Bioactive Properties of Enzymatic Gelatin Hydrolysates Based on In Silico, In Vitro, and In Vivo Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. In Silico Analysis of Protein Sequences
2.2. Proximate Composition of Gelatins
2.3. Degree of Hydrolysis
2.4. Antioxidant Activities
2.5. ACE-I Inhibitory Activity
2.6. Effect of Hydrolysis Time
2.7. Peptides Fractionation
2.8. Animal Behavior Test
2.8.1. Physiological Observation of Treated Mice
2.8.2. Morris Water Maze (MWM) Test for In Vivo Analysis
2.8.3. Antioxidant Capacities on Mice Brain
2.8.4. Histopathology of Mice Brain Tissue
3. Materials and Methods
3.1. Materials
3.2. In Silico Analysis
3.2.1. Homology Study of Bovine, Porcine, and Tilapia Gelatin Sequences
3.2.2. Bioactive Peptides Analysis by BIOPEP-UWM Database Tools
3.3. Proximate Analysis
3.4. Preparation of Gelatin Hydrolysates
3.5. Degree of Hydrolysis (DH)
3.6. Peptide Fractionation
3.7. Antioxidant Analysis
3.7.1. DPPH Radical Scavenging Activity Assay
3.7.2. Metal Ion Chelating Activity Assay
3.7.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.7.4. Superoxide Radical Scavenging Activity (SRSA) Assay
3.8. Angiotensin-I Converting Enzyme (ACE-I) Inhibition Assay
3.9. Anti-Amnestic Activity
3.9.1. Prolyl Endopeptidase (PEP) Inhibition Assay
3.9.2. Acetylcholinesterase (AChE) Inhibition Assay
3.10. Animal Behavior Assessment
3.10.1. Animals
3.10.2. Morris Water Maze (MWM) Test Preparation
3.11. Brain Tissue Collection and Homogenates Preparation
3.12. Thiobarbituric Acid Reactive Substances (TBARS) Assay
3.13. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.14. Superoxide Dismutase (SOD) Assay
3.15. Glutathione Peroxidase (GPx) Assay
3.16. Histopathological Sections and Staining
3.17. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.-J.; Li, H.-L.; Xiong, G.-Q.; Cai, J.; Liao, T.; Zu, X.-Y. Extraction, identification and anti-photoaging activity evaluation of collagen peptides from silver carp (Hypophthalmichthys molitrix) skin. LWT Food Sci. Technol. 2022, 173, 114384. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, K.H.; Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Lim, B.O.; Moon, S.-H.; Jeon, B.-T.; Jeon, Y.-J.; Ahn, C.-B. Biological activity from the gelatin hydrolysates of duck skin by-products. Process Biochem. 2012, 47, 1150–1154. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Canavan, M.; O’Donnell, M.J. Hypertension and cognitive impairment: A review of mechanisms and key concepts. Front. Neurol. 2022, 13, 821135. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Uddin, M.J.; Kader, M.; Alam, A.; Rahman, A.A.; Rashid, M.; Kato, K.; Tanaka, T.; Takeda, M.; Sadik, G. In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aegle marmelos leaf extract: Implications for the treatment of Alzheimer’s disease. Psychogeriatrics 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Oniszczuk, T.; Waksmundzka-Hajnos, M. The influence of common free radicals and antioxidants on development of Alzheimer’s Disease. Biomed. Pharmacother. 2016, 78, 39–49. [Google Scholar] [CrossRef]
