A WO3–CuCrO2 Tandem Photoelectrochemical Cell for Green Hydrogen Production under Simulated Sunlight
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphological Characterization of Thin-Film Electrodes
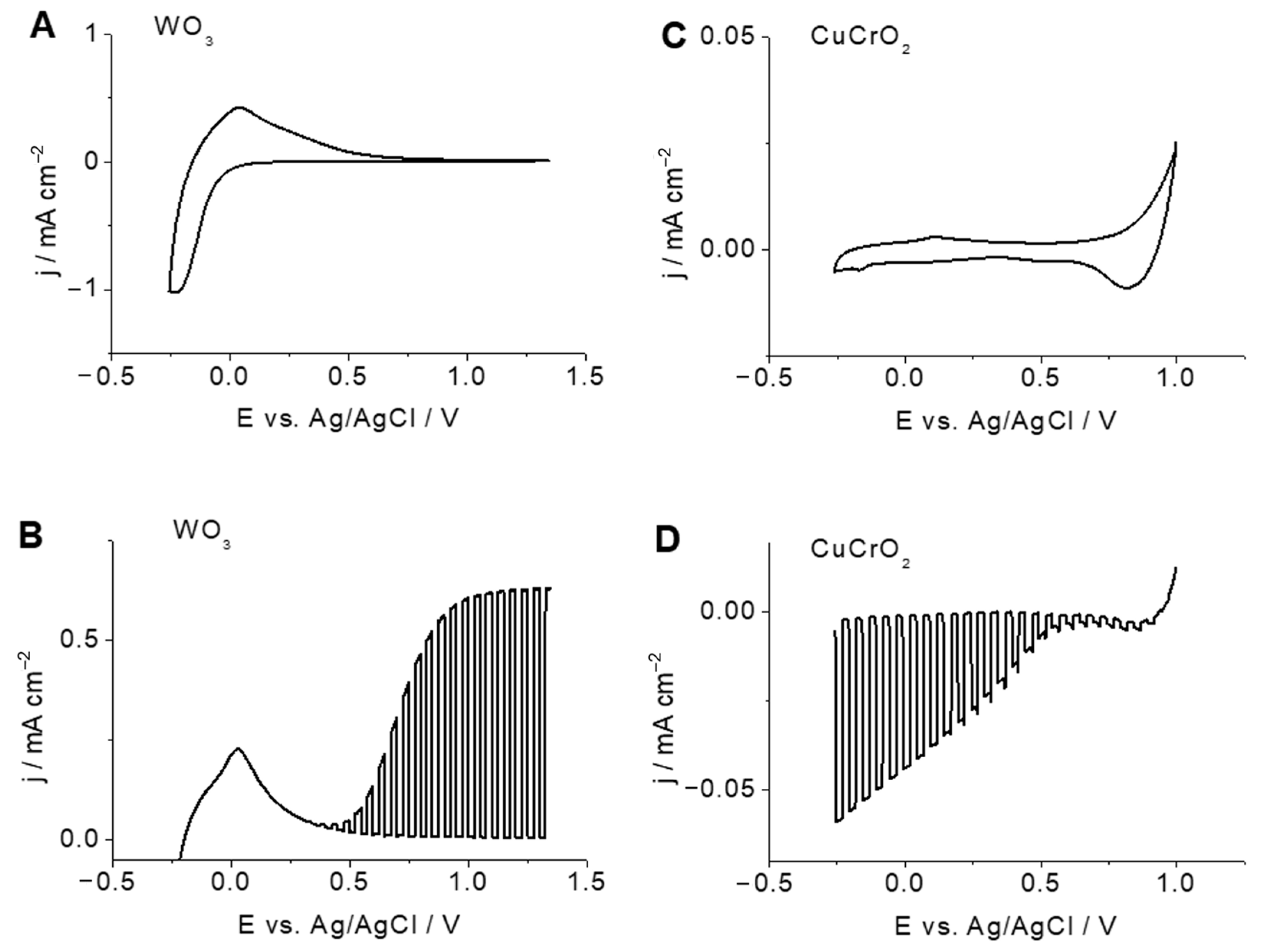
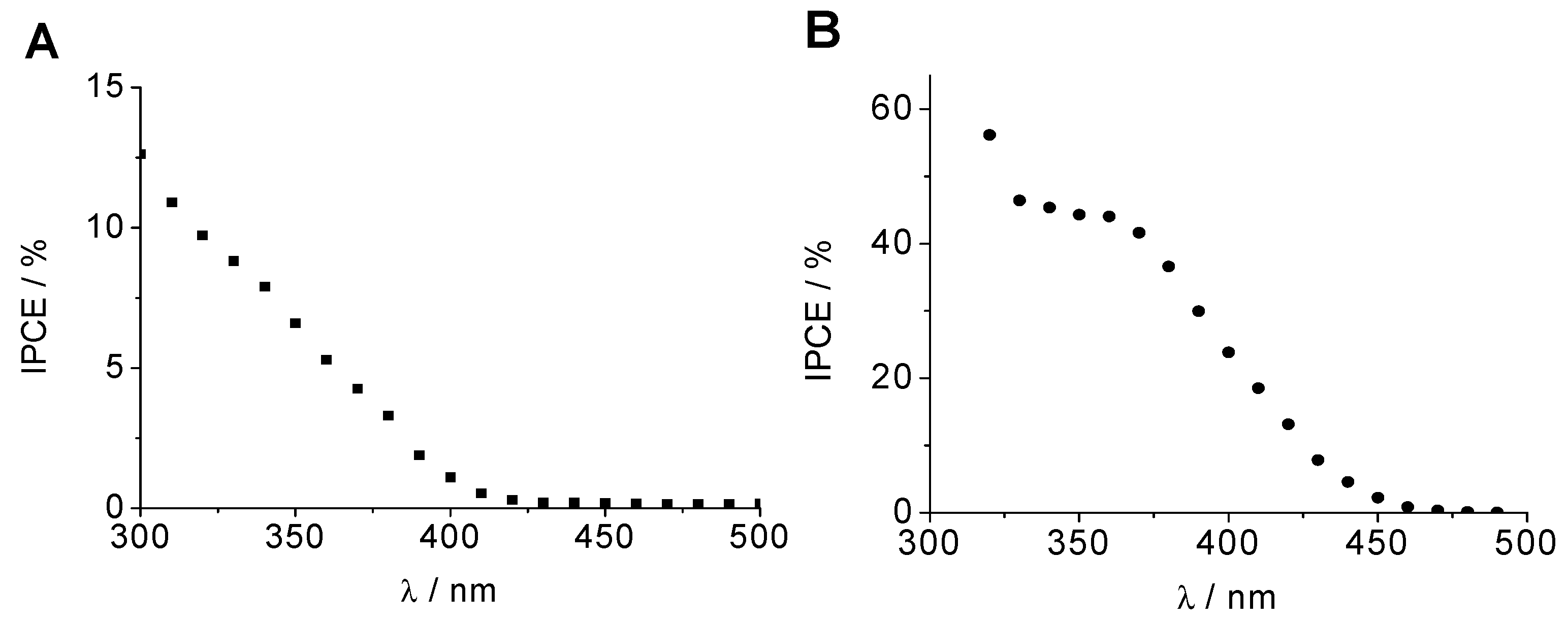
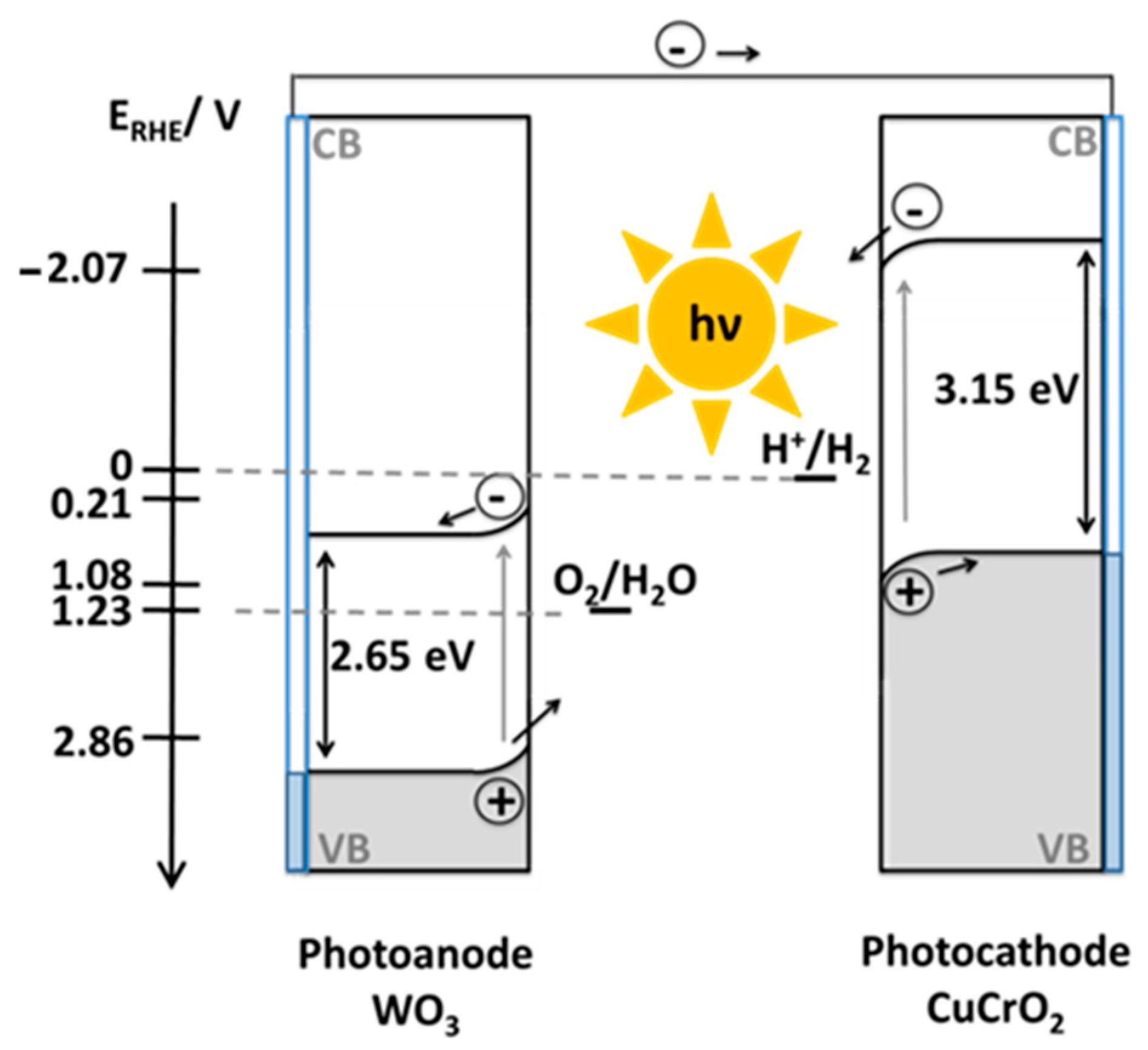
2.2. Photoelectrochemical Properties of WO3 Photoanodes and CuCrO2 Photocathodes
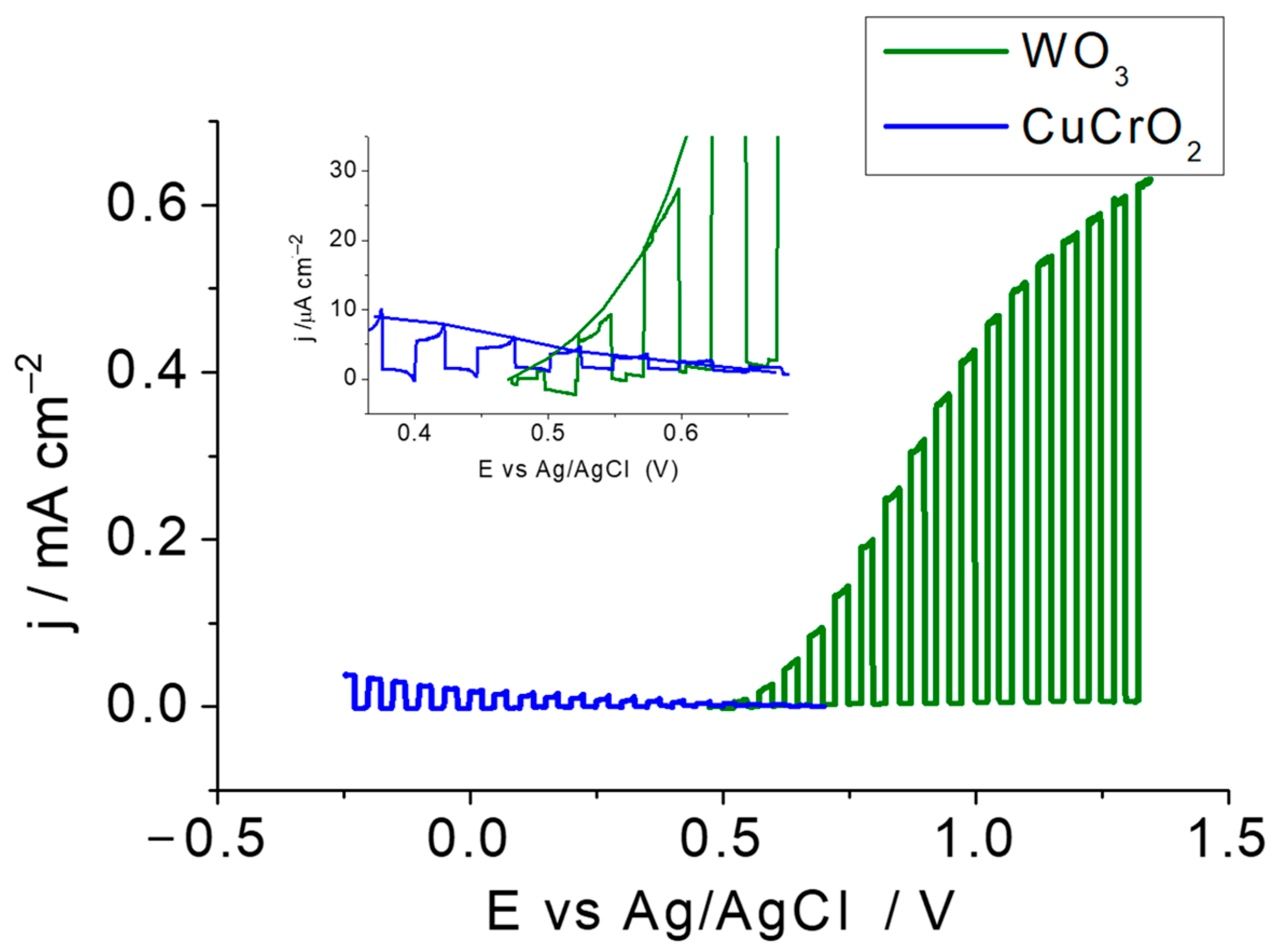
2.3. Operation and Stability of the Tandem Cell
3. Materials and Methods
3.1. Electrode Preparation
3.2. Photoelectrochemical Measurements
3.3. Tandem Cell Measurements
3.4. Hydrogen Evolution Detection by Gas Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van de Krol, R.; Grätzel, M. Photoelectrochemical Hydrogen Production; Springer: New York, NY, USA, 2012. [Google Scholar]
- Prévot, M.S.; Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Lewis, N.S. Developing a scalable artificial photosynthesis technology through nanomaterials by design. Nat. Nanotechnol. 2016, 11, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kim, T.W.; Kubota, S.R.; Cardiel, A.C.; Cha, H.G.; Choi, K.S. Electrochemical Synthesis of Photoelectrodes and Catalysts for Use in Solar Water Splitting. Chem. Rev. 2015, 115, 12839–12887. [Google Scholar] [CrossRef] [PubMed]
- Harris-Lee, T.R.; Marken, F.; Bentley, C.L.; Zhang, J.; Johnson, A.L. A chemist’s guide to photoelectrode development for water splitting—the importance of molecular precursor design. EES. Catal. 2023, 1, 832–873. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Zhang, G.; Gong, Z.; Wu, B.; Wang, T.; Gong, J. Tandem cells for unbiased photoelectrochemical water splitting. Chem. Soc. Rev. 2023, 52, 4644–4671. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Jung, G.; Min, J.; Shin, B. Earth-Abundant Metal Oxides for Monolithic Tandem Photoelectrochemical Water Splitting Devices: Current Trends and Perspectives. ACS Mater. Lett. 2024, 6, 2919–2940. [Google Scholar] [CrossRef]
- Lopes, T.; Andrade, L.; Le Formal, F.; Grätzel, M.; Sivula, K.; Mendes, A. Hematite photoelectrodes for water splitting: Evaluation of the role of film thickness by impedance spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 16515–16523. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Chen, X.; Ye, J.; Ouyang, S.; Kako, T.; Li, Z.; Zou, Z. Enhanced incident photon-to-electron conversion efficiency of tungsten trioxide photoanodes based on 3d-photonic crystal design. ACS Nano 2011, 4310–4318. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 43, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Steier, L.; Son, M.K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Prévot, M.S.; Guijarro, N.; Sivula, K. Enhancing the performance of a robust sol-gel-processed p-type delafossite CuFeO2 photocathode for solar water reduction. ChemSusChem 2015, 8, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Díez-García, M.I.; Gómez, R. Investigating Water Splitting with CaFe2O4 Photocathodes by Electrochemical Impedance Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 21387–21397. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Lana-Villarreal, T.; Gómez, R. Study of Copper Ferrite as a Novel Photocathode for Water Reduction: Improving Its Photoactivity by Electrochemical Pretreatment. ChemSusChem 2016, 9, 1504–1512. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Gómez, R. Metal Doping to Enhance the Photoelectrochemical Behavior of LaFeO3 Photocathodes. ChemSusChem 2017, 10, 2457–2463. [Google Scholar] [CrossRef]
- Díaz-García, A.K.; Lana-Villarreal, T.; Gómez, R. Sol-gel copper chromium delafossite thin films as stable oxide photocathodes for water splitting. J. Mater. Chem. A 2015, 3, 19683–19687. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Gómez, R. Progress in Ternary Metal Oxides as Photocathodes for Water Splitting Cells: Optimization Strategies. Solar RRL 2022, 6, 2100871. [Google Scholar] [CrossRef]
- Huang, Q.; Ye, Z.; Xiao, X. Recent Progress in Photocathodes for Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 15824–15837. [Google Scholar] [CrossRef]
- Ohashi, K.; McCann, J.; Bockris, J.O. Stable photoelectrochemical cells for the splitting of water. Nature 1977, 266, 610–611. [Google Scholar] [CrossRef]
- Ingler, W.B., Jr.; Khan, S.U.M. A Self-Driven p/n-Fe2O3 Tandem Photoelectrochemical Cell for Water Splitting. Electrochem. Solid State Lett. 2006, 9, G144–G146. [Google Scholar] [CrossRef]
- Ida, S.; Yamada, K.; Matsunaga, T.; Hagiwara, H.; Matsumoto, Y.; Ishihara, T. Preparation of p-type CaFe2O4 photocathodes for producing hydrogen from water. J. Am. Chem. Soc. 2010, 132, 17343–17345. [Google Scholar] [CrossRef] [PubMed]
- Bornoz, P.; Abdi, F.F.; Tilley, S.D.; Dam, B.; Van De Krol, R.; Grätzel, M.; Sivula, K. A bismuth vanadate-cuprous oxide tandem cell for overall solar water splitting. J. Phys. Chem. C. 2014, 118, 16959–16966. [Google Scholar] [CrossRef]
