Ionic Liquid/Deep Eutectic Solvent-Mediated Calcining Synthesis of Cobalt-Based Electrocatalysts for Water Splitting
Abstract
1. Introduction
2. Experiments on IL/DES-Mediated Cobalt-Based Electrocatalysts for Water Splitting
2.1. Preparation of Common ILs and DESs
2.1.1. Conventional Synthesis Method of Ionic Liquids
- (1)
- Direct synthesis method
- (2)
- Two-step synthesis method
2.1.2. Synthesis Method of Deep Eutectic Solvents
- (1)
- Melting method
- (2)
- Solution method
- (3)
- Direct mixing method
2.2. Morphology and Composition Characterization of Catalysts [52]
2.3. Evaluation of Catalytic Performance of Electrocatalysts for Water Splitting
2.3.1. Linear Sweep Voltammetry (LSV)
2.3.2. Tafel Slope Analysis
2.3.3. Stability Testing
2.3.4. Electrochemical Impedance Spectroscopy (EIS)
2.3.5. Electrochemical Specific Surface Area (ECSA)
3. IL/DES-Mediated Synthesis of Co-Based Catalysts Using Calcination Method
3.1. One-Step Calcination Method
3.2. Multi-Step Calcination Method
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nordness, O.; Brennecke, J. Ion dissociation in ionic liquids and ionic liquid solutions. Chem. Rev. 2020, 120, 12873–12902. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.G.; Chen, B.H.; Koo, Y.M.; MacFarlane, D.R. Introduction: Lonic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Berezianko, I.; Kostjuk, S. Ionic liquids in cationic polymerization: A review. J. Mol. Liq. 2024, 397, 124037. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kustov, L. Polymeric ionic liquids: Here, there and everywhere. Eur. Polym. J. 2024, 203, 112657. [Google Scholar] [CrossRef]
- Ruan, Q.; Yao, M.; Yuan, D.; Dong, H.; Liu, J.; Yuan, X.; Fang, W.; Zhao, G.; Zhang, H. Ionic liquid crystal electrolytes: Fundamental, applications and prospects. Nano Energy 2023, 106, 108087. [Google Scholar] [CrossRef]
- Luska, K.L.; Moores, A. Functionalized Ionic Liquids for the Synthesis of Metal Nanoparticles and their Application in Catalysis. ChemCatchem 2012, 4, 1534–1546. [Google Scholar] [CrossRef]
- Zhang, L.; Du, J.; Ran, T.; Gao, H.; Liao, Y. Preparation and application of poly(zwitterionic ionic liquid) to enhance the photocatalytic activity of TiO2. J. Mater. Sci. 2016, 51, 7186–7198. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, Q.; Guo, J.; Qin, J.; Mao, H.; Wang, B.; Yan, F. Structure–antibacterial activity relationships of imidazolium-type ionic liquid monomers, poly (ionic liquids) and poly (ionic liquid) membranes: Effect of alkyl chain length and cations. ACS Appl. Mater. Interfaces 2016, 8, 12684–12692. [Google Scholar] [CrossRef]