- Chandran, S.; Binninger, D. Role of Oxidative Stress, Methionine Oxidation and Methionine Sulfoxide Reductases (MSR) in Alzheimer’s Disease. Antioxidants 2024, 13, 21. [Google Scholar] [CrossRef]
- Khurana, V.; Goswami, B. Angiotensin converting enzyme (ACE). Clin. Chim. Acta 2022, 524, 113–122. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res. Int. 2017, 100, 112–120. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Hao, Y.; Zhang, W.; Zhou, G. Antihypertensive effects in vitro and in vivo of novel angiotensin-converting enzyme inhibitory peptides from bovine bone gelatin hydrolysate. J. Agric. Food Chem. 2019, 68, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ngo, D.-H.; Kim, J.-A.; Ryu, B.; Kim, S.-K. An antihypertensive peptide from tilapia gelatin diminishes free radical formation in murine microglial cells. J. Agric. Food Chem. 2011, 59, 12193–12197. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Brown, L.; Malik, K.; Murakami, S. Two opposing functions of angiotensin-converting enzyme (ACE) that links hypertension, dementia, and aging. Int. J. Mol. Sci. 2021, 22, 13178. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.S.; Douglas, V.C.; Johnston, S.C. Alzheimer disease risk and genetic variation in ACE: A meta-analysis. Neurology 2004, 62, 363–368. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.H.B.; Ham, J.-S.; Lee, S.K.; Jang, A. Pig skin gelatin hydrolysates attenuate acetylcholine esterase activity and scopolamine-induced impairment of memory and learning ability of mice. Food Sci. Anim. Resour. 2020, 40, 183. [Google Scholar] [CrossRef]
- Gass, J.; Khosla, C. Prolyl endopeptidases. Cell. Mol. Life Sci. 2007, 64, 345–355. [Google Scholar] [CrossRef]
- Hayes, M. Bioactive peptides in preventative healthcare: An overview of bioactivities and suggested methods to assess potential applications. Curr. Pharm. Des. 2021, 27, 1332–1341. [Google Scholar] [CrossRef]
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme–inhibitory peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1150–1187. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Tolueinia, B.; Hashemi, A.; Ebrahimi, L.; Fesahat, F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimer’s Dis. Other Dement. 