6, 110622–110628. [Google Scholar] [CrossRef]
- Vannucci, A.K. A Molecular/Heterogeneous Nickel Catalyst for Suzuki−Miyaura Coupling. Organometallics 2019, 38, 2007–2014. [Google Scholar]
- Hosseini, S.; Pourmousavi, S.A. Nickel Supported MCM-Functionalized 1,2,3-Triazol-4-ylmethanamine: An Efcient Nano-particle-Heterogeneous Catalyst Activate for Suzuki Reaction. Catal. Lett. 2022, 152, 2186–2199. [Google Scholar] [CrossRef]
- Chen, Z.-M.; Tao, T.-X. Suzuki cross-coupling of aryl halides with phenylboronic acid catalysed by an amidoxime fibres-nickel(0) complex. J. Chem. Res. 2013, 8, 451–454. [Google Scholar]
- Faghihi, K. Synthesis and characterization of new polyesters based on 2,5-bis[(4-chloro carboxyanilino) carbonyl] pyridine and aromatic diols. Chin. Chem. Lett. 2010, 21, 13–17. [Google Scholar] [CrossRef]
- Chhajed, M.; Yadav, C.; Agrawal, A.K. Esterified superhydrophobic nanofibrillated cellulose based aerogel for oil spill treatment. Carbohydr. Polym. 2019, 226, 115286. [Google Scholar] [CrossRef]
- Peng, S.X.; Chang, H.; Kumar, S. A comparative guide to controlled hydrophobization of cellulose nanocrystals via surface esterification. Cellulose 2016, 23, 1825–1846. [Google Scholar] [CrossRef]
- Thompson, L.; Nikzad, M.; Sbarski, I. Esterified cellulose nanocrystals for reinforced epoxy nanocomposites. Prog. Nat. Sci. Mater. Int. 2022, 32, 328–333. [Google Scholar] [CrossRef]
- Qian, Y.-Q.; Han, N.; Bo, Y. Homogeneous synthesis of cellulose acrylate-g-poly (n-alkyl acrylate) solid-solid phase change materials via free radical polymerization. Carbohydr. Polym. 2018, 193, 129–136. [Google Scholar] [CrossRef]
- Okamoto, H.; Taniguchi, T.; Takekuma, M. Revisiting the Synthesis of Cellulose Acrylate and Its Modification via Michael Addition Reactions. Biomacromolecules 2023, 24, 3767–3774. [Google Scholar] [CrossRef]
- Van Waes, F.E.; Drabowicz, J.; Cukalovic, A.; Stevens, C.V. Efficient and catalyst-free condensation of acid chlorides and alcohols using continuous flow. Green Chem. 2012, 14, 2776–2779. [Google Scholar] [CrossRef]

