Exploring Quinazoline Nitro-Derivatives as Potential Antichagasic Agents: Synthesis and In Vitro Evaluation
Abstract
1. Introduction
2. Results
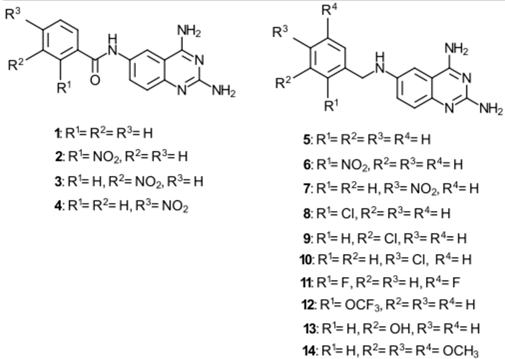
2.1. Chemistry
2.2. Drug-Likeness Predictions
2.3. TAQ-Derivative Synthesis
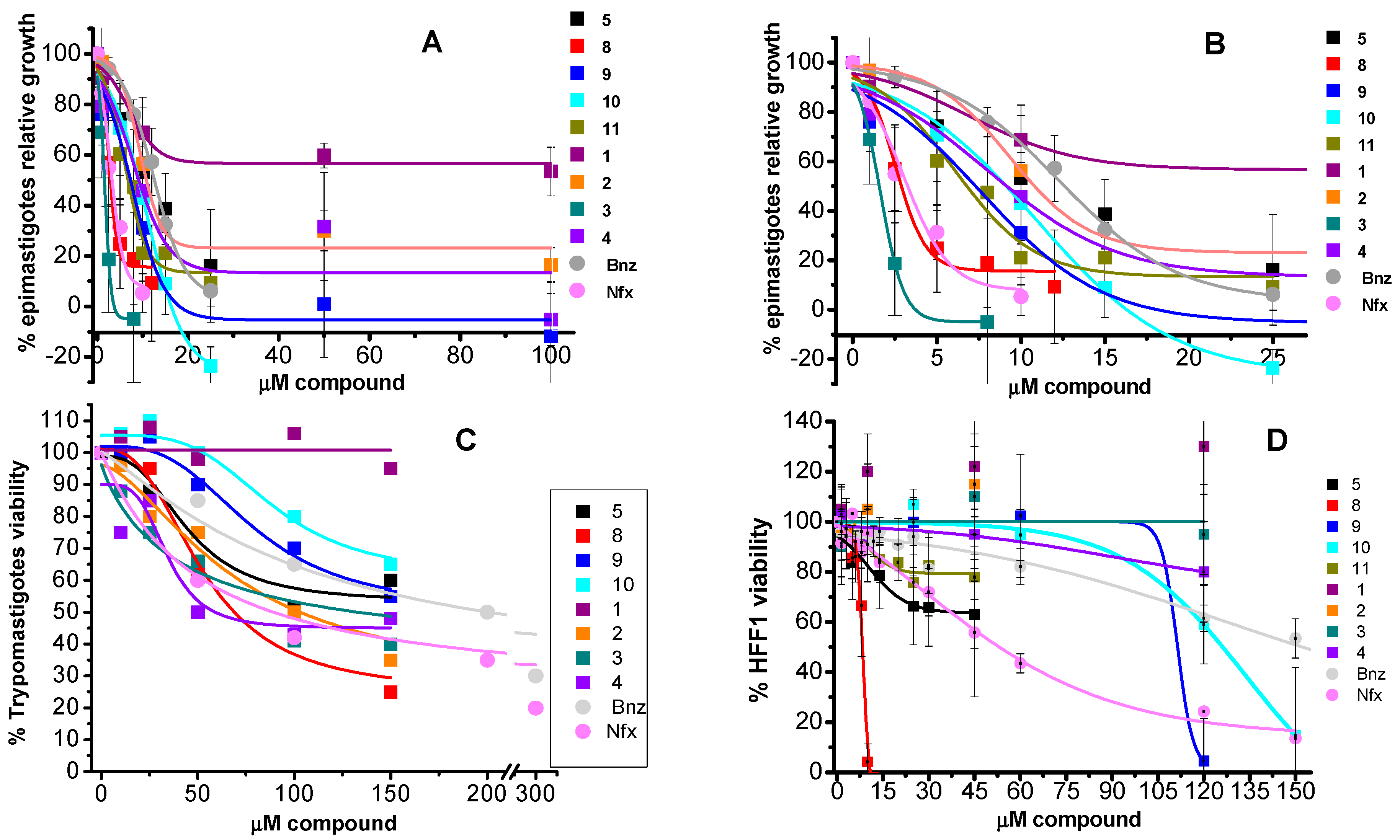
2.4. In Vitro Effect of the Compounds on Parasite Forms and Mammalian Cells at Short Times
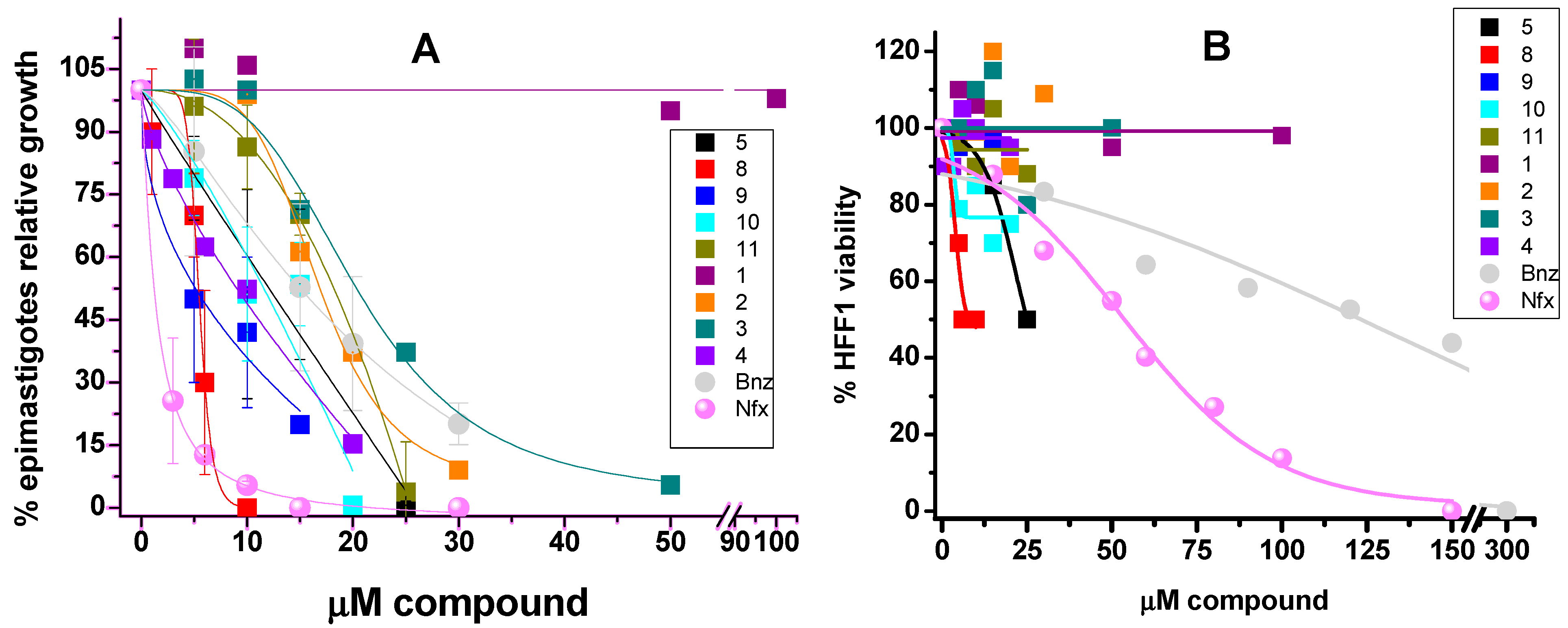
2.5. In Vitro Effect of the Compounds on Parasite Forms and Mammalian Cells after 5 Days
2.6. Inhibitory Concentrations
2.7. Effect of TAQ Derivatives on Antioxidant Thiol Metabolites
3. Discussion
4. Conclusions
5. Methodology
5.1. Analytical Methods
5.2. Preparation of 6-Nitroquinazoline-2,4-diamine
5.3. Preparation of Quinazoline-2,4,6-triamine (TAQ)
5.4. General Procedures for the Synthesis of 1–4
- N-(2,4-Diaminequinazolin-6-yl)benzamide (1)
- N-(2,4-Diaminequinazolin-6-yl)-2-nitrobenzamide (2)
- N-(2,4-Diaminequinazolin-6-yl)-3-nitrobenzamide (3)
- N-(2,4-Diaminequinazolin-6-yl)-4-nitrobenzamide (4)
5.5. General Procedures for the Synthesis of 5–14
- N6-(2,5-Difluorobenzyl)quinazoline-2,4,6-triamine (11)
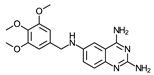
- N6-(3,4,5-Trimethoxybenzyl)quinazoline-2,4,6-triamine (14)
5.6. Cell Culture
5.7. Trypomastigotes Obtention
5.8. Drug Treatment
5.9. Thiol Metabolites Determination
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Available online: https://www-who-int.pbidi.unam.mx:2443/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 28 May 2024).