2013, 28, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Zare-Zardini, H.; Mogharrab, N.; Navapour, L. Purification, characterization and mechanistic evaluation of angiotensin converting enzyme inhibitory peptides derived from Zizyphus jujuba fruit. Sci. Rep. 2020, 10, 3976. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.B.; Norris, R.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide Identification in a Porcine Gelatin Prolyl Endoproteinase Hydrolysate with Angiotensin Converting Enzyme (ACE) Inhibitory and Hypotensive Activity. J. Funct. Foods 2017, 34, 77–88. [Google Scholar] [CrossRef]
- Lin, H.-C.; Alashi, A.M.; Aluko, R.E.; Sun Pan, B.; Chang, Y.-W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2017, 61, 1391666. [Google Scholar] [CrossRef] [PubMed]
- Choonpicharn, S.; Jaturasitha, S.; Rakariyatham, N.; Suree, N.; Niamsup, H. Antioxidant and antihypertensive activity of gelatin hydrolysate from Nile tilapia skin. J. Food Sci. Technol. 2015, 52, 3134–3139. [Google Scholar] [CrossRef]
- Kim, S.-K.; Byun, H.-G.; Park, P.-J.; Shahidi, F. Angiotensin I converting enzyme inhibitory peptides purified from bovine skin gelatin hydrolysate. J. Agric. Food Chem. 2001, 49, 2992–2997. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef]
- Huang, B.-B.; Lin, H.-C.; Chang, Y.-W. Analysis of Proteins and Potential Bioactive Peptides from Tilapia (Oreochromis spp.) Processing Co-products Using Proteomic Techniques Coupled with BIOPEP Database. J. Funct. Foods 2015, 19, 629–640. [Google Scholar] [CrossRef]
- Panjaitan, F.C.A.; Gomez, H.L.R.; Chang, Y.-W. In silico analysis of bioactive peptides released from giant grouper (Epinephelus lanceolatus) roe proteins identified by proteomics approach. Molecules 2018, 23, 2910. [Google Scholar] [CrossRef]
- Alipal, J.; Pu’Ad, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today 2021, 42, 240–250. [Google Scholar] [CrossRef]
- GMIA. Gelatin Handbook; GELITA North America: Sergeant Bluff, LA, USA, 2019. [Google Scholar]
- Sultana, S.; Ali, M.E.; Ahamad, M.N.U. Gelatine, collagen, and single cell proteins as a natural and newly emerging food ingredients. In Preparation and Processing of Religious and Cultural Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 215–239. [Google Scholar]
- Chung, L.; Dinakarpandian, D.; Yoshida, N.; Lauer-Fields, J.L.; Fields, G.B.; Visse, R.; Nagase, H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004, 23, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, Y.; Xing, L.; Zhang, W.; Zhou, G. Structure and physical properties of gelatin from bovine bone collagen influenced by acid pretreatment and pepsin. Food Bioprod. Process 2020, 121, 213–223. [Google Scholar] [CrossRef]
