Abstract
Developing red fluorescence emitters with simple structures via convenient synthetic routes is highly desirable yet challenging. Herein, two novel donor–acceptor-type red emitters, DCFOPV-TPA and SCFOPV-TPA, featuring the intramolecular charge transfer effect were designed by integrating triphenylamine and trifluoromethyl into a CN-substituted oligo(p-phenylene vinylene) backbone. Both chromophores exhibited aggregation-induced enhanced emission and solvatochromic behavior. Moreover, DCFOPV-TPA also displayed reversible mechanofluorochromic properties under external force.
1. Introduction
Mechanofluorochromic (MFC) organic materials, which respond to external physical stimuli, such as shearing, grinding, or compressing [1,2,3], have attracted much attention due to their potential applications in memory sensors [4], security inks [5], data storage [6], fluorescence switches [7], fluorescent probes [8], and optoelectronic devices [9]. Compared with inorganic compounds [10], metal complexes [11,12], dendrimers [13], and polymers [14], organic luminescent molecules have the advantages of low cost, structural tunability, functional controllability, large-scale production, and rich fluorescent performance, making them promising candidates for mechanoresponsive smart materials [15]. However, traditional organic emitters exhibiting strong fluorescence in solution normally contain planar, rigid, and π-conjugated polycyclic structures, which causes non or weakly emissive aggregated or solid state due to aggregation-caused quenching (ACQ) effects [16,17]. To address this issue, Tang and co-workers accidentally discovered that non-planar-twisted molecular structures exhibited aggregation-induced emissions (AIEs), which could effectively suppress the ACQ phenomenon and lead to the fabrication of efficient solid-state fluorescent materials [18,19]. Meanwhile, the discovery of the AIE phenomenon also provides valuable guidance for mechanochromic organic materials, considering that mechanochromism is the common nature of AIE luminogens [20,21,22].
Cyano-substituted oligo(p-phenylene vinylenes) (CN-OPVs) are an important class of conjugated materials with high solid-state efficiency due to the non-planar geometry with reduced π-π stacking interactions [23,24,25,26,27,28,29,30]. Through suitable molecular design, CN-OPVs bearing different functional segments or substituents, such as triphenylamine (TPA) [31,32,33], carbazole [34,35], trifluoromethyl (CF3) [36,37], and tetraphenylethene [38], have been developed with excellent mechanochromic properties. Meanwhile, alkyl (alkoxy) length [39,40,41] and position [42], donor–acceptor interactions [43,44,45], supramolecular interactions [46], and crystallinity [47,48] also play important roles in developing smart mechanochromic materials. Their work mechanisms are usually associated with molecular conformation and packing modes, which undergo a phase transition from a crystalline nature to an amorphous phase [49] or between two different crystalline states [50,51]. The variations in solid-state morphologies further affect the intramolecular charge transfer (ICT) [52] and excited-state switching process [53]. During the above process, the energy level of the molecule is affected, and thus, the solid emission color is changed, which can be converted back to the initial state after exposure to either solvent vapor or thermal treatment.
Organic molecules possessing donor and acceptor moieties are promising candidates for mechanochromic materials due to the possible generation of a twisted intramolecular charge transfer excited state, which is highly sensitive to external mechanical force and can cause shifts in the corresponding photoluminescence spectra [54,55]. Meanwhile, a suitable donor–acceptor functionality in the molecule lowers the optical band gap and results in a red/NIR emission, which is suitable for imaging applications [56]. Our group has reported the self-assembly properties of OPVs via the modulation of the conjugation length, regioregularity, hydrogen bonding, and rigid morphon linker [57,58,59,60]. Recently, we developed solvent-induced multistimuli-responsive properties of CN-OPVs [61]. According to our previous experience and the relevant literature [62,63,64], we herein developed the preparation of two twisted donor–acceptor molecules, DCFOPV-TPA and SCFOPV-TPA, with a different number and position of the CF3 group installed (Scheme 1). TPA was utilized as the electron-donating group, which could regulate molecular packing due to the propeller-like structure. Dicyanovinylbenzene was utilized as the electron-withdrawing group, which also allowed for versatile conformational arrangement through twist elasticity. The introduction of CF3 groups could further tune the electronic configuration and conformational structure. Both compounds exhibited aggregation-induced enhanced emission (AIEE) behavior in the THF/H2O system and bathochromic shift with increasing solvent polarity. The ICT nature of DCFOPV-TPA and SCFOPV-TPA was further confirmed by DFT calculations. Compared with SCFOPV-TPA with no MFC behavior, DCFOPV-TPA displayed an MFC phenomenon with an emission wavelength red-shift of 25 nm, probably due to the larger distortion angle and more pronounced ICT effect of DCFOPV-TPA.
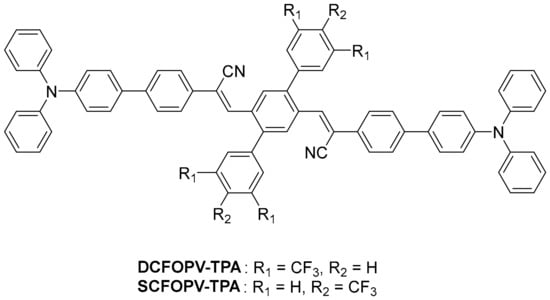
Scheme 1.
The molecular structures of target compounds.
2. Results
2.1. Chemical Synthesis and Characterization
The synthetic procedure for DCFOPV-TPA and SCFOPV-TPA is described in Scheme 2. Compound 1 was prepared via an electrophilic substitution reaction with NBS according to the previous literature [65]. A subsequent Suzuki–Miyaura cross-coupling reaction between aldehyde 1 and trifluoromethyl-substituted phenyl boronic acids was performed to give the corresponding products 2a and 2b 80% and 83% yields, respectively. Next, Knoevenagel condensation between 2 and 2-(4-iodophenyl) acetonitrile using MeONa as the base generated the corresponding products 3a and 3b in an up to 85% yield. Finally, the target compounds, DCFOPV-TPA and SCFOPV-TPA, were obtained via a Pd-catalyzed Suzuki–Miyaura cross-coupling reaction in 78% and 79% yields, respectively. All the new compounds were fully characterized by 1H NMR, 13C NMR, and HRMS analysis (Figures S1–S13).
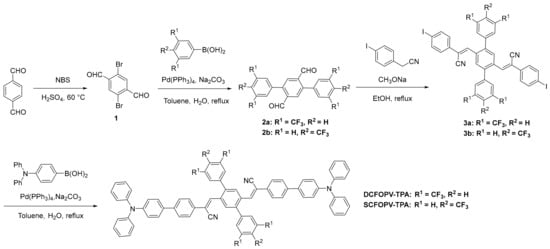
Scheme 2.