- Xu, C.; Diemant, T.; Mariani, A.; Di Pietro, M.E.; Mele, A.; Liu, X.; Passerini, S. Locally Concentrated ionic liquid electrolytes for wide-temperature-range aluminum-sulfur batteries. Angew. Chem. Int. Ed. 2024, 136, e202318204. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, S.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Unprotected organic cations—The dilemma of highly li-concentrated ionic liquid electrolytes. J. Am. Chem. Soc. 2024, 146, 8352–8361. [Google Scholar] [CrossRef]
- Choudhary, G.; Dhariwal, J.; Saha, M.; Trivedi, S.; Banjare, M.K.; Kanaoujiya, R.; Behera, K. Ionic liquids: Environmentally sustainable materials for energy conversion and storage applications. Environ. Sci. Pollut. Res. 2024, 31, 10296–10316. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, F.; Texter, J. Polymerized and colloidal ionic liquids—Syntheses and applications. Chem. Rev. 2024, 124, 3813–3931. [Google Scholar] [CrossRef] [PubMed]
- Naseem, S.; Durrani, A.I.; Rizwan, M.; Yasmeen, F.; Siddiqui, S.; Habib, F. Sono-microwave assisted chlorine free and ionic liquid (smacil) extraction of cellulose from urtica dioica: A benign to green approach. Int. J. Biol. Macromol. 2024, 259, 129059. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Wang, Y.; Hagen, E.; Zachariah, M.R. Electrochemical modulation of the flammability of ionic liquid fuels. J. Am. Chem. Soc. 2023, 145, 16318–16323. [Google Scholar] [CrossRef]
- Zhao, J.; Wilkins, M.; Wang, D. A review on strategies to reduce ionic liquid pretreatment costs for biofuel production. Bioresour. Technol. 2022, 364, 128045. [Google Scholar] [CrossRef]
- Maresca, G.; Ottaviani, M.; Ryan, K.; Brutti, S.; Appetecchi, G.B. Improved compatibility of α-NaMnO2 cathodes at the interface with safer and more reliable electrolytes. ChemSusChem 2024, e202400514. [Google Scholar] [CrossRef]
- Homa, J.; Stachowiak, W.; Olejniczak, A.; Chrzanowski, M.N. Ecotoxicity studies reveal that organic cations in dicamba-derived ionic liquids can pose a greater environmental risk than the herbicide itself. Sci. Total Environ. 2024, 922, 171062. [Google Scholar] [CrossRef]
- He, S.; Yin, F.; Wu, Y.; Wang, M.; Wang, Y.; Row, K.H.; Tang, W. Deep eutectic solvents as extraction media for food-derived biomacromolecules. TrAC Trends Anal. Chem. 2024, 171, 117521. [Google Scholar] [CrossRef]