- Singh, M.R.; Papadantonakis, K.; Xiang, C.; Lewis, N.S. An electrochemical engineering assessment of the operational conditions and constraints for solar-driven water-splitting systems at near-neutral pH. Energy Environ. Sci. 2015, 8, 2760–2767. [Google Scholar] [CrossRef]
- Lichterman, M.F.; Sun, K.; Hu, S.; Zhou, X.; McDowell, M.T.; Shaner, M.R.; Richter, M.H.; Crumlin, E.J.; Carim, A.I.; Saadi, F.H.; et al. Protection of inorganic semiconductors for sustained, efficient photoelectrochemical water oxidation. Catal. Today 2016, 262, 11–23. [Google Scholar] [CrossRef]
- Kim, J.H.; Kaneko, H.; Minegishi, T.; Kubota, J.; Domen, K.; Lee, J.S. Overall photoelectrochemical water splitting using tandem cell under simulated sunlight. ChemSusChem 2016, 9, 61–66. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y. A Cu2O/Cu2S-ZnO/CdS tandem photoelectrochemical cell for self-driven solar water splitting. J. Alloys Compd. 2017, 698, 133–140. [Google Scholar] [CrossRef]
- Arunachalam, M.; Kanase, R.S.; Badiger, J.G.; Sayed, S.A.; Ahn, K.-S.; Ha, J.-S.; Ryu, S.-W.; Kang, S.H. Durable bias-free solar Water-Splitting cell composed of n+ p-Si/Nb2O5/NiPt photocathode and W:BiVO4/NiCo(O-OH)2 photoanode. Chem. Eng. J. 2023, 474, 145262. [Google Scholar] [CrossRef]
- Osterloh, F.F. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Borja, L.; Rodes, A.; Gómez, R.; Salvador, P. Photoelectrochemical Behavior of Nanostructured WO3 Thin-Film Electrodes: The Oxidation of Formic Acid. ChemPhysChem 2006, 7, 2540–2551. [Google Scholar] [CrossRef]
