Enhancement of hMSC In Vitro Proliferation by Surface Immobilization of a Heparin-Binding Peptide
Abstract
1. Introduction
2. Results
2.1. pKL Synthesis
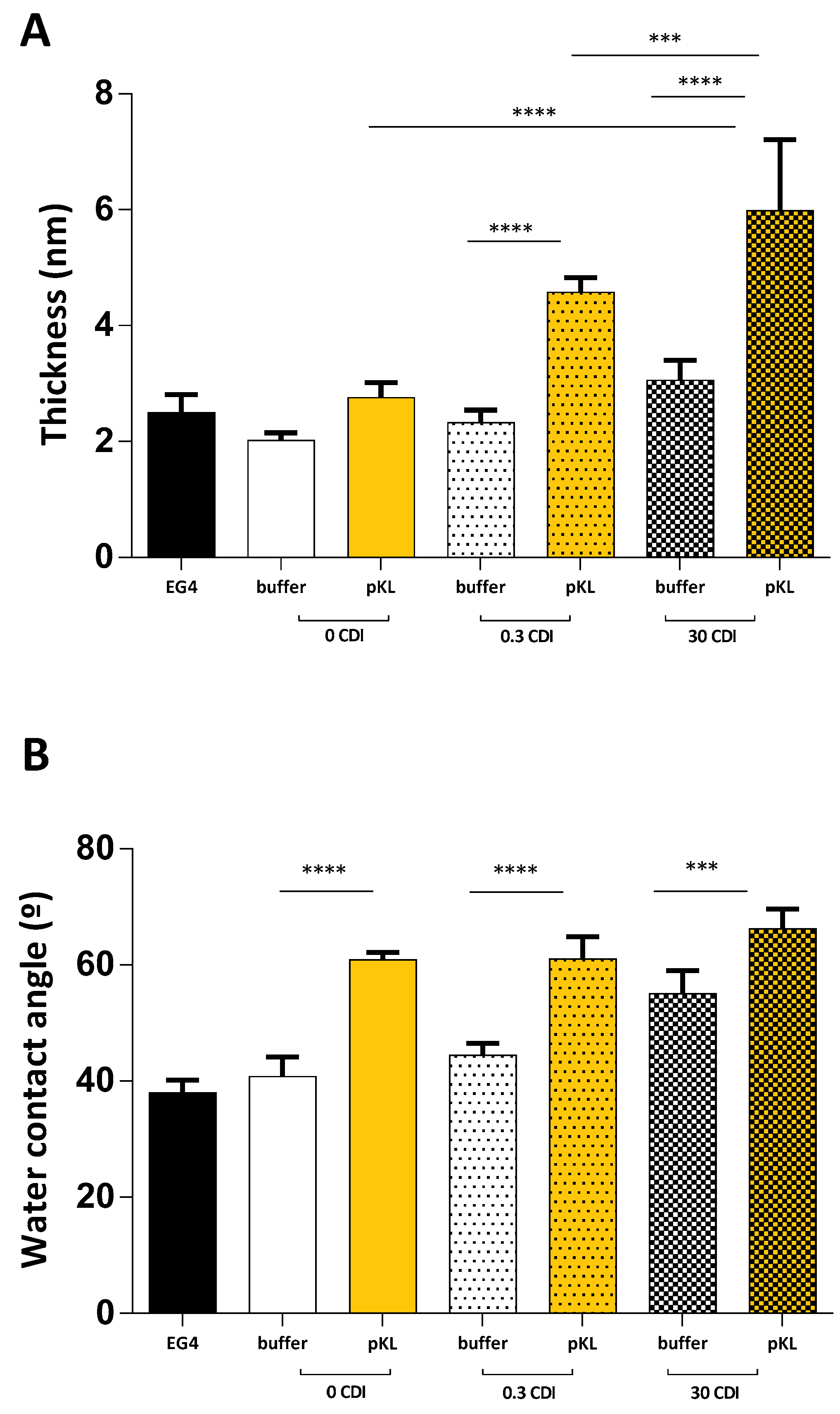
2.2. pKL-SAMs
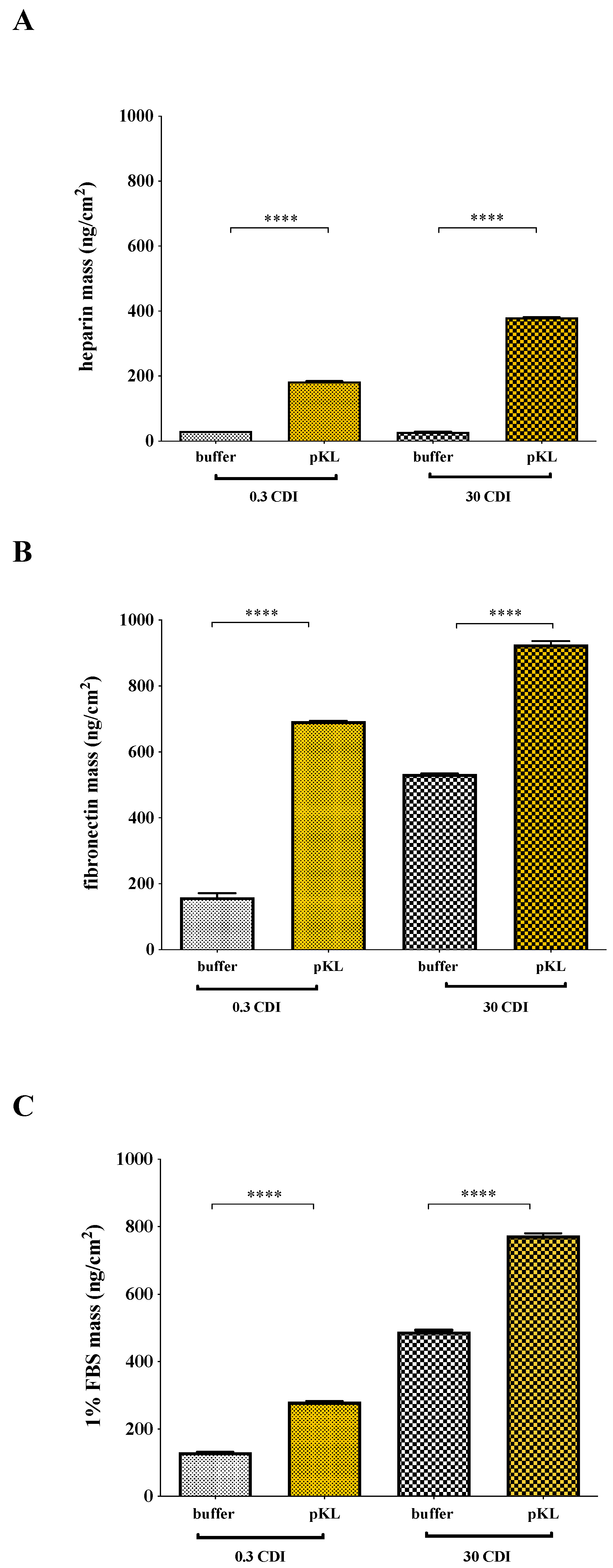
2.3. Heparin and Fibronectin Adsorption on pKL-SAMs
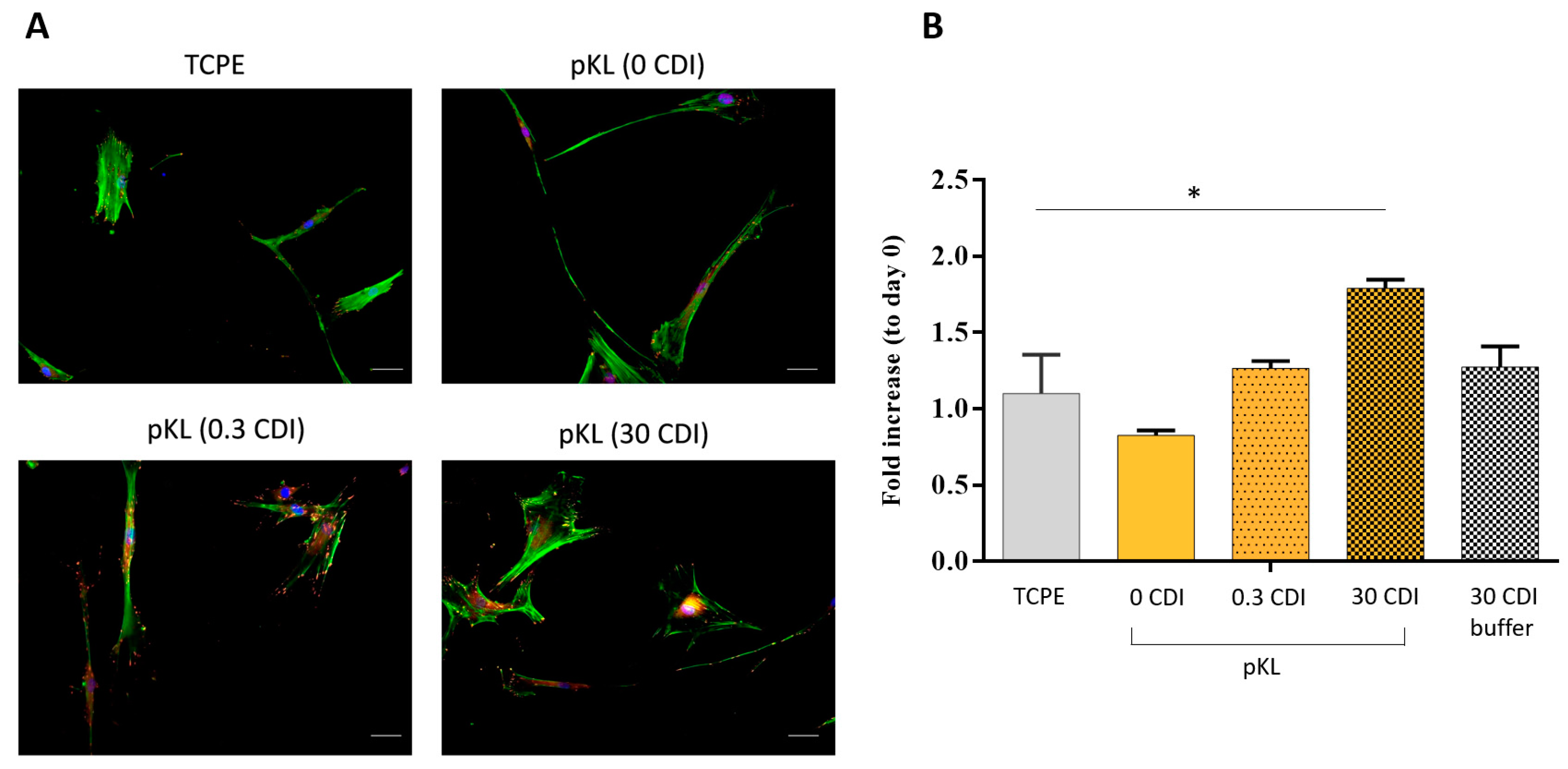
2.4. hMSC Adhesion and Morphology on pKL-SAMs
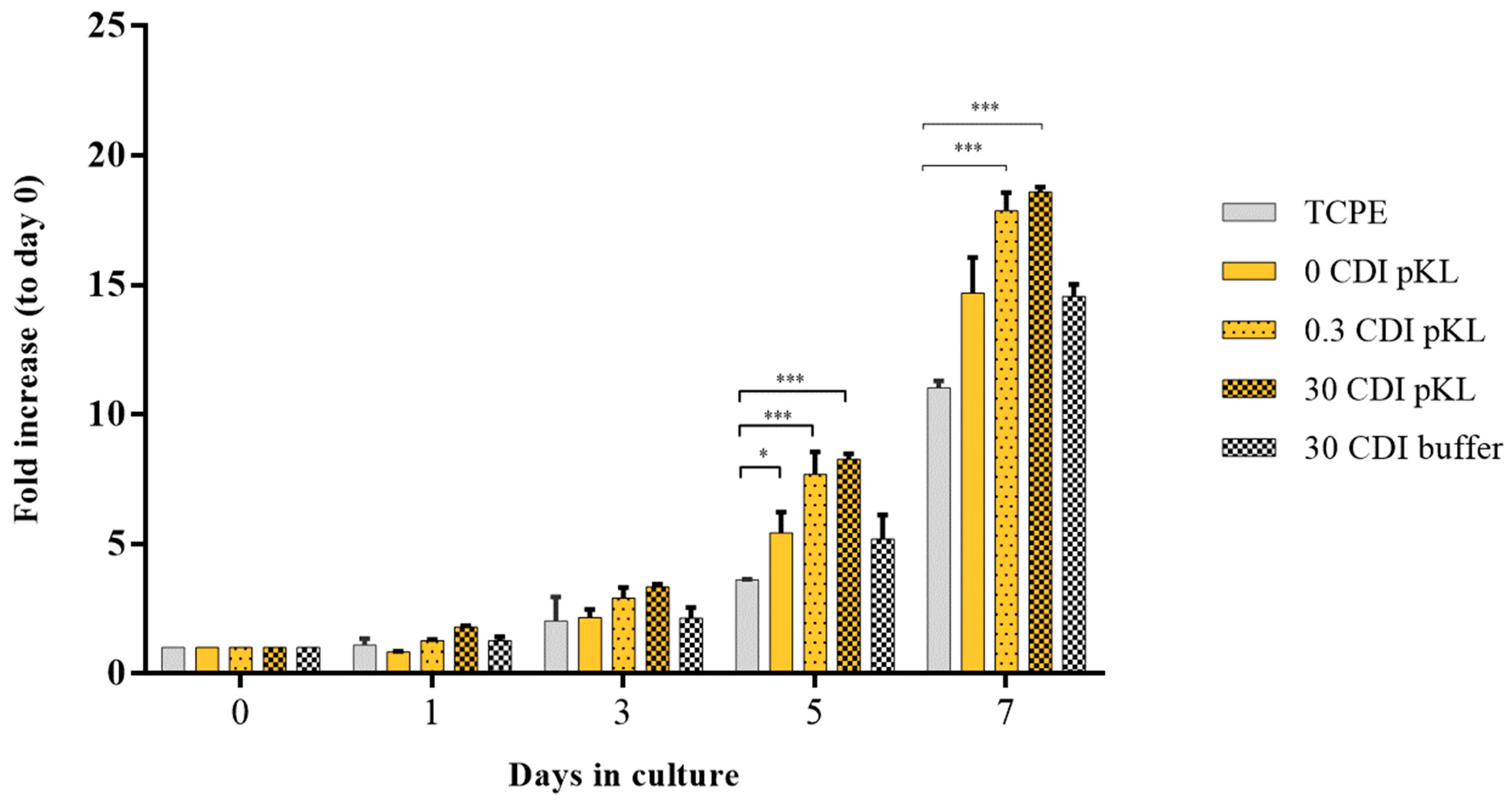
2.5. hMSC Metabolic Activity/Growth on pKL-SAMs
3. Discussion
4. Materials and Methods
4.1. pKL Synthesis and Characterization
4.2. pKL Surfaces Preparation and Characterization
4.2.1. Gold Substrates
For Surface Characterization and Biological Assays
For Adsorption Assays Using Quartz Crystal Microbalance with Dissipation (QCM-D)
4.2.2. EG4-SAMs
4.2.3. pKL-SAMs