- Shiao, W.-C.; Wu, T.-C.; Kuo, C.-H.; Tsai, Y.-H.; Tsai, M.-L.; Hong, Y.-H.; Huang, C.-Y. Physicochemical and antioxidant properties of gelatin and gelatin hydrolysates obtained from extrusion-pretreated fish (Oreochromis sp.) scales. Mar. Drugs 2021, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, Z.-R.; Luo, H.-Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Pattamapun, K.; Manosroi, W.; Manosroi, J. Antioxidant and gelatinolytic activities of papain from papaya latex and bromelain from pineapple fruits. Chiang Mai J. Sci. 2014, 41, 635–648. [Google Scholar]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2018, 77–106. [Google Scholar]
- Alemán, A.; Giménez, B.; Montero, P.; Gómez-Guillén, M. Antioxidant activity of several marine skin gelatins. LWT Food Sci. Technol. 2011, 44, 407–413. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and Identification of Novel Antioxidant Peptides from Enzymatic Hydrolysates of Sardinelle (Sardinella aurita) By-products Proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Vaidya, S.; Sheshappa, M.B. Functional properties of protein hydrolyzate from ribbon fish (Lepturacanthus Savala) as prepared by enzymatic hydrolysis. Int. J. Food Prop. 2022, 25, 187–203. [Google Scholar] [CrossRef]
- Wu, H.; Xu, N.; Sun, X.; Yu, H.; Zhou, C. Hydrolysis and purification of ACE inhibitory peptides from the marine microalga Isochrysis galbana. J. Appl. Phycol. 2015, 27, 351–361. [Google Scholar] [CrossRef]
- Noman, A.; Xu, Y.; AL-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W. Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- UG, Y.; Bhat, I.; Karunasagar, I.; BS, M. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- Weng, W.; Tang, L.; Wang, B.; Chen, J.; Su, W.; Osako, K.; Tanaka, M. Antioxidant properties of fractions isolated from blue shark (Prionace glauca) skin gelatin hydrolysates. J. Funct. Foods 2014, 11, 342–351. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.-S.; Wang, R. Neuroprotective activities of enzymatically hydrolyzed peptides from porcine hide gelatin. Int. J. Clin. Exp. Med. 2008, 1, 283–293. [Google Scholar]
- Sila, A.; Martinez-Alvarez, O.; Haddar, A.; Gómez-Guillén, M.C.; Nasri, M.; Montero, M.P.; Bougatef, A. Recovery, viscoelastic and functional properties of Barbel skin gelatine: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatine polypeptides. Food Chem. 2015, 168, 478–486. [Google Scholar] [CrossRef]
- Männistö, P.T.; García-Horsman, J.A. Mechanism of Action of Prolyl Oligopeptidase (PREP) in Degenerative Brain Diseases: Has Peptidase Activity Only a Modulatory Role on the Interactions of PREP with Proteins? Front. Aging Neurosci. 2017, 9, 27. [Google Scholar] [CrossRef]