- Shishov, A.; El-Deen, A.; Godunov, P.; Bulatov, A. Atypical deep eutectic solvents: New opportunities for chemical analysis. TrAC Trends Anal. Chem. 2024, 176, 117752. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
- Elhamarnah, Y.; Alkhouzaam, A.; Qiblawey, H.; Nasser, M. Enhancing the properties and performance of polysulfone ultrafiltration membranes using citric acid based deep eutectic solvent additives. J. Environ. Chem. Eng. 2024, 12, 112110. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Pant, K.; Mishra, B. Investigating the effect of mono di carboxylic acids as hydrogen bond donor on solvation of copper in choline chloride-based deep eutectic solvents. J. Mol. Liq. 2023, 383, 122142. [Google Scholar] [CrossRef]
- Rozas, S.; Benito, C.; Alcalde, R.; Atilhan, M.; Aparicio, S. Insights on the water effect on deep eutectic solvents properties and structuring: The archetypical case of choline chloride+ ethylene glycol. J. Mol. Liq. 2021, 344, 117717. [Google Scholar] [CrossRef]
- Odegova, V.; Lavrinenko, A.; Rakhmanov, T.; Sysuev, G.; Dmitrenko, A.; Vinogradov, V. DES solvents: An open platform for the search and prediction of the physicochemical properties of deep eutectic solvents. Chem. Sci. 2024, 26, 3958–3967. [Google Scholar] [CrossRef]
- Kinaci, E.; Francis, C.; Lima, T.A.; Alvarez, N.J.; Palmese, G.R. Synthesis and characterization of a di-methacrylated polymerizable ionic liquid through two-step esterification and ion exchange process. React. Funct. Polym. 2024, 197, 105865. [Google Scholar] [CrossRef]
- Walunj, G.; Waware, U.; Hamouda, A.; Borkar, T. Electrodeposition of duplex Ni–B–Zn/Co composite coatings. JOM 2020, 72, 4296–4304. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Zhang, H.; Huang, S.; Nan, X.; Wang, Q.; Zhu, H.; Liu, Y.; Zhao, X. Solvothermally Synthesized Co(CoO)/Ti3C2Tx/TiO2 Nanocomposites for Enhanced Microwave Absorption. ACS Appl. Nano Mater. 2024, 7, 5488–5497. [Google Scholar] [CrossRef]
- Xiong, Q.; Lou, J.; Zhou, Y.; Shi, S.; Ji, Z. Ultrafast synthesis of Mn0.8Co0.2CO3/graphene composite as anode material by microwave solvothermal strategy with enhanced Li storage properties. Mater. Lett. 2018, 210, 267–270. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, K.Q.; Gao, X.J.; Shi, X.M.; Han, J.W.; Pan, J.Q. Solid octahedral NiO micro-particles prepared by a calcination method in the presence of an ionic liquid and their application as anode for lithium ion batteries. Int. J. Electrochem. Sci. 2020, 15, 1498–1508. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, X.; Hu, C.; Wang, F.; Hu, S. Role of partial limestone calcination in carbonated lime-based binders. Cem. Concr. Res. 2024, 183, 107572. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Jaszczur, M.; Al-Jiboory, A.K.; Al Musawi, T.J.; Ali, B.M.; Viktor, P.; Fodor, M.; Ahsan, M.; Salman, H.M.; et al. Hydrogen role in energy transition: A comparative review. Process Saf. Environ. Prot. 2024, 184, 1069–1093. [Google Scholar] [CrossRef]