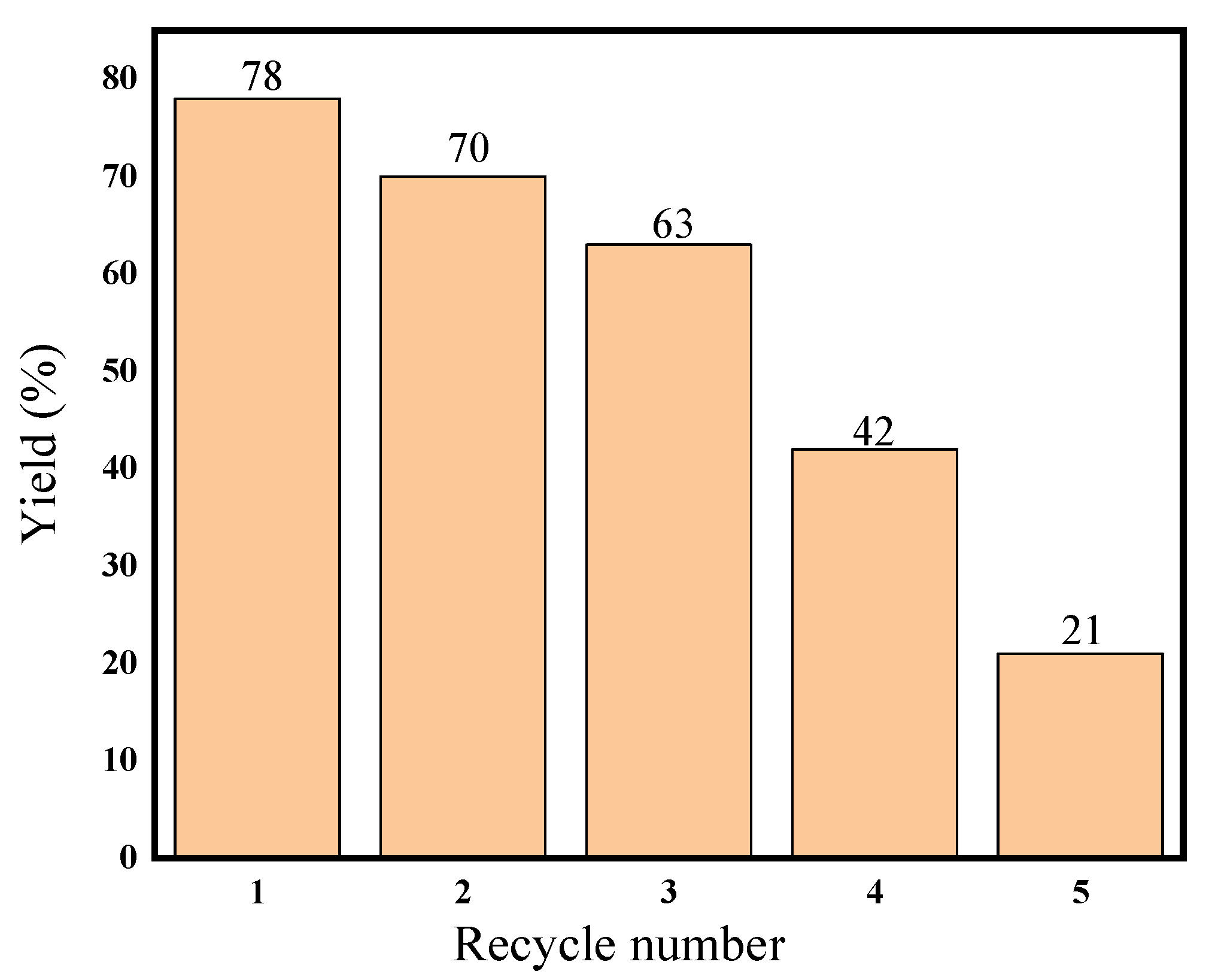


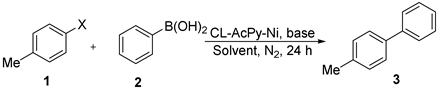 | ||||
|---|---|---|---|---|
| Entry a | Base | Solvent | Temperature | Yield (%) b |
| 1 | Et3N | THF | 100 | none |
| 2 | NaOH | THF | 100 | 24 |
| 3 | KOH | THF | 100 | 27 |
| 4 | Cs2CO3 | THF | 100 | trace |
| 5 | K2CO3 | THF | 100 | 8 |
| 6 | K3PO4 | THF | 100 | 60 |
| 7 | K3PO4 | THF | 120 | 78 |
| 8 c | K3PO4 | THF | 120 | 50 |
| 9 | K3PO4 | 1,4-dioxane | 120 | 73 |
| 10 | K3PO4 | toluene | 120 | trace |
| 11 | K3PO4 | H2O | 120 | trace |
 |
| Run | Fresh | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Nickel content (mmol/g) | 0.216 | 0.174 | 0.110 | 0.044 | 0.026 | 0.0108 |
| Leaching ratio | 78% | 70 | 63% | 42% | 21% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhou, G.; Sun, Y.; Mao, Y.; Zeng, F.; Wang, Z.; Zhang, Y.; Li, B. Eco-Friendly Cellulose-Supported Nickel Complex as an Efficient and Recyclable Heterogeneous Catalyst for Suzuki Cross-Coupling Reaction. Molecules 2024, 29, 4525. https://doi.org/10.3390/molecules29194525
Li Z, Zhou G, Sun Y, Mao Y, Zeng F, Wang Z, Zhang Y, Li B. Eco-Friendly Cellulose-Supported Nickel Complex as an Efficient and Recyclable Heterogeneous Catalyst for Suzuki Cross-Coupling Reaction. Molecules. 2024; 29(19):4525. https://doi.org/10.3390/molecules29194525
Chicago/Turabian StyleLi, Zhanyu, Guohao Zhou, Yu Sun, Yingning Mao, Fanxiang Zeng, Zhihui Wang, Yuanyuan Zhang, and Bin Li. 2024. "Eco-Friendly Cellulose-Supported Nickel Complex as an Efficient and Recyclable Heterogeneous Catalyst for Suzuki Cross-Coupling Reaction" Molecules 29, no. 19: 4525. https://doi.org/10.3390/molecules29194525
APA StyleLi, Z., Zhou, G., Sun, Y., Mao, Y., Zeng, F., Wang, Z., Zhang, Y., & Li, B. (2024). Eco-Friendly Cellulose-Supported Nickel Complex as an Efficient and Recyclable Heterogeneous Catalyst for Suzuki Cross-Coupling Reaction. Molecules, 29(19), 4525. https://doi.org/10.3390/molecules29194525