- Parameshwaran, K.; Irwin, M.H.; Steliou, K.; Pinkert, C.A. D-galactose effectiveness in modeling aging and therapeutic antioxidant treatment in mice. Rejuvenation Res. 2010, 13, 729–735. [CrossRef]
- Zhen, Y.Z.; Lin, Y.J.; Li, K.J.; Zhang, G.L.; Zhao, Y.F.; Wang, M.M.; Wei, J.B.; Wei, J.; Hu, G. Effects of rhein lysinate on D-galactose-induced aging mice. Exp. Ther. Med. 2016, 11, 303–308. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhao, J.; Chen, Y.; Ren, C.; Chen, Y. Antioxidant effects of compound walnut oil capsule in mice aging model induced by D-galactose. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Effect and mechanism of oyster hydrolytic peptides on spatial learning and memory in mice. RSC Adv. 2018, 8, 6125–6135. [Google Scholar] [CrossRef]
- Liu, Y.T.; Cheng, F.Y.; Takeda, S.; Lai, K.M.; Lin, L.C.; Sakata, R. Effects of porcine brain hydrolysate on impairment of cognitive learning ability in amyloid β (1–40)-infused rats. Anim. Sci. J. 2019, 90, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.T.; Fenton, A.A. On how the dentate gyrus contributes to memory discrimination. Neuron 2018, 98, 832–845.e5. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2003. [Google Scholar]
- Lowry, O.; Rosebrough, N.; Farr, A. Protein measurement with folin phenol reagent, Lowry protein. J. Biol. Chem. 1951, 193, 165–275. [Google Scholar] [CrossRef]
- Charoenphun, N.; Cheirsilp, B.; Sirinupong, N.; Youravong, W. Calcium-binding peptides derived from tilapia (Oreochromis niloticus) protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 57–63. [Google Scholar] [CrossRef]
- Girgih Abraham, T.; Udenigwe Chibuike, C.; Aluko Rotimi, E. In Vitro Antioxidant Properties of Hemp Seed (Cannabis sativa L.) Protein Hydrolysate Fractions. J. Am. Oil Chem. Soc. 2010, 88, 381–389. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant Activity of Peptides Isolated from Alfalfa Leaf Protein Hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Siswoyo, T.A.; Mardiana, E.; Lee, K.O.; Hoshokawa, K. Isolation and Characterization of Antioxidant Protein Fractions from Melinjo (Gnetum gnemon) Seeds. J. Agric. Food Chem. 2011, 59, 5648–5656. [Google Scholar] [CrossRef]
- Girgih, A.T.; Nwachukwu, I.D.; Hasan, F.; Fagbemi, T.N.; Gill, T.; Aluko, R.E. Kinetics of the inhibition of renin and angiotensin I-converting enzyme by cod (Gadus morhua) protein hydrolysates and their antihypertensive effects in spontaneously hypertensive rats. Food Nutr. Res. 2015, 59, 29788. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. In Vitro Acetylcholinesterase-Inhibitory Properties of Enzymatic Hemp Seed Protein Hydrolysates. J. Am. Oil Chem. Soc. 2015, 93, 411–420. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.-M.; Zheng, Y.-L.; Hu, B.; Zhang, Z.-F.; Ye, Q.; Liu, C.