- Quan, L.; Jiang, H.; Mei, G.; Sun, Y.; You, B. Bifunctional electrocatalysts for overall and hybrid water splitting. Chem. Rev. 2024, 124, 3694–3812. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ni, C.; Gao, X.; Lin, S.; He, X.; Tian, L.; Li, Z. Constructing built-in-electric field for boosting electrocatalytic water splitting. ChemSusChem 2024, e202400977. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sammi, A.; Srivastava, A.; Azad, U.P.; Chandra, P. Emerging 3D nanomaterials as electrocatalysts for water splitting reactions. Int. J. Hydrogen Energy 2024, 74, 214–231. [Google Scholar] [CrossRef]
- Zhong, Y.; Lu, Y.; Pan, Z.; Yang, J.; Du, G.; Chen, J.; Zhang, Q.; Zhou, H.; Wang, J.; Wang, C.; et al. Efficient water splitting system enabled by multifunctional platinum-free electrocatalysts. Adv. Funct. Mater. 2021, 31, 2009853. [Google Scholar] [CrossRef]
- Li, D.; Huang, Y.; Li, Z.; Zhong, L.; Liu, C.; Peng, X. Deep eutectic solvents derived carbon-based efficient electrocatalyst for boosting H2 production coupled with glucose oxidation. Chem. Eng. J. 2022, 430, 132783. [Google Scholar] [CrossRef]
- Jiang, A.; Nidamanuri, N.; Zhang, C.; Li, Z. Ionic-liquid-assisted one-step synthesis of CoO nanosheets as electrocatalysts for oxygen evolution reaction. ACS Omega 2018, 3, 10092–10098. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Mane, V.S.; Bandal, H.A.; Kim, H.; Kumbhar, A.S. Ionic Liquid-Derived Co3O4-N/S-Doped Carbon Catalysts for the Enhanced Water Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 14889–14898. [Google Scholar] [CrossRef]
- Thorat, G.; Jadhav, H.; Roy, A.; Chung, W.; Seo, J. Dual role of deep eutectic solvent as a solvent and template for the synthesis of octahedral cobalt vanadate for an oxygen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 16255–16266. [Google Scholar] [CrossRef]
- Li, T.; Tang, D.; Li, C. A high active hydrogen evolution reaction electrocatalyst from ionic liquids-originated cobalt phosphide/carbon nanotubes. Int. J. Hydrogen Energy 2017, 42, 21786–21792. [Google Scholar] [CrossRef]