- Hochberg, N.S.; Montgomery, S.P. Chagas Disease. Ann. Intern. Med. 2023, 176, ITC17–ITC23. [Google Scholar] [CrossRef] [PubMed]
- Silvestre de Sousa, A.; Vermeij, S.; Novaes Ramos, A.; Luquetti, A.O. Chagas Disease. Lancet 2024, 403, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Lascano, F.; García-Bournissen, F.; Altcheh, J. Review of pharmacological options for the treatment of Chagas disease. Br. J. Clin. Pharmacol. 2022, 88, 383–402. [Google Scholar] [PubMed]
- Guarner, J. Chagas disease as example of a reemerging parasite. Semin. Diagn. Pathol. 2019, 36, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Martínez, S.J.; Romano, P.S.; Engman, D.M. Precision Health for Chagas disease: Integrating parasite and host factors to predict outcome of infection and response to theraphy. Front. Cell. Infect. Microbiol. 2020, 10, 210. [Google Scholar] [CrossRef]
- Tarleton, R.L. Effective drug discovery in Chagas disease. Trends Parasitol. 2023, 39, 423–431. [Google Scholar] [CrossRef]
- Losada-Galván, I.; Alonso-Padilla, A.; Cortés-Serra, N.; Alonso-Vega, C.; Gascón, J.; Pinazzo, M.J. Benznidazole for the treatment of Chagas disease. Expert Rev. Anti-Infect. Ther. 2021, 19, 547–556. [Google Scholar] [CrossRef]
- Murta, S.M.F.; Lemos Santana, P.A.; Jacques Dit Lapierre, T.J.W.; Penteado, A.B.; El Hajje, M.; Navarro Vinha, T.C.; Liarte, D.B.; de Souza, M.L.; Goulart Trossini, G.H.; de Oliveira Rezende Júnior, C.; et al. New drug discovery strategies for the treatment of benznidazole-resistance in Trypanosoma cruzi, the causative agent of Chagas disease. Expert Opin. Drug Discov. 2024, 19, 741–753. [Google Scholar] [CrossRef]
- Jaime, L.D.; Aracely, L.M.; Paulina, O.M.; Dumonteil, E.; Barnabé, C.; Waleckx, E.; Hernández-Giles, R.G.; Ramos-Ligonio, A. Molecular Characterization of Four Mexican Isolates of Trypanosoma cruzi and Their Profile Susceptibility to Nifurtimox. Acta Parasitol. 2022, 67, 1584–1593. [Google Scholar] [CrossRef]
- Bisio, M.M.C.; Jurado Medina, L.S.; García-Bournissen, F.; Gulin, J.E.N. Listen to what the animals say: A systematic review and meta-analysis of sterol 14-demethylase inhibitor efficacy for in vivo models of Trypanosoma cruzi infection. Parasitol. Res. 2024, 123, 248. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Forsyth, C.; Losada, I.; Esteban, E.T.; García-Rodríguez, M.; Villegas, M.L.; Molina, I.; Crespillo-Andújar, C.; Gállego, M.; Ballart, C.; et al. FEXI-12 Study Team. Efficacy and safety of fexinidazole for treatment of chronic indeterminate Chagas disease (FEXI-12): A multicentre, randomised, double-blind, phase 2 trial. Lancet Infect. Dis. 2024, 24, 395–403. [Google Scholar] [PubMed]