-M.; Shan, Q.; Wang, Y.-J. Ursolic Acid Attenuates D-Galactose-Induced Inflammatory Response in Mouse Prefrontal Cortex through Inhibiting AGEs/RAGE/NF-κB Pathway Activation. Cereb. Cortex 2010, 20, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, O.; Gutiérrez-Smith, Y.; Guzmán-Muñiz, J.; Moy-López, N.A. Intrauterine stress impairs spatial learning in the progeny of Wistar rats. Rev. Invest. Clín. 2011, 63, 279–286. [Google Scholar] [PubMed]
- Chan, C.-J.; Tseng, J.-K.; Wang, S.-Y.; Lin, Y.-L.; Wu, Y.-H.S.; Chen, J.-W.; Chen, Y.-C. Ameliorative effects of functional chalaza hydrolysates prepared from protease-A digestion on cognitive dysfunction and brain oxidative damages. Poult. Sci. 2020, 99, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-Y.; Fu, T.Y.-C.; Shih, P.-H.; Lee, C.-P.; Yen, G.-C. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibits CCl4-induced hepatic damage in rats. Food Chem. Toxicol. 2006, 44, 1424–1431. [Google Scholar] [CrossRef]
- Mueller, A.S.; Bosse, A.C.; Most, E.; Klomann, S.D.; Schneider, S.; Pallauf, J. Regulation of the insulin antagonistic protein tyrosine phosphatase 1B by dietary Se studied in growing rats. J. Nutr. Biochem. 2009, 20, 235–247. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
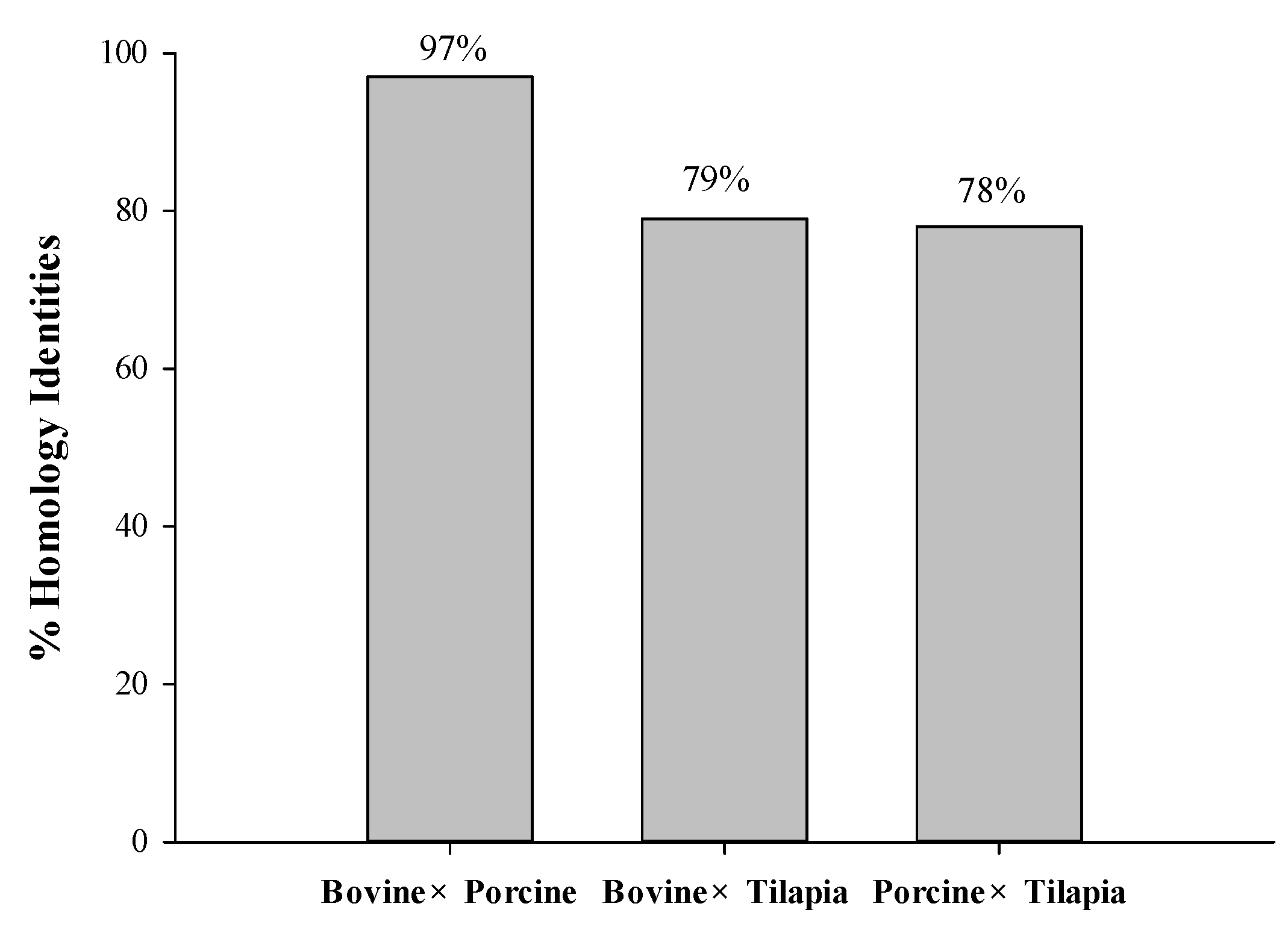
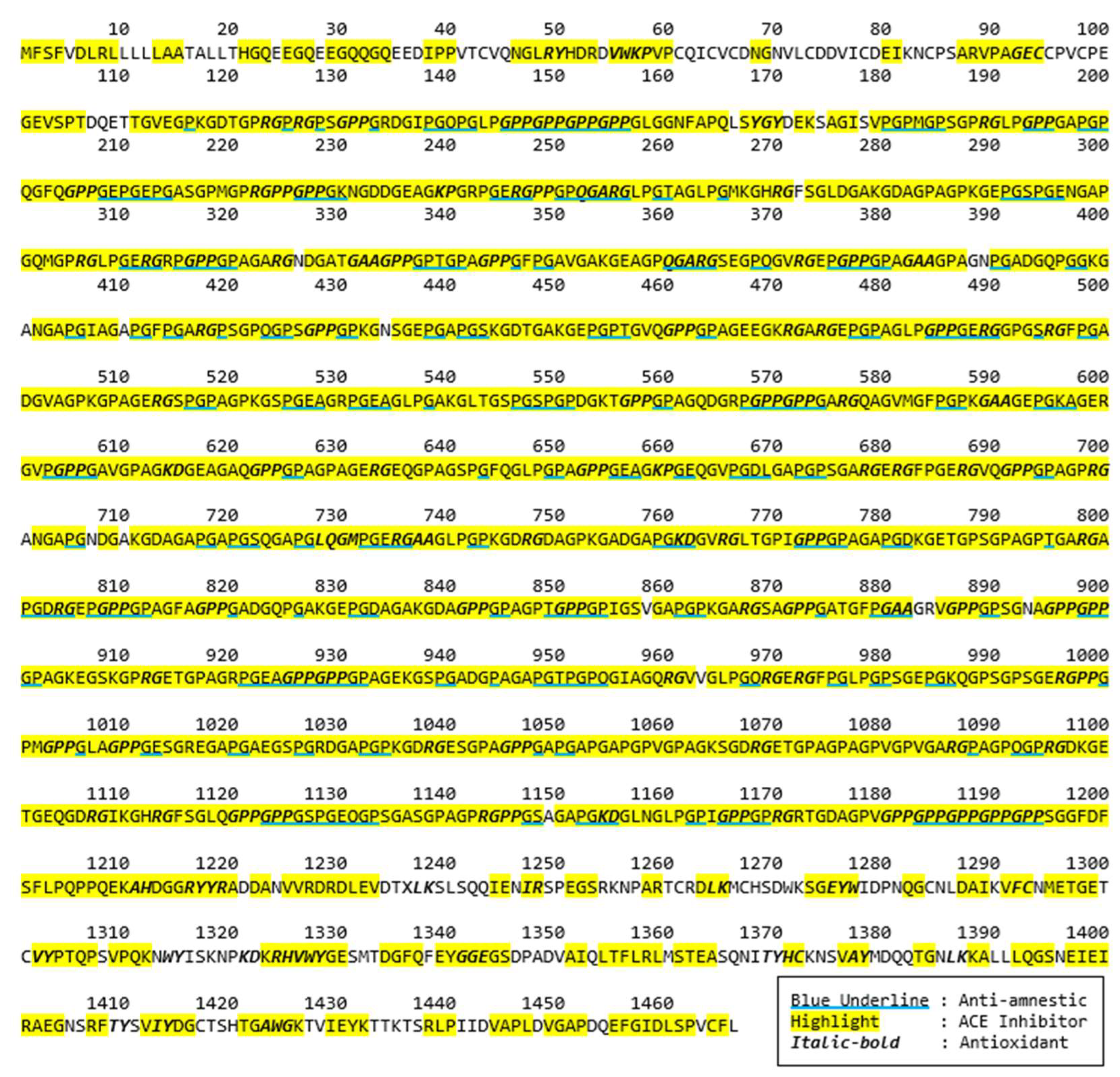





| Bioactivities | Bovine | Porcine | Tilapia | |||
|---|---|---|---|---|---|---|
| Bromelain | Papain | Bromelain | Papain | Bromelain | Papain | |
| Antioxidant | 0.0014 | 0.0007 | 0.0014 | 0.0007 | 0.0014 | 0.0014 |
| ACE inhibitor | 0.0971 | 0.1053 | 0.0969 | 0.1091 | 0.0919 | 0.1113 |
| Anti-amnestic | 0.0410 | 0.0178 | 0.0402 | 0.0177 | 0.0428 | 0.0207 |
| Composition | Bovine (B) | Porcine (P) | Tilapia (T) |
|---|---|---|---|
| Moisture | 13.29 ± 0.00 a | 14.63 ± 0.18 c | 14.01 ± 0.07 b |
| Ash | 0.42 ± 0.01 c | 0.04 ± 0.01 a | 0.21 ± 0.00 b |
| Crude Protein | 83.56 ± 0.60 a | 83.31 ± 0.37 a | 85.10 ± 0.82 b |
| Crude fat | 2.38 ± 0.24 a | 2.50 ± 0.23 a | 2.65 ± 0.01 a |
| Sample | DH (%) | Protein Contents (%) | Yield *** (%) | DPPH Scavenging Activity (%) | FRAP Activity (mM Fe2+/mg Protein) | Metal Ion Chelating Activity (%) | SRSA Activity (%) | ACE-I Activity (%) |
|---|---|---|---|---|---|---|---|---|
| Gelatin Hydrolysates * | ||||||||
| Bovine–Bromelain (BB) | 24.17 ± 0.03 a | 69.49 ± 0.22 a | 29.84 ± 0.93 a | 19.20 ± 1.77 def | 0.073 ± 0.00 cde | 13.24 ± 0.40 a | 14.89 ± 1.32 ab | 27.73 ± 2.06 a |
| Bovine–Collagenase (BC) | 48.95 ± 0.26 h | 76.13 ± 0.20 b | 59.87 ± 4.31 b | 22.53 ± 2.81 f | 0.086 ± 0.00 ef | 46.66 ± 0.23 e | 32.82 ± 1.32 e | 44.15 ± 3.75 b |