- Tang, D.; Li, T.; Li, C. Metal and phosphonium-based ionic liquid: A new Co and P dual-source for synthesis of cobalt phosphide toward hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 1720–1726. [Google Scholar] [CrossRef]
- Mou, H.; Xue, Z.; Zhang, B.; Lan, X.; Mu, T. A deep eutectic solvent strategy to form defect-rich N, S, and O tridoped carbon/Co9S8 hybrid materials for a pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 2099–2103. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.Y.; Zhang, B.H.; Li, Z.H.; Hao, J.C. All-In-One Deep Eutectic Solvent toward Cobalt-Based Electrocatalyst for Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 8964–8971. [Google Scholar] [CrossRef]
- Zhang, D.; Xing, M.; Mou, X.; Song, C.; Wang, D. Deep eutectic solvent induced ultrathin Co4N/N-doped carbon nanosheets self-supporting electrode for boosting hydrogen evolution integrated with biomass electrooxidation. Appl. Surf. Sci. 2023, 608, 155283. [Google Scholar] [CrossRef]
- Gao, J.; Ma, N.; Zheng, Y.; Zhang, J.; Gui, J.; Guo, C.; An, H.; Tan, X.; Yin, Z.; Ma, D. Cobalt/nitrogen-doped porous carbon nanosheets derived from polymerizable ionic liquids as bifunctional electrocatalyst for oxygen evolution and oxygen reduction reaction. ChemCatChem 2017, 9, 1601–1609. [Google Scholar] [CrossRef]
- Jiang, J.; Chang, L.; Zhao, W.; Tian, Q.; Xu, Q. An advanced FeCoNi nitro-sulfide hierarchical structure from deep eutectic solvents for enhanced oxygen evolution reaction. Chem. Commun. 2019, 55, 10174–10177. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, B.; Chen, T.; Ying, H.; Li, Z.; Hao, J. Deep eutectic solvent strategy enables an octahedral Ni–Co precursor for creating high-performance NiCo2O4 catalyst toward oxygen evolution reaction. Green Energy Environ. 2022, 7, 1217–1227. [Google Scholar] [CrossRef]
- Rong, K.; Wei, J.; Huang, L.; Fang, Y.; Dong, S. Synthesis of low dimensional hierarchical transition metal oxides via a direct deep eutectic solvent calcining method for enhanced oxygen evolution catalysis. Nanoscale 2020, 12, 20719–20725. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Q.; Xu, G.; Ning, Y.; Huang, K.; He, F.; Wu, Z.; Zhang, J. Phosphorus and fluorine Co-doping induced enhancement of oxygen evolution reaction in bimetallic nitride nanorods arrays: Ionic liquid-driven and mechanism clarification. Chem. Eur. J. 2017, 23, 16862–16870. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, T. Preparation of Fe-doped transition metal phosphides using ionic liquid as precursor for efficient oxygen evolution reaction. ChemCatChem 2023, 15, e202300132. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Xin, B.W.; Xi, Z.C.; Zhang, B.H.; Li, Z.Y.; Zhang, H.; Li, Z.H.; Hao, J.C. Phosphonium-Based Ionic Liquid: A New Phosphorus Source toward Microwave-Driven Synthesis of Nickel Phosphide for Efficient Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 1468–1477. [Google Scholar] [CrossRef]