The synthetic route for target molecules.
2.2. UV–Vis Absorption and Fluorescent Emission Spectra in Solutions
The UV-vis absorption and fluorescent emission spectra of DCFOPV-TPA and SCFOPV-TPA in various solvents were initially investigated (Figure 1), and the key photophysical parameters are summarized in Table S1. Due to the inclusion of the electron-donating TPA group and electron-withdrawing cyano moiety, both DCFOPV-TPA and SCFOPV-TPA are donor–acceptor (D-A)-type molecules, which exhibit a typical ICT process. As expected, both compounds exhibited two intense absorption bands in the 280–550 nm region (Figure 1a,b). The absorption peaks located at around 335 nm originated from the π–π* transition, while the absorption bands ranging from 380 nm to 550 nm can be ascribed to the ICT transition from the electron-rich TPA segment to the electron-deficient cyano-substituted vinylbenzene moiety. With the increasing solvent polarity, both DCFOPV-TPA and SCFOPV-TPA exhibit similar absorption profiles with very slightly blue-shifted phenomena, suggesting little difference in the dipole moment in the ground state. For example, in the nonpolar solvents cyclohexane and toluene, the ICT band of DCFOPV-TPA is located at 436 nm and 438 nm, which is blue-shifted to 431 nm, 423 nm, 415 nm, and 419 nm in the polar solvents CHCl3, DCM, THF, and 1,4-dioxane. It could be that larger dihedral angles in more polar solvents lead to a lower degree of conjugation, which further causes the absorption peak to be blue-shifted [66,67]. To support this hypothesis, density functional theory (DFT) calculations were performed for DCFOPV-TPA (Table S2). Clearly, the dihedral angles of DCFOPV-TPA in the CHCl3, DCM, and THF solvents are larger compared with those in cyclohexane and toluene, which, consequently, leads to the blue-shifted simulated UV-vis absorption peaks.
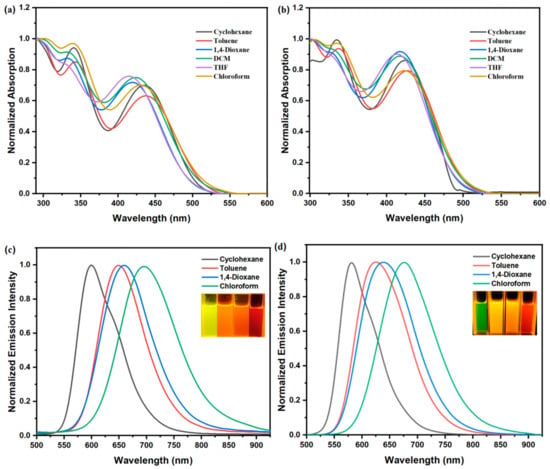
Figure 1.
Normalized UV–vis absorption spectra of DCFOPV-TPA (a) and SCFOPV-TPA (b), and normalized PL spectra of DCFOPV-TPA ((c), λex = 480 nm) and SCFOPV-TPA ((d), λex = 480 nm) in different solvents (1.0 × 10−5 mol L−1). Photos of DCFOPV-TPA and SCFOPV-TPA in different solvents under UV light irradiation (from left to right: cyclohexane, toluene, 1,4-dioxane, chloroform, and λex = 365 nm).
Figure 1c,d show the fluorescence spectra of the compounds DCFOPV-TPA and SCFOPV-TPA in solvents varying in polarity, which demonstrate prominent solvatochromic behavior, consistent with the strong ICT character of a solvent-relaxed emissive state. By increasing the solvent polarity, a significant bathochromic shift and reduction in the vibration structure in emission spectra were observed, suggesting the enhanced dipole moment of the polarized excited state when stabilized by more polar solvents. For example, in nonpolar cyclohexane, DCFOPV-TPA exhibited yellow light centered at 600 nm, with a vibrational structure appearing as a shoulder in the emission band. With increasing solvent polarity, the emission bands were gradually red-shifted to 649 nm (in toluene), 659 nm (in 1,4-dioxane), and 695 nm (in CHCl3). For SCFOPV-TPA, the emission wavelength was located at 581 nm in cyclohexane with a green color, which was red-shifted to 625 nm (in toluene), 638 nm (in 1,4-dioxane), and 676 nm (in CHCl3). Compared with SCFOPV-TPA, DCFOPV-TPA exhibited an increased ICT effect, considering the larger maximum ICT absorption and emission bands of DCFOPV-TPA. The fluorescence quantum yielded (Φf) of DCFOPV-TPA and SCFOPV-TPA, which were determined using fluorescein (Φf = 0.92, 0.1 mol L−1) as the standard, and both showed stronger emissions in the nonpolar solvent (0.72 and 0.69 in cyclohexane) than polar solvent (0.20 and 0.08 in CHCl3) because of the restricted ICT transitions. The further increasing solvent polarity in THF and DCM led to a completely quenched emission efficiency, indicating a positive solvatokinetic effect.
2.3. Theoretical Calculation
To better understand their optical properties, DFT calculations were performed at the B3LYP/6-311++G (d, p) level of theory via the Gaussian 16 program package to clarify the geometric and electronic structures of DCFOPV-TPA and SCFOPV-TPA. As shown in Figure 2, the highest occupied molecular orbitals (HOMOs) of DCFOPV-TPA and SCFOPV-TPA were mainly delocalized on the TPA moiety, while the lowest unoccupied molecular orbitals (LUMOs) were generally distributed over the dicyanovinylbenzene framework, suggesting a typical ICT process. According to their optimized lowest energy state, both DCFOPV-TPA and SCFOPV-TPA adopted distorted molecular conformations, which could suppress close packing arrangements and consequently reduce the effective π-π interaction between neighboring molecules. For DCFOPV-TPA, in detail, the dihedral angles between the central benzene and the vinyl plane were 30.45°. Meanwhile, the dihedral angle between the central core and the CF3-substituted benzene arms was 54.89°. Compared with DCFOPV-TPA, the smaller value of 53.81° was observed in the case of SCFOPV-TPA, probably due to the relatively smaller steric hindrance. Such large twist angles in both DCFOPV-TPA and SCFOPV-TPA could be beneficial to AIE characteristics and MFC behavior.
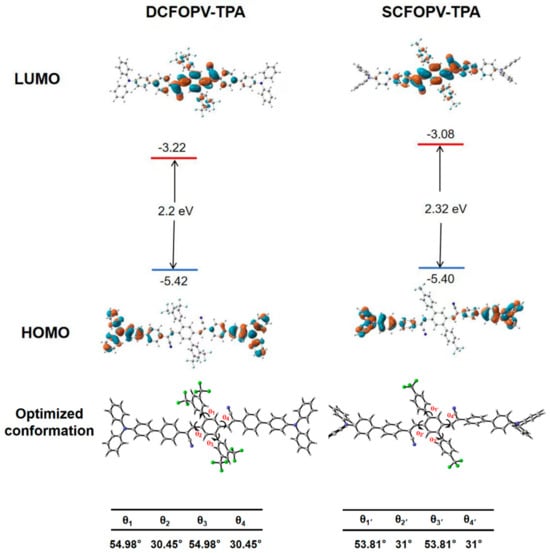
Figure 2.