4.3. Surface Characterization
4.3.1. Water Contact Angle
4.3.2. Ellipsometry
4.4. Heparin and Fibronectin Adsorption to pKL-SAMs
4.5. Human Mesenchymal Stem Cells (hMSC) Adhesion and Proliferation on pKL-SAMs
4.5.1. hMSC Culture
4.5.2. hMSC Cytoskeleton Organization
4.5.3. hMSC Metabolic Activity
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoogduijn, M.; Dor, F. Mesenchymal Stem Cells: Are We Ready for Clinical Application in Transplantation and Tissue Regeneration? Front. Immunol. 2013, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Liu, W.-F.; Bai, Y.-Y.; Zhou, Y.-Y.; Zhang, Y.; Wang, C.-M.; Lin, S.; He, H.-F. Transplantation of mesenchymal stem cells for spinal cord injury: A systematic review and network meta-analysis. J. Transl. Med. 2021, 19, 178. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-S.; Jeong, E.-J.; Kim, J.-Y.; Park, S.J.; Ju, W.S.; Kim, C.-H.; Kim, J.-S.; Choo, Y.-K. Application of Mesenchymal Stem Cells in Inflammatory and Fibrotic Diseases. Int. J. Mol. Sci. 2020, 21, 8366. [Google Scholar] [CrossRef]
- Kelly, K.; Rasko, J.E.J. Mesenchymal Stromal Cells for the Treatment of Graft Versus Host Disease. Front. Immunol. 2021, 12, 761616. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Hynes, R.O. Extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Shimokawa, K.; Kimura-Yoshida, C.; Nagai, N.; Mukai, K.; Matsubara, K.; Watanabe, H.; Matsuda, Y.; Mochida, K.; Matsuo, I. Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev. Cell 2011, 21, 257–272. [Google Scholar] [CrossRef]