- Paracchino, A.; Brauer, J.C.; Moser, J.E.; Thimsen, E.; Graetzel, M. Synthesis and characterization of high-photoactivity electrodeposited Cu2O solar absorber by photoelectrochemistry and ultrafast spectroscopy. J. Phys. Chem. C 2012, 116, 7341–7350. [Google Scholar] [CrossRef]
- Dotan, H.; Mathews, N.; Hisatomi, T.; Grätzel, M.; Rothschild, A. On the solar to hydrogen conversion efficiency of photoelectrodes for water splitting. J. Phys. Chem. Lett. 2014, 5, 3330–3334. [Google Scholar] [CrossRef] [PubMed]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Du, C.; Yang, J.; Yang, J.; Zhao, Y.; Chen, R.; Shan, B. An iron oxide -copper bismuth oxide photoelectrochemical cell for spontaneous water splitting. Int. J. Hydrogen Energy 2018, 43, 22807–22814. [Google Scholar] [CrossRef]
- Ye, S.; Shi, W.; Liu, Y.; Li, D.; Yin, H.; Chi, H.; Luo, Y.; Ta, N.; Fan, F.; Wang, X.; et al. Unassisted Photoelectrochemical Cell with Multimediator Modulation for Solar Water Splitting Exceeding 4% Solar-to-Hydrogen Efficiency. J. Am. Chem. Soc. 2021, 143, 12499–12508. [Google Scholar] [CrossRef]
- Prévot, M.S.; Li, Y.; Guijarro, N.; Sivula, K. Improving charge collection with delafossite photocathodes: A host–guest CuAlO2/CuFeO2 approach. J. Mater. Chem. A 2016, 4, 3018–3026. [Google Scholar] [CrossRef]
- Lalanne, M.; Barnabé, A.; Mathieu, F.; Tailhades, P. Synthesis and thermostructural studies of a CuFe1−xCrxO2 delafossite solid solution with 0 ≤ x ≤ 1. Inorg. Chem. 2009, 48, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Taddee, C.; Kamwanna, T.; Amornkitbamrung, V. Characterization of transparent superconductivity Fe-doped CuCrO2 delafossite oxide. Appl. Surf. Sci. 2016, 380, 237–242. [Google Scholar] [CrossRef]