- Xu, M.; Sheng, B.; Cheng, Y.; Lu, J.; Chen, M.; Wang, P.; Liu, B.; Chen, J.; Han, X.; Wang, M.S.; et al. One-step calcination synthesis of interface-coherent crystallized and surface-passivated LiNi0.5Mn1.5O4 for high-voltage lithium-ion battery. Nano Res. 2024, 17, 4192–4202. [Google Scholar] [CrossRef]
- Sewnet, A.; Alemayehu, E.; Abebe, M.; Mani, D.; Thomas, S.; Kalarikkal, N.; Lennartz, B. Single-Step Synthesis of Graphitic Carbon Nitride Nanomaterials by Directly Calcining the Mixture of Urea and Thiourea: Application for Rhodamine B (RhB) Dye Degradation. Nanomaterials 2023, 13, 762. [Google Scholar] [CrossRef]
- Zhang, T.; Doert, T.; Wang, H.; Zhang, S.J.; Ruck, M. Inorganic Synthesis Based on Reactions of Ionic Liquids and Deep Eutectic Solvents. Angew. Chem. Int. Ed. 2021, 60, 22148–22165. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Lai, M.C.; Huang, H.L.; Sun, I.W. Speciation of cobalt-chloride-based ionic liquids and electrodeposition of Co wires. Electrochim. Acta 2014, 117, 217–223. [Google Scholar] [CrossRef]
- Zdolšek, N.; Vujković, M.; Metin, Ö.; Brković, S.; Jocić, A.; Dimitrijević, A.; Trtić-Petrović, T.; Šljukić, B. Boosting electrocatalysis of oxygen reduction and evolution reactions with cost-effective cobalt and nitrogen-doped carbons prepared by simple carbonization of ionic liquids. Int. J. Hydrogen Energy 2022, 47, 14847–14858. [Google Scholar] [CrossRef]
- Shi, R.F.; Zhou, F.Y.; Chen, Y.; Liu, Z.H.; Liu, S.Z.; Mu, T.C. Magnetic deep eutectic solvents: Formation and properties. Phys. Chem. Chem. Phys. 2022, 24, 20073–20081. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, C.C.; Liu, L.R.; Zhu, M.Y. A review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2023, 8, 406–437. [Google Scholar] [CrossRef]
- Elumeeva, K.; Ren, J.; Antonietti, M.; Fellinger, T.P. High surface iron/cobalt containing nitrogen-doped carbon aerogels as non-precious advanced electrocatalysts for oxygen reduction. ChemElectroChem 2015, 2, 584–591. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, Z.; Zhang, H.; Wu, P.; Cai, C. Active site structures in nitrogen-doped carbon-supported cobalt catalysts for the oxygen reduction reaction. ACS Appl. Mater. Inter. 