Illustration of spatial electron distribution of HOMOs and LUMOs and optimized molecular geometry of DCFOPV-TPA and SCFOPV-TPA. The DFT calculations were carried out using the Gaussian 16 program at the B3LYP/6-311++G(d, p) level of theory.
2.4. Aggregation-Induced Enhanced Emission (AIEE)
CN-OPVs and TPA are common molecular skeletons with excellent aggregation-induced optical properties, which have been regarded as an effective strategy for developing anti-ACQ chromophores. To examine the AIEE behavior of DCFOPV-TPA and SCFOPV-TPA, the UV-vis absorption and fluorescence spectra were investigated in dilute mixtures of THF/water with different volume fractions of water (fw). As shown in Figure 3, the absorption profiles of DCFOPV-TPA and SCFOPV-TPA showed a significant difference when fw was above 60%, accompanied by a bathochromic shift from 415 (fw = 0%) to 429 (fw = 90%) and from 413 nm (fw = 0%) to 441 nm (fw = 90%), respectively. Meanwhile, the appearance of a leveling-off tail in the visible region due to the Mie effect further suggests the formation of nanoaggregation in the solvent mixture (fw ≥ 60%).
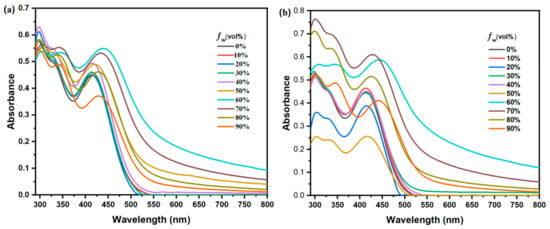
Figure 3.
UV–vis absorption spectra of DCFOPV-TPA (a) and SCFOPV-TPA (b) in THF/water mixtures with different fw.
Similarly, both DCFOPV-TPA and SCFOPV-TPA emit weak fluorescence in a pure THF solution excited by light (Figure 4). When fw is less than 60%, little change in the emission bands is observed, probably caused by the balance between the solvation effect and the molecular aggregation effect. When fw is above 50–60%, the fluorescence intensity increases significantly due to the formation of nanoaggregates. In the aggregation state, intramolecular rotation and vibration are inhibited. The rotation and vibration of the chemical bonds require energy consumption, and non-radiative dissipation is suppressed, resulting in increased luminous intensity. Further increasing the water fractions (fw) leads to the gradual enhancement of fluorescence efficiency accompanied by a blue-shifted emission wavelength, ascribed to the encapsulation of DCFOPV-TPA and SCFOPV-TPA in a less polar environment once nanoaggregates are formed. The maximum fluorescence efficiency of DCFOPV-TPA and SCFOPV-TPA was obtained at fw = 90%, which was about 14-fold and 12-fold, respectively, relative to the original efficiency in a pure THF solution. Finally, according to a dynamic light scattering (DLS) experiment, the average particle sizes (Zaverage) of DCFOPV-TPA and SCFOPV-TPA at the aggregate state (fw = 90%) were found to be 274.9 and 298.7 nm, respectively, which further confirmed the formation of nanoparticles in solutions.
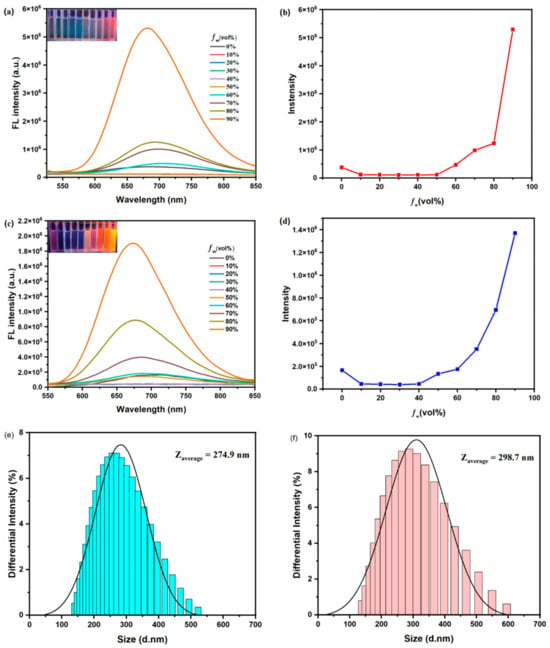
Figure 4.
PL spectra of DCFOPV-TPA (a), λex = 480 nm and SCFOPV-TPA (c), λex = 480 nm in THF/water mixtures with different fw. FL intensity versus water fraction of DCFOPV-TPA (b) and SCFOPV-TPA (d). The insets of (a) and (c) depict the fluorescence images of DCFOPV-TPA and SCFOPV-TPA with various water fractions (from 0% to 90%, excitation wavelength: 365 nm), respectively. DLS of DCFOPV-TPA (e) and SCFOPV-TPA (f) in 90% THF/water mixtures. The concentration was 10 μM.
2.5. Mechanofluorochromic (MFC) Properties
Considering the MFC properties of most AIEE chromophores, the fluorescent emission of DCFOPV-TPA and SCFOPV-TPA in the solid was studied (Figure 5 and Figure 6). Upon excitation, both DCFOPV-TPA and SCFOPV-TPA exhibited a bright reddish color, with emission maxima determined to be 607 nm and 639 nm, respectively. Interestingly, only compound DCFOPV-TPA displayed reversible MCF behavior, while SCFOPV-TPA had no MFC properties, probably due to the more steric hindrance nature of DCFOPV-TPA. Specifically, the as-prepared DCFOPV-TPA exhibited an emission peak at 607 nm, which was red-shifted to 632 nm in the ground state with a dark red color. To check the reversibility of MFC behavior, the ground sample of DCFOPV-TPA was fumed with ethyl acetate for 1 min. Luckily, the dark-red-emitting ground powder could be transferred into a reddish color accompanied by a blue-shifted emission wavelength, which corresponded to the as-prepared DCFOPV-TPA solid.
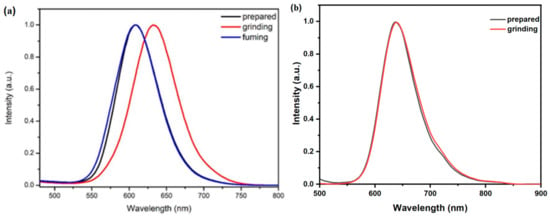
Figure 5.