| Bovine–Papain (BP) | 27.52 ± 0.43 b | 75.58 ± 1.86 b | 53.49 ± 4.71 b | 19.77 ± 0.72 ef | 0.090 ± 0.00 f | 24.02 ± 0.50 b | 20.99 ± 0.00 c | 35.62 ± 2.07 ab |
| Porcine–Bromelain (PB) | 43.27 ± 0.97 f | 87.17 ± 0.45 ef | 59.51 ± 0.92 b | 14.60 ± 0.20 abc | 0.086 ± 0.01 ef | 32.36 ± 0.83 c | 16.03 ± 0.66 b | 34.08 ± 0.64 ab |
| Porcine–Collagenase (PC) | 53.89 ± 0.53 i | 84.35 ± 1.98 de | 61.77 ± 9.84 b | 18.05 ± 0.72 cde | 0.051 ± 0.00 ab | 60.95 ± 0.23 g | 32.44 ± 1.15 e | 44.86 ± 1.08 ab |
| Porcine–Papain (PP) | 37.46 ± 0.24 d | 89.57 ± 0.75 f | 62.52 ± 2.85 b | 15.63 ± 0.40 bcd | 0.079 ± 0.00 efg | 34.41 ± 1.00 d | 21.76 ± 1.75 c | 60.94 ± 2.94 c |
| Tilapia–Bromelain (TB) | 31.26 ± 0.53 c | 87.54 ± 0.91 ef | 67.98 ± 15.67 b | 11.38 ± 1.92 a | 0.063 ± 0.01 bc | 56.25 ± 0.98 f | 12.21 ± 1.75 a | 38.40 ± 0.53 ab |
| Tilapia–Collagenase (TC) | 45.44 ± 0.32 e | 79.02 ± 1.58 bc | 60.25 ± 6.92 b | 13.56 ± 1.11 ab | 0.043 ± 0.00 a | 69.76 ± 0.11 h | 29.01 ± 0.11 d | 48.74 ± 1.14 bc |
| Tilapia–Papain (TP) | 42.30 ± 0.15 g | 82.64 ± 1.02 cd | 60.30 ± 3.84 b | 15.52 ± 0.34 bcd | 0.068 ± 0.00 cd | 34.22 ± 1.13 d | 22.90 ± 0.66 c | 39.32 ± 4.69 ab |
| Porcine–Papain (PP) Hydrolysates ** | ||||||||
| PP1 (PP 1-hour hydrolysis) | 29.82 ± 0.17 a | 94.48 ± 0.55 b | 65.23 ± 12.81 a | 8.53 ± 0.91 a | 0.085 ± 0.01 a | 11.83 ± 0.91 a | 18.53 ± 0.36 a | 96.56 ± 1.37 a |
| PP4 (PP 4-hour hydrolysis) | 37.46 ± 0.24 b | 89.11 ± 2.86 a | 62.52 ± 2.85 a | 8.08 ± 0.46 a | 0.068 ± 0.02 b | 16.74 ± 1.02 a | 17.69 ± 1.31 a | 94.76 ± 2.00 a |
| Amino Acid | PP1 Hydrolysate (g/100 g) |
|---|---|
| Alanine | 7.96 ± 0.09 |
| Arginine | 7.20 ± 0.30 |
| Aspartic acid | 5.45 ± 0.12 |
| Cystine | 0.10 ± 0.00 |
| Glutamic acid | 9.48 ± 0.23 |
| Glycine | 20.63 ± 0.40 |
| Histidine | 0.62 ± 0.03 |
| Isoleucine | 1.17 ± 0.00 |
| Leucine | 2.55 ± 0.00 |
| Lysine | 3.75 ± 0.04 |
| Methionine | 0.80 ± 0.03 |
| Phenylalanine | 1.72 ± 0.12 |
| Proline | 12.23 ± 0.23 |
| Serine | 3.44 ± 0.18 |
| Threonine | 1.76 ± 0.05 |
| Tryptophan | – |
| Tyrosine | 0.82 ± 0.05 |
| Valine | 2.13 ± 0.01 |
| Bioactivities * | PP1 Fractions | ||
|---|---|---|---|
| <1 kDa | 1–5 kDa | 5–10 kDa | |
| Antioxidant | |||
| -DPPH-scavenging activity (%) | ND ** | 2.95 ± 0.20 | ND |
| -Metal ion chelating activity (%) | ND | 3.97 ± 0.58 | 4.57 ± 0.58 |
| -FRAP activity (mM Fe2+/mg protein) | 0.22 ± 0.01 b | 0.17 ± 0.01 a | 0.17 ± 0.01 a |
| -SRSA activity (%) | 18.24 ± 0.44 b | 15.47 ± 0.00 a | 14.72 ± 1.31 a |
| Antihypertensive | |||
| -ACE-I inhibition | 87.42 ± 3.20 c | 69.47 ± 4.87 b | 10.82 ± 1.96 a |
| Anti-amestic | |||
| -AChE inhibition | 21.24 ± 2.36 b | 2.30 ± 2.76 a | 3.02 ± 1.40 a |
| -PEP inhibition | 48.07 ± 13.65 | ND | ND |
| Parameters | ICR Mice Groups | |||
|---|---|---|---|---|