2016, 8, 32875–32886. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, L.L.; Liu, Z.G.; Wang, Y. Ionic-Liquid-Derived Boron-Doped Cobalt-Coordinating Nitrogen-Doped Carbon Materials for Enhanced Catalytic Activity. ChemCatchem 2016, 8, 1782–1787. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, J.L.; Xu, Q.Q.; Zhang, F.Z.; Sun, Q.; Xie, H.B. Noble-metal-free Co-N-C catalyst derived from cellulose-based poly(ionic liquid)s for highly efficient oxygen reduction reaction. Int. J. Biol. Macromol. 2023, 242, 125110. [Google Scholar] [CrossRef]
- Pariiska, O.; Mazur, D.; Cherchenko, K.; Kurys, Y.; Koshechko, V.; Pokhodenko, V. Efficient Co-N-C electrocatalysts for oxygen reduction derived from deep eutectic solvents. Electrochim. Acta 2022, 413, 140132. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Jin, A.L.; Huang, K.; Liu, F.J.; Tao, D.J. Co-N-C catalysts synthesized by pyrolysis of Co-based deep eutectic solvents for aerobic oxidation of alcohols. New J. Chem. 2018, 42, 15871–15878. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Fard, M.R.; Fadaeerayeni, S.; Yaripour, F. Effect of Calcination Conditions on Crystalline Structure and Pore Size Distribution for a Mesoporous Alumina. Chem. Eng. Commun. 2015, 202, 493–499. [Google Scholar] [CrossRef]
- Afonso, J.; Mezzetta, A.; Marrucho, I.M.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green Chem. 2023, 25, 59–105. [Google Scholar] [CrossRef]

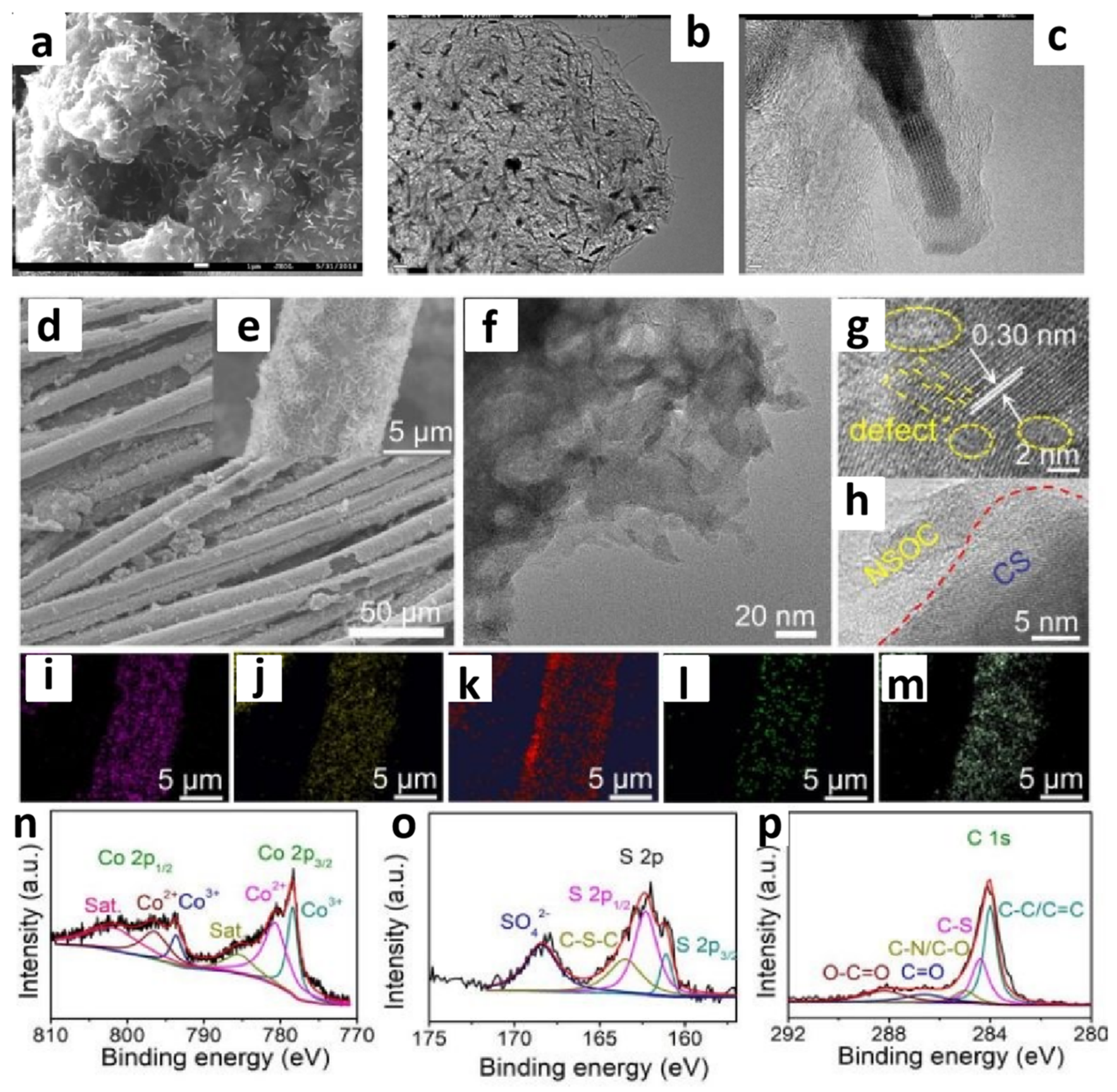
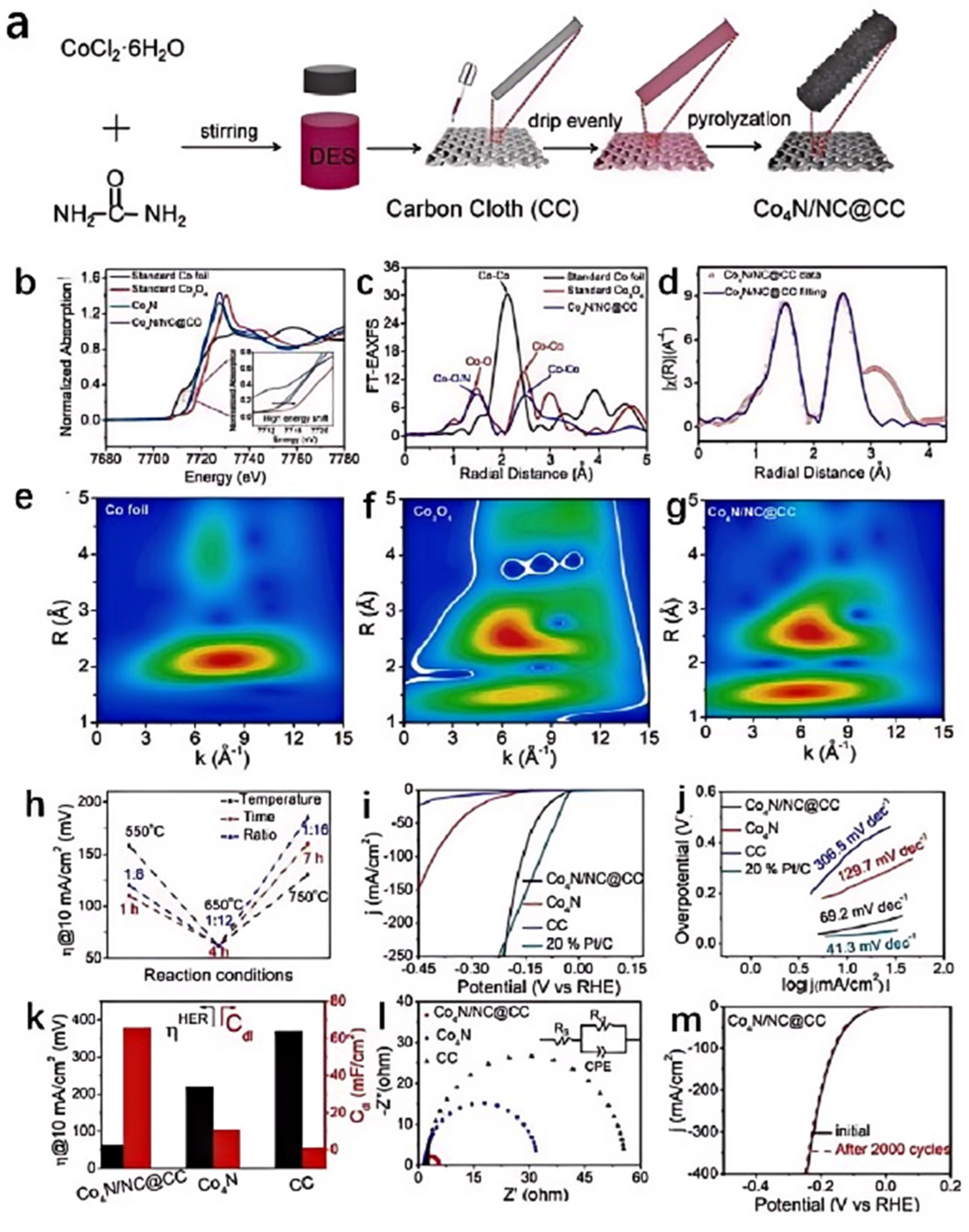
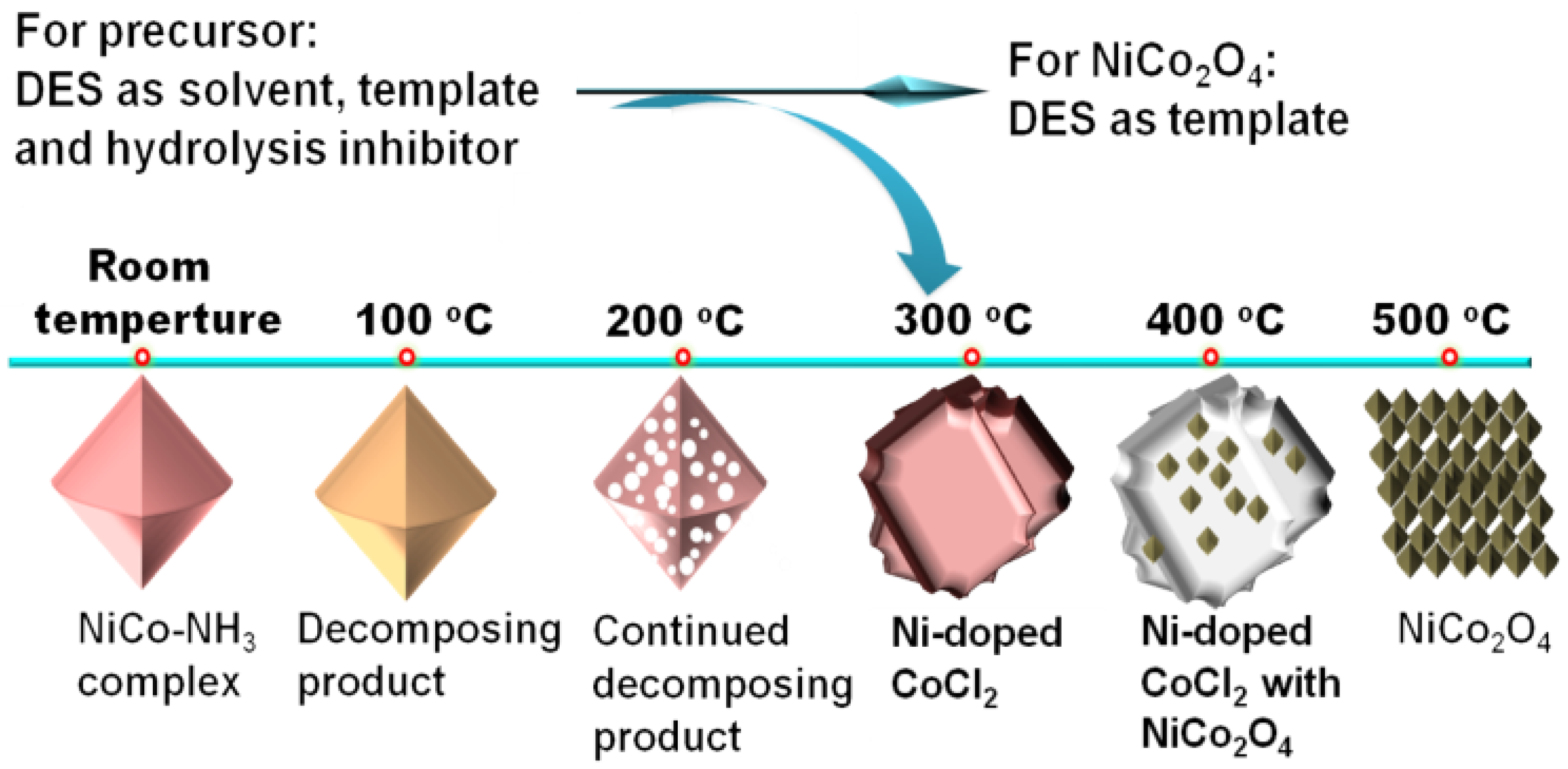
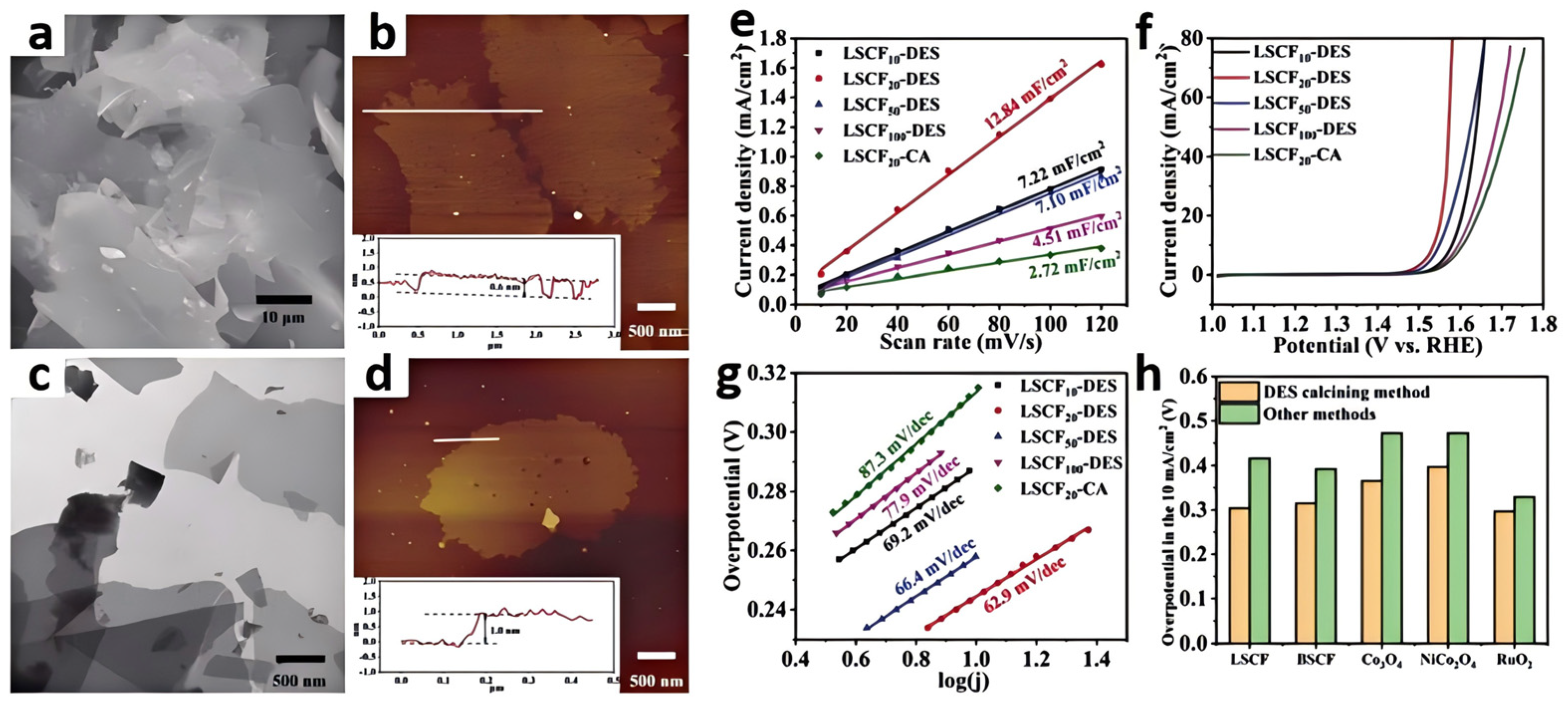
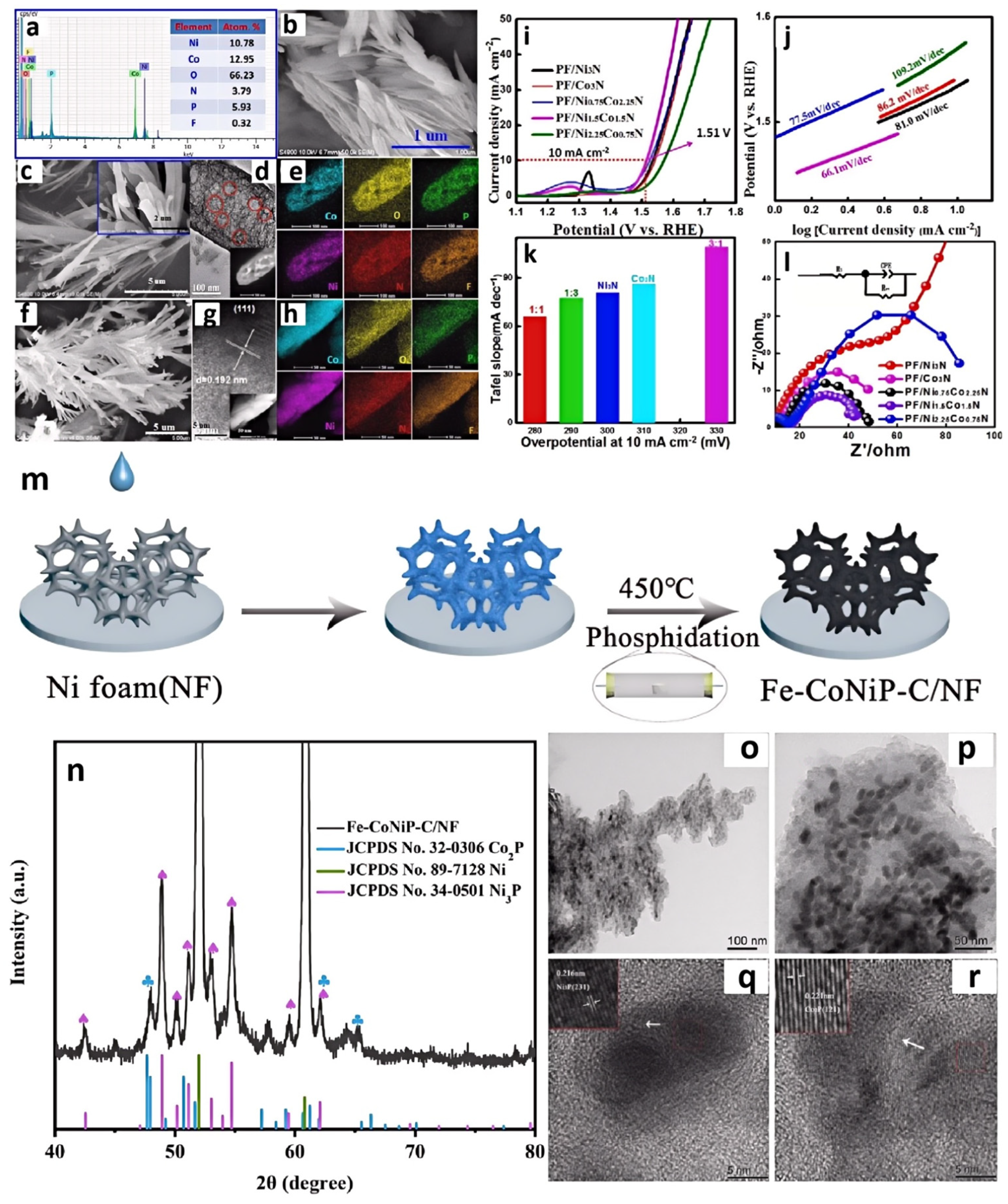
| Catalyst | Applied IL/DES | Catalytic Performance | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| HER | OER | |||||||
| Electrolyte | ƞ(mV)@Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Electrolyte | ƞ(mV)@Current Density (mA cm−2) | Tafel Slope (mV dec−1) | |||
| Co@NPC | ChCl/urea/gluconic acid | 0.5 M H2SO4 | 215@10 | 70 | -- | -- | -- | [36] |
| Co@NPC | ChCl/urea/gluconic acid | 1 M KOH | 274@10 | 91 | 1 M KOH | 430@10 | 87 | [36] |
| CoO | [BMIM]Tf2N | -- | -- | -- | 1 M KOH | 320@10 | 70 | [37] |
| Co3O4-N/S | [1-vinylimidazolium]HSO4 | -- | -- | -- | 1 M KOH | 350@10 | 67 | [38] |
| CoV2O6 | ChCl/malonic acid | -- | -- | -- | 1 M KOH | 324@10 | -- | [39] |
| CoP/CNT | [MBMG]2CoCl2Br2 | 0.5 M H2SO4 | 135@10 | 58 | -- | -- | -- | [40] |
| Co2P/CNT | [P66614]2CoCl4 | 0.5 M H2SO4 | 150@10 | 47 | -- | -- | -- | [41] |