(a) Normalized fluorescent spectra of DCFOPV-TPA (a) and SCFOPV-TPA (b) in the following different solid states: as-prepared, grinding, and fuming (λex = 425 nm).
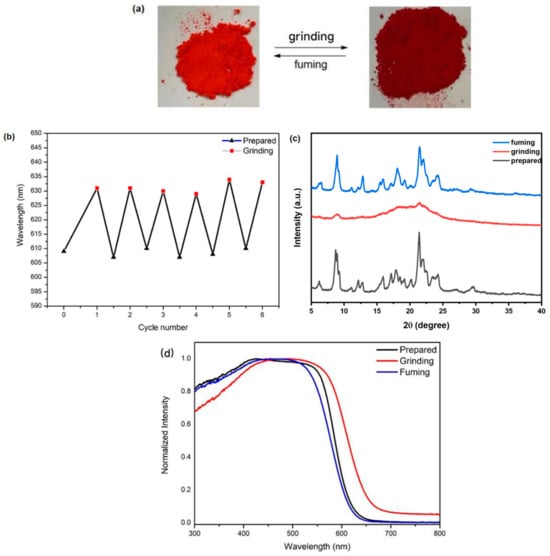
Figure 6.
(a) Photographs of DCFOPV-TPA color changes under grinding and fuming stimuli. (b) Cyclic diagram of stimulus–response performance of DCFOPV-TPA. (c) PXRD patterns of DCFOPV-TPA in the following different solid states: as-prepared, grinding, and fuming. (d) Normalized UV-vis spectra of DCFOPV-TPA in different states.
Importantly, the above grinding–fuming process could be repeated several times, suggesting the reversible nature of MFC properties of DCFOPV-TPA under external stimuli. To further clarify the mechanism of the MFC phenomenon, powder X-ray diffraction (PXRD) patterns for DCFOPV-TPA in different solids were examined. As shown in Figure 6c, the as-prepared DCFOPV-TPA solid in PXRD displayed multiple intense and sharp diffraction peaks, suggesting the existence of crystalline forms. On the contrary, very weak and broad diffraction peaks were observed for the ground state of DCFOPV-TPA, suggesting the formation of an amorphous state. It was hypothesized that external force leads to a collapse in the crystalline lattice, leading to red-shifted emissions due to the disordered molecular packing and greater planar conformation. Compared with solid absorption peaks of as-prepared (425 nm) DCFOPV-TPA, the grinding state was red-shifted to 447 nm, further proving a more effective conjugation length after grinding.
3. Materials and Methods
3.1. General Methods
All chemicals were commercially available and utilized without further purification unless otherwise specified. Column chromatography was performed using 200–300 mesh silica gel. 1H NMR, 13C NMR, and 19F NMR spectra were recorded on a Bruker DPX 600 instrument (Brooke Technologies Co., Ltd., Zurich, Switzerland) using TMS as the internal standard. HRMS was determined on a waters UPLC G2-XS Qtof system ESI spectrometer (Waters World Science & Technology Co., Ltd., Wilmslow, UK). The UV-vis absorption and fluorescent emission spectra were assessed by a dual-beam UV-vis spectrophotometer (TU-1901) (Agilent, Inc., Santa Clara, CA, USA) and Hitachi F-7000 spectrofluorimeter (Hitachi High-tech (Shanghai) International Trade Co., Ltd., Shanghai, China), respectively. Powder X-ray diffraction (PXRD) was performed on an X’Pert PRO Powder X-ray diffraction instrument (Holland PANalytical, Almelo, The Netherlands). The DFT calculations were carried out using the Gaussian 16 program at the B3LYP/6-311++G (d, p) level of theory, and the solvent effects of THF were simulated by the SMD mode.
3.2. General Procedure for Preparation of 2,5-Dibromoterephthalaldehyde 1
A 100 mL two-necked round-bottom flask had terephthalaldehyde (3.0 g, 22.39 mmol) added to it in concentrated H2SO4 (30 mL), which was heated at 60 °C, followed by the addition of NBS (7.97 g, 44.78 mmol) after 15 min. After stirring at 60 °C for 3 h, the reaction mixture was poured into ice water (30 mL), and the precipitation was filtered. Next, the precipitation was redissolved in CH2Cl2 (30 mL), which was washed with NaHCO3 three times. After the removal of the organic solvent, the crude product was purified via recrystallization in CHCl3 to yield compound 1 as the yellow solid (2.61 g, 40% yield). Compound 1 was a known compound, and 1H NMR data were the same as in the previous report [65]. 1H NMR (600 MHz, CDCl3): δ ppm 10.35 (s, 2H), 8.15 (s, 2H).
3.3. General Procedure for Preparation of Compound 2
To a 100 mL Schlenk flask, compound 1 (0.5 g, 1.71 mmol), (3,5-bis(trifluoromethyl)phenyl)boronic acid (1.25 g, 4.79 mmol) or (4-(trifluoromethyl)phenyl)boronic acid (0.91 g, 4.79 mmol), Pd(PPh3)4 (18 mg, 0.015 mmol) and Na2CO3 (997 mg, 9.41 mmol) were added in a mixed solvent of toluene and water (37.5 mL, v/v = 3:1) under Ar. After refluxing for 24 h, the reaction mixture was cooled down to room temperature and the organic layer was collected. The aqueous layer was washed with CH2Cl2 three times. The organic layer was combined and concentrated. After the removal of the organic solvent, the organic residue was purified via column chromatography using petroleum ether/CH2Cl2 (v/v = 2/1) as an eluent to afford the corresponding products 2a and 2b. Compound 2 was a known compound, and 1H NMR data were the same as in the previous report [68].
2a: white solid (763 mg, 80% yield). 1H NMR (600 MHz, CDCl3): δ ppm 10.06 (s, 2H), 8.15 (s, 2H), 8.06 (s, 2H), 7.91 (s, 4H).
2b: white solid (599 mg, 83% yield). 1H NMR (600 MHz, CDCl3): δ ppm 10.07 (s, 2H), 8.12 (s, 2H), 7.81 (d, J = 8.0 Hz, 4H), 7.59 (d, J = 8.0 Hz, 4H).
3.4. General Procedure for Preparation of Compounds 3
To a 100 mL Schlenk flask, compound 2a (1.0 g, 1.79 mmol) or 2b (756 mg, 1.79 mmol) and 2-(4-iodophenyl)acetonitrile (1.25 g, 4.79 mmol) were added in dry EtOH (40 mL) under Ar, followed by the addition of MeONa (193 mg, 3.58 mmol). After refluxing for 8 h, the reaction mixture was cooled down to room temperature, and the precipitation was filtered. The obtained precipitation was washed with water, EtOH, and warm EtOAc in sequence to afford the corresponding products 3a and 3b.