| CON | DG | DG_LPP1 | DG_HPP1 | |
| Initial weight (g) | 36.38 ± 0.94 a | 36.48 ± 0.71 a | 37.08 ± 0.87 a | 37.08 ± 0.99 a |
| Final weight (g) | 40.92 ± 2.23 a | 38.85 ± 0.95 a | 39.41 ± 1.58 a | 39.78 ± 1.61 a |
| Food intake (g/mouse/day) | 6.46 ± 0.43 a | 6.30 ± 0.33 a | 6.41 ± 0.31 a | 6.68 ± 0.22 a |
| Water intake (g/mouse/day) | 8.56 ± 0.39 a | 8.32 ± 0.37 a | 8.72 ± 0.50 a | 8.88 ± 0.25 a |
| Brain (g/100 g BW) | 1.33 ± 0.08 a | 1.28 ± 0.07 a | 1.26 ± 0.07 a | 1.29 ± 0.07 a |
| Liver (g/100 g BW) | 3.98 ± 0.14 a | 4.14 ± 0.18 a | 4.14 ± 0.18 a | 4.12 ± 0.05 a |
| Epididymal fat (g/100 g BW) | 0.52 ± 0.12 a | 0.46 ± 0.12 a | 0.48 ± 0.17 a | 0.48 ± 0.17 a |
| Perirenal fat (g/100 g BW) | 1.30 ± 0.22 a | 1.00 ± 0.14 a | 1.07 ± 0.30 a | 1.03 ± 0.15 a |
| TBARS (nmol MDA eq/mg protein) | 31.97 ± 1.67 a | 30.68 ± 2.65 a | 32.76 ± 1.07 a | 31.46 ± 0.84 a |
| TEAC (nmol Trolox equivalents/mg protein) | 300.81 ± 12.07 a | 269.59 ± 10.50 a | 280.28 ± 15.97 a | 277.84 ± 16.58 a |
| SOD activity (unit/mg protein) | 2.03 ± 0.24 a | 1.34 ± 0.13 b | 1.75 ± 0.08 ab | 1.88 ± 0.15 a |
| GPx activity (munit/mg protein) | 49.00 ± 1.61 a | 37.58 ± 2.44 b | 43.14 ± 2.71 ab | 42.66 ± 2.89 ab |
| Group. | Subcutaneous Injection on the Back | Oral Gavage |
|---|---|---|
| CON | Saline (0.9%) | Distilled water |
| DG | D-galactose (300 mg/kg BW/day) | Distilled water |
| DG_LPP1 | D-galactose (300 mg/kg BW/day) | PP1 (100 mg/kg BW/day) |
| DG_HPP1 | D-galactose (300 mg/kg BW/day) | PP1 (500 mg/kg BW/day) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panjaitan, F.C.A.; Shie, S.-T.; Park, S.H.; Sevi, T.; Ko, W.-L.; Aluko, R.E.; Chang, Y.-W. Bioactive Properties of Enzymatic Gelatin Hydrolysates Based on In Silico, In Vitro, and In Vivo Studies. Molecules 2024, 29, 4402. https://doi.org/10.3390/molecules29184402
Panjaitan FCA, Shie S-T, Park SH, Sevi T, Ko W-L, Aluko RE, Chang Y-W. Bioactive Properties of Enzymatic Gelatin Hydrolysates Based on In Silico, In Vitro, and In Vivo Studies. Molecules. 2024; 29(18):4402. https://doi.org/10.3390/molecules29184402
Chicago/Turabian StylePanjaitan, Fenny Crista A., Sin-Ting Shie, Sung Hoon Park, Tesalonika Sevi, Wen-Ling Ko, Rotimi E. Aluko, and Yu-Wei Chang. 2024. "Bioactive Properties of Enzymatic Gelatin Hydrolysates Based on In Silico, In Vitro, and In Vivo Studies" Molecules 29, no. 18: 4402. https://doi.org/10.3390/molecules29184402
APA StylePanjaitan, F. C. A., Shie, S.-T., Park, S. H., Sevi, T., Ko, W.-L., Aluko, R. E., & Chang, Y.-W. (2024). Bioactive Properties of Enzymatic Gelatin Hydrolysates Based on In Silico, In Vitro, and In Vivo Studies. Molecules, 29(18), 4402. https://doi.org/10.3390/molecules29184402