- Ramos, L.G.; de Souza, K.R.; Júnior, P.A.S.; Câmara, C.C.; Castelo-Branco, F.S.; Boechat, N.; Carvalho, S.A. Tackling the challenges of human Chagas disease: A comprehensive review of treatment strategies in the chronic phase and emerging therapeutic approaches. Acta Trop. 2024, 256, 107264. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Goel, T.; Thakar, S.; Jadhav, M.; Bansode, D. An Explicative Review on the Progress of Quinazoline Scaffold as Bioactive Agents in the Past Decade. Med. Chem. 2023, 19, 211–245. [Google Scholar] [CrossRef] [PubMed]
- Bansal, B.; Malhotra, A. Therapeutic progression of quinazolines as targeted chemotherapeutic agents. Eur. J. Med. Chem. 2021, 211, 2111–2127. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 1. Anti-infective drugs for regulatory registration. Parasites Vectors 2023, 16, 82. [Google Scholar] [CrossRef]
- Mendoza-Martínez, C.; Galindo-Sevilla, N.; Correa-Basurto, J.; Ugalde-Saldivar, V.M.; Rodríguez-Delgado, R.G.; Hernández-Pineda, J.; Padierna-Mota, C.; Flores-Alamo, M.; Hernández-Luis, F. Antileishmanial activity of quinazoline derivatives: Synthesis, docking screens, molecular dynamic simulations and electrochemical studies. Eur. J. Med. Chem. 2015, 92, 314–331. [Google Scholar] [CrossRef]
- Mendoza-Martínez, C.; Correa-Basurto, J.; Nieto-Meneses, R.; Marquez-Navarro, A.; Aguilar-Suarez, R.; Montero-Cortes, M.D.; Nogueda-Torres, B.; Suarez-Contreras, E.; Galindo-Sevilla, N.; Rojas-Rojas, A.; et al. Design, synthesis and biological evaluation of quinazoline derivatives as anti-trypanosomatid and anti-plasmodial agents. Eur. J. Med. Chem. 2015, 96, 296–307. [Google Scholar] [CrossRef]
- Matus-Meza, A.S.; Velasco-Velázquez, M.A.; Hernández-Luis, F. Design, synthesis and cytotoxic evaluation of quinazoline-2,4,6-triamine and 2,6-diaminoquinazolin-4(3H)-one derivatives. Med. Chem. Res. 2018, 27, 1748–1756. [Google Scholar] [CrossRef]
- Daina, A.; Micheilin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Swarit Jasial, S.; Hu, Y.; Bajorath, J. How Frequently Are Pan-Assay Interference Compounds Active? Large-Scale Analysis of Screening Data Reveals Diverse Activity Profiles, Low Global Hit Frequency, and Many Consistently Inactive Compounds. J. Med. Chem. 2017, 60, 3879–3886. [Google Scholar] [CrossRef]
- Lopez-Olmos, V.; Perez-Nasser, N.; Pinero, D.; Ortega, E.; Hernandez, R.; Espinoza, B. Biological characterization and genetic diversity of Mexican isolates of Trypanosoma cruzi. Acta Trop. 1998, 69, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Olin-Sandoval, V.; Moreno-Sánchez, R.; Saavedra, E. Targeting trypanothione metabolism in trypanosomatid human parasites. Curr. Drug Targets 2010, 11, 1614–1630. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.; Barrias, E.S. May the epimastigote form of Trypanosoma cruzi be infective? Acta Trop. 2020, 212, 105688. [Google Scholar] [CrossRef] [PubMed]