3a: yellowish green solid (1.53 g, 85% yield). m.p.: 280 °C. 1H NMR (600 MHz, CDCl3): δ ppm 8.30 (s, 2H), 8.02 (s, 2H), 7.97 (s, 4H), 7.78 (d, J = 8.6 Hz, 4H), 7.43 (s, 2H), 7.29 (d, J = 8.6 Hz, 4H). HR-MS (ESI, m/z): [M + K]+ calcd for [C40H18I2F12N2K]+, 1046.8999. found, 1046.9003.
3b: yellowish green solid (1.12 g, 72% yield). m.p.: 292 °C. 1H NMR (600 MHz, CDCl3): δ ppm 8.25 (s, 2H), 7.82–7.73 (m, 8H), 7.62 (d, J = 8.0 Hz, 4H), 7.48 (s, 2H), 7.30 (d, J = 8.5 Hz, 4H). HR-MS (ESI, m/z): [M + Na]+ calcd for [C38H20F6I2N2Na]+, 894.9512. found, 894.9512.
3.5. General Procedure for Preparation of the Compounds DCFOPV-TPA and SCFOPV-TPA
To a 100 mL Schlenk flask, compound 3a (800 mg, 0.79 mmol) or 3b (1.56 g, 1.79 mmol), (4-(diphenylamino)phenyl)boronic acid (639 mg, 2.21 mmol), Pd(PPh3)4 (27.39 mg, 0.02 mmol) and Na2CO3 (460 mg, 4.35 mmol) were added in a mixed solvent of toluene and water (64 mL, v/v = 3:1) under Ar. After refluxing for 24 h, the reaction mixture was cooled down to room temperature, and the organic layer was collected. The aqueous layer was washed with CH2Cl2 three times. The organic layer was combined and concentrated. After removal of the organic solvent, the organic residue was purified via column chromatography using petroleum ether/CH2Cl2 (v/v = 2/1) as an eluent to afford the corresponding products DCFOPV-TPA and SCFOPV-TPA.
DCFOPV-TPA: red solid (765 mg, 78% yield). m.p.: 305 °C. 1H NMR (600 MHz, CDCl3): δ ppm 8.36 (s, 2H), 8.05 (d, J = 3.3 Hz, 6H), 7.68–7.63 (m, 8H), 7.53–7.50 (m, 4H), 7.49 (s, 2H), 7.31 (dd, J = 8.4, 7.5 Hz, 8H), 7.17 (dd, J = 8.5, 1.2 Hz, 12H), 7.08 (t, J = 7.4 Hz, 4H). 13C NMR (151 MHz, CDCl3) δ ppm 147.4, 142.5, 140.5, 139.3, 137.2, 134.0, 133.0, 132.7, 132.7, 132.5, 132.3, 131.1, 131.0, 129.4, 127.7, 127.2, 126.6, 125.8, 124.7, 124.0, 123.4, 123.3, 122.4, 122.1, 120.3, 117.5, 116.7, 100.0, 77.2, 77.0, 76.8. HRMS (ESI, m/z): [M + Na]+ calcd for [C76H46F12N4Na]+, 1265.3423. found, 1265.3424.
SCFOPV-TPA: yellowish green solid (1.56 g, 79% yield). m.p.: >320 °C. 1H NMR (600 MHz, CDCl3): δ ppm 8.29 (s, 2H), 7.79 (d, J = 8.1 Hz, 4H), 7.70–7.62 (m, 12H), 7.52 (s, 2H), 7.49 (d, J = 8.6 Hz, 4H), 7.28 (t, J = 7.9 Hz, 8H), 7.14 (dd, J = 8.2, 3.1 Hz, 12H), 7.06 (t, J = 7.4 Hz, 4H). 13C NMR (151 MHz, CDCl3) δ ppm 147.5, 142.2, 140.7, 138.9, 133.8, 133.1, 131.7, 130.9, 130.4, 129.4, 127.7, 127.2, 126.5, 125.8, 124.8, 123.4, 123.3, 117.8, 115.0. 77.2, 77.0, 76.8. HRMS (ESI, m/z): [M + Na]+ calcd for [C74H48F6N4Na]+, 1107.3856. found, 1107.3865.
4. Conclusions
In conclusion, two novel CN-substituted OPVs, DCFOPV-TPA and SCFOPV-TPA, equipped with TPA and CF3 segments, were designed and synthesized to investigate their photophysical properties. Both DCFOPV-TPA and SCFOPV-TPA exhibited a typical AIEE effect. Also, remarkable solvatochromic behavior was observed due to the highly distorted conformation and ICT transition within the D-A-type molecules. Moreover, DCFOPV-TPA demonstrated reversible MCF phenomena under external force due to phase transition from the crystalline to the amorphous state. The AIEE features and MCF performance of DCFOPV-TPA are beneficial for the development of smart materials with potential applications in mechanical sensors, data encryption, and security protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29184447/s1, Figures S1–S13: 1H NMR, 13C NMR, and HRMS analysis; Table S1: Photophysical properties of compounds DCFOPV-TPA and SCFOPV-TPA; Table S2: Optimized molecular geometry of DCFOPV-TPA in different solvents. Ref. [69] is cited in the Supplementary Materials.