| N,S,O-C/Co9S8 | CoCl2·6H2O/TU | 1 M KOH | 53@10 | 45.3 | -- | -- | -- | [42] |
| N,S,O-C/Co9S8 | CoCl2·6H2O/TU | 1 M PBS | 103@10 | 113.7 | -- | -- | -- | [42] |
| N,S,O-C/Co9S8 | CoCl2·6H2O/TU | 0.5 M H2SO4 | 68@10 | 31 | -- | -- | -- | [42] |
| CoOOH | CoCl2/urea | -- | -- | -- | 1 M KOH | 291@10 | 65 | [43] |
| Co4N/NC@CC | CoCl2·6H2O/urea | 0.5 M H2SO4 | 62@10 | -- | -- | -- | -- | [44] |
| Co4N/NC@CC | CoCl2·6H2O/urea | 1 M PBS | 98@10 | -- | -- | -- | -- | [44] |
| Co4N/NC@CC | CoCl2·6H2O/urea | 1 M KOH | 60@10 | 69.2 | -- | -- | -- | [44] |
| Co-N-C | hydrolyzed vinyl imidazolium nitrate | 1 M KOH | 400@10 | 127.4 | [45] | |||
| N,S-NiCoFe | FeCl3·6H2O/CoCl2·6H2O/NiCl2·6H2O/L-cysteine | -- | -- | -- | 1 M KOH | 251@10 | 58 | [46] |
| NiCo2O4 | ChCl/glycerol | -- | -- | -- | 1 M KOH | 320@10 | 61 | [47] |
| La0.5Sr0.5Co0.8Fe0.2O3 | Glucose/urea DES | -- | -- | -- | 1 M KOH | 304@10 | 62.9 | [48] |
| Ba0.5Sr0.5Co0.8Fe0.2O3 | Glucose/urea DES | -- | -- | -- | 1 M KOH | 315@10 | -- | [48] |
| NiCo2O4 | Glucose/urea DES | -- | -- | -- | 1 M KOH | 365@10 | -- | [48] |
| Co3O4 | Glucose/urea DES | -- | -- | -- | 1 M KOH | 397@10 | -- | [48] |
| P,F-Ni1.5Co1.5N | [BMIM]PF6 | -- | -- | -- | 1 M KOH | 280@10 | 66.1 | [49] |
| Fe-CoNiP | [P(C6H13)3C14H29] [FeCl4], [P(C6H13)3C14H29]2 [CoCl4] | -- | -- | -- | 1 M KOH | 355@50 424@100 | 45 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Jin, J.; Wang, J.; Sun, F.; Xu, J.; Wang, S.; Xu, L.; Zhang, J.; Xin, B. Ionic Liquid/Deep Eutectic Solvent-Mediated Calcining Synthesis of Cobalt-Based Electrocatalysts for Water Splitting. Molecules 2024, 29, 4435. https://doi.org/10.3390/molecules29184435
Zhang C, Jin J, Wang J, Sun F, Xu J, Wang S, Xu L, Zhang J, Xin B. Ionic Liquid/Deep Eutectic Solvent-Mediated Calcining Synthesis of Cobalt-Based Electrocatalysts for Water Splitting. Molecules. 2024; 29(18):4435. https://doi.org/10.3390/molecules29184435
Chicago/Turabian StyleZhang, Chenyun, Jianjiao Jin, Jiahao Wang, Fangfang Sun, Jiacheng Xu, Shun Wang, Lihua Xu, Jing Zhang, and Bingwei Xin. 2024. "Ionic Liquid/Deep Eutectic Solvent-Mediated Calcining Synthesis of Cobalt-Based Electrocatalysts for Water Splitting" Molecules 29, no. 18: 4435. https://doi.org/10.3390/molecules29184435
APA StyleZhang, C., Jin, J., Wang, J., Sun, F., Xu, J., Wang, S., Xu, L., Zhang, J., & Xin, B. (2024). Ionic Liquid/Deep Eutectic Solvent-Mediated Calcining Synthesis of Cobalt-Based Electrocatalysts for Water Splitting. Molecules, 29(18), 4435. https://doi.org/10.3390/molecules29184435