- Khabnadideh, S.; Pez, D.; Musso, A.; Brun, R.; Ruiz Pérez, L.M.; González-Pacanowska, D.; Gilbert, I.H. Design, synthesis and evaluation of 2,4-diaminoquinazolines as inhibitors of trypanosomal and leishmanial dihydrofolate reductase. Bioorg. Med. Chem. 2005, 13, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Fernando da Silva Santos-Júnior, P.; Rocha Silva, L.; José Quintans-Júnior, L.; Ferreira da Silva-Júnior, E. Nitro compounds against trypanosomatidae parasites: Heroes or villains? Bioorg. Med. Chem. Lett. 2022, 75, 128930. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Bot, C.; Kelly, J.M.; Hall, B.S. Trypanocidal activity of nitroaromatic prodrugs: Current treatments and future perspectives. Curr. Top. Med. Chem. 2011, 11, 2072–2084. [Google Scholar] [CrossRef]
- Vázquez, C.; Mejia-Tlachi, M.; González-Chávez, Z.; Silva, A.; Rodríguez-Zavala, J.S.; Moreno-Sánchez, R.; Saavedra, E. Buthionine sulfoximine is a multitarget inhibitor of trypanothione synthesis in Trypanosoma cruzi. FEBS Lett. 2017, 591, 3881–3894. [Google Scholar] [CrossRef]
- González-Chávez, Z.; Vázquez, C.; Mejia-Tlachi, M.; Márquez-Dueñas, C.; Manning-Cela, R.; Encalada, R.; Rodríguez-Enríquez, S.; Michels, P.A.M.; Moreno-Sánchez, R.; Saavedra, E. Gamma-glutamylcysteine synthetase and tryparedoxin 1 exert high control on the antioxidant system in Trypanosoma cruzi contributing to drug resistance and infectivity. Redox Biol. 2019, 26, 101231. [Google Scholar] [CrossRef]


 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | MW g/mol | S a mg/mL | ClogP b o/w | TPSA Å2 | Lipinsky Rule Violations c | Veber Rule Violations d | %ABS e | GI-ab f | PAINS g Alert |
| 1 | 279.30 | 0.22 | 1.64 | 106.92 | 0 | 0 | 72.11 | High | 0 |
| 2 | 324.29 | 0.30 | 0.98 | 152.74 | 0 | 1 | 56.30 | Low | 0 |
| 3 | 324.29 | 0.30 | 1.01 | 152.74 | 0 | 1 | 56.30 | Low | 0 |
| 4 | 324.29 | 0.30 | 1.03 | 152.74 | 0 | 1 | 56.30 | Low | 0 |
| 5 | 265.31 | 0.09 | 2.02 | 89.85 | 0 | 0 | 78.01 | High | 0 |
| 6 | 310.31 | 0.13 | 1.44 | 135.67 | 0 | 0 | 62.19 | High | 0 |
| 7 | 310.31 | 0.13 | 1.44 | 135.67 | 0 | 0 | 62.19 | High | 0 |
| 8 | 299.76 | 0.03 | 2.57 | 89.85 | 0 | 0 | 78.01 | High | 0 |
| 9 | 299.76 | 0.03 | 2.53 | 89.85 | 0 | 0 | 78.01 | High | 0 |
| 10 | 299.76 | 0.02 | 2.58 | 89.85 | 0 | 0 | 78.01 | High | 0 |
| 11 | 301.29 | 0.06 | 2.64 | 89.85 | 0 | 0 | 78.01 | High | 0 |
| 12 | 333.31 | 0.02 | 2.99 | 89.85 | 0 | 0 | 78.01 | High | 0 |
| 13 | 281.31 | 0.18 | 1.63 | 110.08 | 0 | 0 | 71.02 | High | 0 |
| 14 | 355.39 | 0.10 | 1.96 | 117.54 | 0 | 0 | 68.44 | High | 0 |
| 24 h | 5 days | 3 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Epi ED50 | HFF1 LD50 | S.I. LD50/ED50 | Epi ED50 | HFF1 LD50 | S.I. LD50/ED50 | Trypo LD50 | |
 | Bnz | 9.4 ± 1.3 (3) | 165 ± 27 (3) | 18 | 17 ± 10 (3) | 118 ± 14 (3) | 7 | 183 (2) |
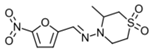 | Nfx | 3.8 ± 1.2 (3) | 55 ± 6 (3) | 15 | 11.8 ± 0.1 (3) | 50 ± 7 (3) | 4 | 86 (2) |