Author Contributions
Y.P., X.Z. (Xinran Zhao) and X.Y. performed the experiments; Y.Y., Z.Z., B.S. and L.S. analyzed the data; W.Z. conducted the DFT calculations; X.Z. (Xinju Zhu), Y.C. and X.H. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the National Natural Science Foundation of China (Nos. 21803059, U1904212, and U2004191) is gratefully appreciated.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Chi, Z.-G.; Zhang, X.-Q.; Xu, B.-J.; Zhou, X.; Ma, C.-P.; Zhang, Y.; Liu, S.-W.; Xu, J.-R. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, C. Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials. Adv. Mater. 2016, 28, 1073–1095. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.-Y.; Bose, P.; Gao, Q.; Li, Y.-X.; Ganguly, R.; Zhao, Y.-L. Halogen-Assisted Piezochromic Supramolecular Assemblies for Versatile Haptic Memory. J. Am. Chem. Soc. 2017, 139, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-B.; Liu, S.-J.; Lin, W.-P.; Zhang, K.-Y.; Lv, W.; Huang, X.; Huo, F.-W.; Yang, H.-R.; Jenkins, G.; Zhao, Q.; et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014, 5, 3601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-Y.; Chen, X.-J.; Sun, G.-L.; Zhang, T.-W.; Liu, S.-J.; Zhao, Q.; Huang, W. Utilization of Electrochromically Luminescent Transition-Metal Complexes for Erasable Information Recording and Temperature-Related Information Protection. Adv. Mater. 2016, 28, 7137–7142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, X.; Zhang, Y.-M.; Zhu, S.-Y.; Zhang, L.-A.; Yu, B.-H.; Wang, K.; Yang, B.; Li, M.-J.; Zou, B.; et al. Dynamic Behavior of Molecular Switches in Crystal under Pressure and Its Reflection on Tactile Sensing. J. Am. Chem. Soc. 2015, 137, 931–939. [Google Scholar] [CrossRef]
- Ma, J.; Sun, R.; Xia, K.; Xia, Q.; Liu, Y.; Zhang, X. Design and Application of Fluorescent Probes to Detect Cellular Physical Microenvironments. Chem. Rev. 2024, 124, 1738–1861. [Google Scholar] [CrossRef]
- Pan, C.-F.; Chen, M.-X.; Yu, R.-M.; Yang, Q.; Hu, Y.-F.; Zhang, Y.; Wang, Z.-L. Progress in Piezo-Phototronic-Effect-Enhanced Light-Emitting Diodes and Pressure Imaging. Adv. Mater. 2016, 28, 1535–1552. [Google Scholar] [CrossRef]
- Xiao, G.-J.; Cao, Y.; Qi, G.-Y.; Wang, L.-R.; Liu, C.; Ma, Z.-W.; Yang, X.-Y.; Sui, Y.-M.; Zheng, W.-T.; Zou, B. Pressure Effects on Structure and Optical Properties in Cesium Lead Bromide Perovskite Nanocrystals. J. Am. Chem. Soc. 2017, 139, 10087–10094. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Chi, Z.-G.; Zhang, Y.; Liu, S.-W.; Xu, J.-R. Recent advances in mechanochromic luminescent metal complexes. J. Mater. Chem. C 2013, 1, 3376–3390. [Google Scholar] [CrossRef]
- Xue, P.-C.; Ding, J.-P.; Wang, P.-P.; Lu, R. Recent progress in the mechanochromism of phosphorescent organic molecules and metal complexes. J. Mater. Chem. C 2016, 4, 6688–6706. [Google Scholar] [CrossRef]
- Sagara, Y.; Komatsu, T.; Ueno, T.; Hanaoka, K.; Kato, T.; Nagano, T. Covalent Attachment of Mechanoresponsive Luminescent Micelles to Glasses and Polymers in Aqueous Conditions. J. Am. Chem. Soc. 2014, 136, 4273–4280. [Google Scholar] [CrossRef]
- Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-containing polymers: Methods for preparation of mechanochromic materials. Chem. Soc. Rev. 2013, 42, 857–870. [Google Scholar] [CrossRef]
- Xie, Y.-X.; Li, Z. The development of mechanoluminescence from organic compounds: Breakthrough and deep insight. Mater. Chem. Front. 2020, 4, 317–331. [Google Scholar] [CrossRef]
- Ma, X.-F.; Sun, R.; Cheng, J.-H.; Liu, J.-H.; Gou, F.; Xiang, H.-F.; Zhou, X.-G. Fluorescence Aggregation-Caused Quenching versus Aggregation-Induced Emission: A Visual Teaching Technology for Undergraduate Chemistry Students. J. Chem. Educ. 2016, 93, 345–350. [Google Scholar] [CrossRef]
- Watson, M.-D.; Fechtenkötter, A.; Müllen, K. Big Is Beautiful—“Aromaticity” Revisited from the Viewpoint of Macromolecular and Supramolecular Benzene Chemistry. Chem. Rev. 2001, 101, 1267–1300. [Google Scholar] [CrossRef]
- Luo, J.-D.; Xie, Z.-L.; Lam, J.-W.Y.; Cheng, L.; Chen, H.-Y.; Qiu, C.-F.; Kwok, H.-S.; Zhan, X.-W.; Liu, Y.-Q.; Zhu, D.-B.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.-L.C.; Kwok, R.-T.K.; Lam, J.-W.Y.; Tang, B.-Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Chi, Z.-G.; Li, H.-Y.; Xu, B.-J.; Li, X.-F.; Zhou, W.; Liu, S.-W.; Zhang, Y.; Xu, J.-R. Piezofluorochromism of an Aggregation-Induced Emission Compound Derived from Tetraphenylethylene. Chem. Asian J. 2011, 6, 808–811. [Google Scholar] [CrossRef]
- Zhao, J.; Chi, Z.-C.; Zhang, Y.; Mao, Z.; Yang, Z.-Y.; Ubba, E.; Chi, Z.-G. Recent progress in the mechanofluorochromism of cyanoethylene derivatives with aggregation-induced emission. J. Mater. Chem. C 2018, 6, 6327–6353. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Lei, Z.-Q.; Ma, H.-C. Twisted aggregation-induced emission luminogens (AIEgens) contribute to mechanochromism materials: A review. J. Mater. Chem. C 2022, 10, 14834. [Google Scholar] [CrossRef]
- Löwe, C.; Weder, C. Oligo(p-phenylene vinylene) Excimers as Molecular Probes: Deformation-Induced Color Change in Photoluminescent Polymer Blends. Adv. Mater. 2002, 14, 1625–1629. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Chung, J.-W.; Gierschner, J.; Kim, K.-S.; Choi, M.-G.; Kim, D.; Park, S.-Y. Multistimuli Two-Color Luminescence Switching via Different Slip-Stacking of Highly Fluorescent Molecular Sheets. J. Am. Chem. Soc. 2010, 132, 13675–13683. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.-K.; Vijayakumar, S.; Mal, A.; Karunakaran, V.; Janardhanan, J.-C.; Maiti, K.-K.; Praveen, V.-K.; Ajayaghosh, A. Bimodal detection of carbon dioxide using fluorescent molecular aggregates. Chem. Commun. 2019, 55, 6046–6049. [Google Scholar] [CrossRef] [PubMed]