- Liew, S.L.; Subramanian, G.S.; Seng Chua, C.; Luo, H.-K. Studies into the Yb-doping effects on photoelectrochemical properties of WO3 photocatalysts. RSC Adv. 2016, 6, 19452–19458. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef]
- Costa, M.B.; de Araújo, M.A.; Tinoco, M.V.D.L.; Brito, J.F.D.; Mascaro, L.H. Current trending and beyond for solar-driven water splitting reaction on WO3 photoanodes. J. Energy Chem. 2022, 73, 88–113. [Google Scholar] [CrossRef]
- Luo, J.; Hepel, M. Photoelectrochemical degradation of naphthol blue black diazo dye on WO3 film electrode. Electrochim. Acta 2001, 46, 2913–2922. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-García, A.K.; Gómez, R. A WO3–CuCrO2 Tandem Photoelectrochemical Cell for Green Hydrogen Production under Simulated Sunlight. Molecules 2024, 29, 4462. https://doi.org/10.3390/molecules29184462
Díaz-García AK, Gómez R. A WO3–CuCrO2 Tandem Photoelectrochemical Cell for Green Hydrogen Production under Simulated Sunlight. Molecules. 2024; 29(18):4462. https://doi.org/10.3390/molecules29184462
Chicago/Turabian StyleDíaz-García, Ana K., and Roberto Gómez. 2024. "A WO3–CuCrO2 Tandem Photoelectrochemical Cell for Green Hydrogen Production under Simulated Sunlight" Molecules 29, no. 18: 4462. https://doi.org/10.3390/molecules29184462
APA StyleDíaz-García, A. K., & Gómez, R. (2024). A WO3–CuCrO2 Tandem Photoelectrochemical Cell for Green Hydrogen Production under Simulated Sunlight. Molecules, 29(18), 4462. https://doi.org/10.3390/molecules29184462