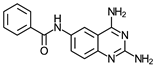 | 1 | >100 (5) | >100 (6) | 1 | >100 (1) | >100 (2) | 1 | >150 (2) |
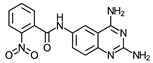 | 2 | 19 ± 2 (5) | >100 (6) | ≥5 | 15 ± 3 (3) | >100 (2) | ≥6.7 | 95 (2) |
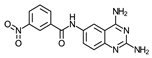 | 3 | 2.4 ± 0.4 (5) | >100 (6) | ≥42 | 8.9 (2) | >100 (2) | ≥11 | 50 (2) |
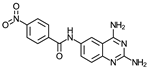 | 4 | 10 ± 0.4 (4) | >100 (6) | ≥10 | 10 (2) | >100 (2) | ≥10 | 60 (2) |
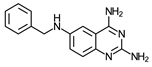 | 5 | 13 ± 3 (5) | >60 (4) | ≥5 | 14 ± 2 (3) | 26 (2) | ≥6 | >150 (2) |
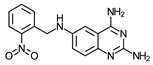 | 6 | 143 * (2) | – | – | – | – | – | – |
 | 7 | >100 * (2) | – | – | – | – | – | – |
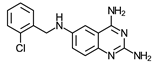 | 8 | 2.3 ± 0.5 (5) | 8.6 ± 2 (4) | 4 | 6.6 ± 0.5 (3) | 8 (2) | ≥2 | 59 (2) |
 | 9 | 7.6 ± 2.5 (5) | 85 ± 7 (6) | 11 | 8.1 ± 1.8 (3) | >100 (2) | ≥12 | >150 (2) |
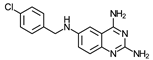 | 10 | 8.9 ± 2 (6) | 116 ± 13 (6) | 13 | 14 ± 1.2 (3) | >100 (2) | ≥7 | >150 (2) |
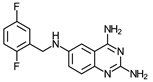 | 11 | 9 ± 1.4 (5) | >60 (4) | ≥7 | 20 ± 2 (3) | >60 (1) | ≥3 | >150 (2) |
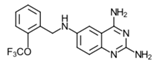 | 12 | 44 ± 3 (3) * | – | – | – | – | – | – |
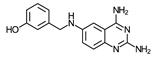 | 13 | >100 * (2) | – | – | – | – | – | – |
 | 14 | >100 * (2) | – | – | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, C.; Matus-Meza, A.-S.; Nuñez-Moreno, O.; Barbosa-Sánchez, B.M.; Farías-Gutiérrez, V.M.; Mendoza-Conde, M.; Hernández-Luis, F.; Saavedra, E. Exploring Quinazoline Nitro-Derivatives as Potential Antichagasic Agents: Synthesis and In Vitro Evaluation. Molecules 2024, 29, 4501. https://doi.org/10.3390/molecules29184501
Vázquez C, Matus-Meza A-S, Nuñez-Moreno O, Barbosa-Sánchez BM, Farías-Gutiérrez VM, Mendoza-Conde M, Hernández-Luis F, Saavedra E. Exploring Quinazoline Nitro-Derivatives as Potential Antichagasic Agents: Synthesis and In Vitro Evaluation. Molecules. 2024; 29(18):4501. https://doi.org/10.3390/molecules29184501
Chicago/Turabian StyleVázquez, Citlali, Audifás-Salvador Matus-Meza, Oswaldo Nuñez-Moreno, Brenda Michelle Barbosa-Sánchez, Victor Manuel Farías-Gutiérrez, Mariana Mendoza-Conde, Francisco Hernández-Luis, and Emma Saavedra. 2024. "Exploring Quinazoline Nitro-Derivatives as Potential Antichagasic Agents: Synthesis and In Vitro Evaluation" Molecules 29, no. 18: 4501. https://doi.org/10.3390/molecules29184501
APA StyleVázquez, C., Matus-Meza, A.-S., Nuñez-Moreno, O., Barbosa-Sánchez, B. M., Farías-Gutiérrez, V. M., Mendoza-Conde, M., Hernández-Luis, F., & Saavedra, E. (2024). Exploring Quinazoline Nitro-Derivatives as Potential Antichagasic Agents: Synthesis and In Vitro Evaluation. Molecules, 29(18), 4501. https://doi.org/10.3390/molecules29184501