- Gierschner, J.; Park, S.-Y. Luminescent distyrylbenzenes: Tailoring molecular structure and crystalline morphology. J. Mater. Chem. C 2013, 1, 5818–5832. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Zhao, Y.-L. Cyanostilbene-based intelligent organic optoelectronic materials. J. Mater. Chem. C 2013, 1, 1059–1065. [Google Scholar] [CrossRef]
- Martínez-Abadía, M.; Giménez, R.; Ros, M.-B. Self-Assembled α-Cyanostilbenes for Advanced Functional Materials. Adv. Mater. 2018, 30, 1704161. [Google Scholar] [CrossRef]
- Gao, A.-P.; Wang, Q.-Q.; Wu, H.-J.; Zhao, J.-W.; Cao, X.-H. Research progress on AIE cyanostilbene-based self-assembly gels: Design, regulation and applications. Coord. Chem. Rev. 2022, 471, 214753. [Google Scholar] [CrossRef]
- Mahalingavelar, P.; Kanvah, S. a-Cyanostilbene: A multifunctional spectral engineering motif. Phys. Chem. Chem. Phys. 2022, 24, 23049–23075. [Google Scholar] [CrossRef]
- Shimizu, M.; Kaki, R.; Takeda, Y.-H.; Hiyama, T.; Nagai, N.; Yamagish, H.; Furutani, H. 1,4-Bis(diarylamino)-2,5-bis(4-cyanophenylethenyl)benzenes: Fluorophores Exhibiting Efficient Red and Near-Infrared Emissions in Solid State. Angew. Chem. Int. Ed. 2012, 51, 4095. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-W.; Lv, X.-J.; Wang, P.-J.; Zhang, Y.-J.; Dai, Y.-Y.; Wu, Q.-C.; Yang, M.-Y.; Zhang, C. A donor-acceptor cruciform π-system: High contrast mechanochromic properties and multicolour electrochromic behavior. J. Mater. Chem. C 2014, 2, 5365–5371. [Google Scholar] [CrossRef]
- Gayathri, P.; Pannipara, M.; Al-Sehemi, A.-G.; Anthony, S.-P. Triphenylamine-based stimuli-responsive solid state fluorescent materials. New J. Chem. 2020, 44, 8680–8696. [Google Scholar] [CrossRef]
- Feng, C.-F.; Wang, K.; Xu, Y.-X.; Liu, L.-Q.; Zou, B.; Lu, P. Unique piezochromic fluorescence behavior of organic crystal of carbazole-substituted CNDSB. Chem. Commun. 2016, 52, 3836–3839. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-F.; Hao, J.-Y.; Gao, H.-Z.; Wang, Y.-H.; Wang, Y.; Liu, X.-L.; Han, A.-X.; Zhang, C. Twisted donor-acceptor cruciform fluorophores exhibiting strong solid emission, efficient aggregation-induced emission and high contrast mechanofluorochromism. Dye. Pigment. 2018, 150, 293–300. [Google Scholar]
- Kwon, M.-S.; Gierschner, J.; Yoon, S.-J.; Park, S.-Y. Unique Piezochromic Fluorescence Behavior of Dicyanodistyrylbenzene Based Donor-Acceptor-Donor Triad: Mechanically Controlled Photo-Induced Electron Transfer (eT) in Molecular Assemblies. Adv. Mater. 2012, 24, 5487–5492. [Google Scholar] [CrossRef]
- Kwon, M.-S.; Gierschner, J.; Seo, J.; Park, S.-Y. Rationally designed molecular D–A–D triad for piezochromic and acidochromic fluorescence on–off switching. J. Mater. Chem. C 2014, 2, 2552–2557. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Xu, D.-F.; Gao, H.-Z.; Wang, Y.; Liu, X.-L.; Han, A.-X.; Zhang, C.; Zang, L. Mechanofluorochromic properties of aggregation-induced emission-active tetraphenylethene-containing cruciform luminophores. Dye. Pigment. 2018, 156, 291–298. [Google Scholar] [CrossRef]
- Kunzelman, J.; Kinami, M.; Crenshaw, B.-R.; Protasiewicz, J.-D.; Weder, C. Oligo(p-phenylene vinylene)s as a “New” Class of Piezochromic Fluorophores. Adv. Mater. 2008, 20, 119–122. [Google Scholar] [CrossRef]
- Kim, H.-J.; Gierschner, J.; Park, S.-Y. Tricolor fluorescence switching in a single component mechanochromic molecular material. J. Mater. Chem. C 2020, 8, 7417–7421. [Google Scholar] [CrossRef]
- Li, P.-Y.; Wang, J.-X.; Li, P.-F.; Lai, L.-M.; Yin, M.-Z. Minor alkyl modifications for manipulating the fluorescence and photomechanical properties in molecular crystals. Mater. Chem. Front. 2021, 5, 1355–1363. [Google Scholar] [CrossRef]
- Ramya, N.-K.; Femina, C.; Suresh, S.; Mohanakumari, D.S.; Krishnan, R.; Thomas, R. Dicyanodistyrylbenzene based positional isomers: A comparative study of AIEE and stimuli responsive multicolour fluorescence switching. New J. Chem. 2022, 46, 1339–1346. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Varghese, S.; Park, S.-K.; Wannemacher, R.; Gierschner, J.; Park, S.Y. Color-Tuned, Highly Emissive Dicyanodistyrylbenzene Single Crystals: Manipulating Intermolecular Stacking Interactions for Spontaneous and Stimulated Emission Characteristics. Adv. Opt. Mater. 2013, 1, 232–237. [Google Scholar] [CrossRef]
- Park, S.-K.; Cho, I.; Gierschner, J.; Kim, J.-H.; Kim, J.-H.; Kwon, J.-E.; Kwon, O.-K.; Whang, D.-R.; Park, J.-H.; An, B.-K.; et al. Stimuli-Responsive Reversible Fluorescence Switching in a Crystalline Donor-Acceptor Mixture Film: Mixed Stack Charge-Transfer Emission versus Segregated Stack Monomer Emission. Angew. Chem. Int. Ed. 2016, 55, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Zeng, Q.-X.; Zou, B.; Liu, Y.; Xu, B.; Tian, W.-J. Piezochromic Luminescence of Donor-Acceptor Cocrystals: Distinct Responses to Anisotropic Grinding and Isotropic Compression. Angew. Chem. Int. Ed. 2018, 57, 15670–15674. [Google Scholar] [CrossRef] [PubMed]
- Lavrenova, A.; Balkenende, D.-W.R.; Sagara, Y.; Schrettl, S.; Simon, Y.-C.; Weder, C. Mechano- and Thermoresponsive Photoluminescent Supramolecular Polymer. J. Am. Chem. Soc. 2017, 139, 4302–4305. [Google Scholar] [CrossRef] [PubMed]
- Gierschner, J.; Shi, J.; Milián-Medina, B.; Roca-Sanjuán, D.; Varghese, S.; Park, S.-Y. Luminescence in Crystalline Organic Materials: From Molecules to Molecular Solids. Adv. Opt. Mater. 2021, 9, 2002251. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Wang, K.; Zhang, Y.-J.; Xie, Z.-Q.; Zou, B.; Ma, Y. Fluorescence mutation and structural evolution of a p-conjugated molecular crystal during phase transition. J. Mater. Chem. C 2016, 4, 1257–1262. [Google Scholar] [CrossRef]
- Sagara, Y.; Kubo, K.; Nakamura, T.; Tamaoki, N.; Weder, C. Temperature-Dependent Mechanochromic Behavior of Mechanoresponsive Luminescent Compounds. Chem. Mater. 2017, 29, 1273–1278. [Google Scholar] [CrossRef]
- Pauk, K.; Luňák, S., Jr.; Růžička, A.; Marková, A.; Mausová, A.; Kratochvíl, M.; Melánová, K.; Weiter, M.; Imramovský, A.; Vala, M. Green-, Red-, and Infrared-Emitting Polymorphs of Sterically Hindered Push–Pull Substituted Stilbenes. Chem. Eur. J. 2021, 27, 4341–4348. [Google Scholar] [CrossRef]
- Sagara, Y.; Lavrenova, A.; Crochet, A.; Simon, Y.-C.; Fromm, K.-M.; Weder, C. A Thermo- and Mechanoresponsive Cyano-Substituted Oligo(p-phenylene vinylene) Derivative with Five Emissive States. Chem. Eur. J. 2016, 22, 4374–4378. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-W.; Dai, Y.-Y.; Ouyang, M.; Zhang, Y.-J.; Zhan, L.-L.; Zhang, C. Unique torsional cruciform p-architectures composed of donor and acceptor axes exhibiting mechanochromic and electrochromic properties. J. Mater. Chem. C 2015, 3, 3356–3363. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wang, K.; Zhuang, G.-L.; Xie, Z.-A.; Zhang, C.; Cao, F.; Pan, G.-X.; Chen, H.-F.; Zou, B.; Ma, Y.-G. Multicolored-Fluorescence Switching of ICT-Type Organic Solids with Clear Color Difference: Mechanically Controlled Excited State. Chem. Eur. J. 2015, 21, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Zhang, J.-X.; Shen, J.-H.; Sun, J.-W.; Wang, K.; Xie, Z.-G.; Gao, H.-W.; Zou, B. Solid-State TICT-Emissive Cruciform: Aggregation-Enhanced Emission, Deep-Red to Near-Infrared Piezochromism and Imaging In Vivo. Adv. Opt. Mater. 2018, 6, 1800956. [Google Scholar] [CrossRef]
- Zhu, C.-F.; Li, C.-J.; Wen, L.; Song, Q.-B.; Wang, K.; Lv, C.-Y.; Zhang, Y.-J. Piezochromism of cyanostilbene derivatives: A small structural alteration makes a big photophysical difference. New J. Chem. 2021, 45, 12895–12901. [Google Scholar] [CrossRef]
- Lu, H.-G.; Zheng, Y.-D.; Zhao, X.-W.; Wang, L.-J.; Ma, S.-Q.; Han, X.-Q.; Xu, B.; Tian, W.-J.; Gao, H. Highly Efficient Far Red/Near-Infrared Solid Fluorophores: Aggregation-Induced Emission, Intramolecular Charge Transfer, Twisted Molecular Conformation, and Bioimaging Applications. Angew. Chem. Int. Ed. 2015, 54, 155–159. [Google Scholar]
- Zhu, X.-J.; Traub, M.-C.; Bout, D.-A.V.; Plunkett, K.-N. Well-Defifined Alternating Copolymers of Oligo(phenylenevinylene)s and Flexible Chains. Macromolecules 2012, 45, 5051–5057. [Google Scholar] [CrossRef]
- Zhu, X.-J.; Plunkett, K.-N. Controlled Regioregularity in Oligo(2-methoxy-5-(2′-ethylhexyloxy)-1,4-phenylenevinylenes. J. Org. Chem. 2014, 79, 7093–7102. [Google Scholar] [CrossRef]
- Zhu, X.-J.; Shao, B.-Y.; Bout, D.-A.V.; Plunkett, K.-N. Directing the Conformation of Oligo(phenylenevinylene) Polychromophores with Rigid, Nonconjugatable Morphons. Macromolecules 2016, 49, 3838–3844. [Google Scholar] [CrossRef]
- Shao, B.-Y.; Zhu, X.-J.; Plunkett, K.-N.; Bout, D.-A.V. Controlling the folding of conjugated polymers at the single molecule level via hydrogen bonding. Polym. Chem. 2017, 8, 1188–1195. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, Y.; Ren, X.; Li, Y.; Shi, L.; Zhang, W.; Zhu, X.; Hao, X.-Q.; Song, M.-P. Solvent-induced MultiStimuli-Responsive properties of cyano-substituted Oligo(p-phenylene vinylene) derivatives. Dye. Pigment. 2023, 214, 111195. [Google Scholar] [CrossRef]
- Ren, X.; Wang, W.; Meng, Z.; Li, Y.; Wang, Q.; Zhang, W.; Zhang, H.; Zhu, X.; Hao, X.-Q.; Song, M.-P. Highly emissive tridentate fluorophores based on bis-imidazo [1,2-α]pyridine for deep-blue photoluminescence with CIE y ≤ 0.08. J. Lumin. 2023, 263, 120097. [Google Scholar] [CrossRef]
- Meng, Z.; Li, Y.; Liao, K.; Sun, Y.; Zhang, W.; Song, B.; Zhu, X.; Hao, X.-Q. Dual state emissive tridentate imidazopyridines with applications in acidochromism, metal ions detection, data encryption, and bioimaging. Dye. Pigment. 2024, 222, 111882. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, K.; Li, Y.; Zhang, W.; Song, B.; Hao, X.-Q.; Zhu, X. Dual-state emissive imidazo[1,2-α]pyridines with full color emission, acidochromism, viscosity-dependent fluorescence, and bioimaging applications. Dye. Pigment. 2024, 224, 112004. [Google Scholar] [CrossRef]
- Prusinowska, N.; Bardziński, M.; Janiak, A.; Skowronek, P.; Kwit, M. Sterically Crowded Trianglimines—Synthesis, Structure, Solid State Self-Assembly and Unexpected Chiroptical Properties. Chem. Asian J. 2018, 13, 2691–2699. [Google Scholar] [CrossRef]
- Xue, P.; Yao, B.; Sun, J.; Xu, Q.; Chen, P.; Zhang, Z.; Lu, R. Phenothiazine-based benzoxazole derivates exhibiting mechanochromic luminescence: The effect of a bromine atom. J. Mater. Chem. C 2014, 2, 3942–3950. [Google Scholar] [CrossRef]
- Xue, P.; Chen, P.; Jia, J.; Xu, Q.; Sun, J.; Yao, B.; Zhang, Z.; Lu, R. A triphenylamine-based benzoxazole derivative as a high-contrast piezofluorochromic material induced by protonation. J. Mater. Chem. C 2014, 2, 2569–2571. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, D.; Zhou, H.; Liu, X.; Wang, Y.; Han, A.; Zhang, C. Reversible solid-state mechanochromic luminescence originated from aggregation-induced enhanced emission-active Donor–Acceptor cruciform luminophores containing triphenylamine. Dye. Pigment. 2009, 171, 107689. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).