- Mohammadi, M.; Olsen, S.K.; Goetz, R. A protein canyon in the FGF–FGF receptor dimer selects from an à la carte menu of heparan sulfate motifs. Curr. Opin. Struct. Biol. 2005, 15, 506–516. [Google Scholar] [CrossRef]
- Makarenkova, H.P.; Hoffman, M.P.; Beenken, A.; Eliseenkova, A.V.; Meech, R.; Tsau, C.; Patel, V.N.; Lang, R.A.; Mohammadi, M. Differential Interactions of FGFs with Heparan Sulfate Control Gradient Formation and Branching Morphogenesis. Sci. Signal. 2009, 2, ra55. [Google Scholar] [CrossRef] [PubMed]
- Blau, A. Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: An introductory overview and critical discussion. Curr. Opin. Colloid Interface Sci. 2013, 18, 481–492. [Google Scholar] [CrossRef]
- Barrias, C.C.; Martins, M.C.L.; Almeida-Porada, G.; Barbosa, M.A.; Granja, P.L. The correlation between the adsorption of adhesive proteins and cell behaviour on hydroxyl-methyl mixed self-assembled monolayers. Biomaterials 2009, 30, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Cimino, M.; Gonçalves, R.; Bauman, E.; Barroso-Vilares, M.; Logarinho, E.; Barrias, C.; Martins, M. Optimization of the use of a pharmaceutical grade xeno-free medium for in vitro expansion of human mesenchymal stem/stromal cells. J. Tissue Eng. Regen. Med. 2017, 12, e1785–e1795. [Google Scholar] [CrossRef]
- Bellosta, P.; Iwahori, A.; Plotnikov, A.N.; Eliseenkova, A.V.; Basilico, C.; Mohammadi, M. Identification of receptor and heparin binding sites in fibroblast growth factor 4 by structure-based mutagenesis. Mol. Cell. Biol. 2001, 21, 5946–5957. [Google Scholar] [CrossRef]
- Li, B.; Lin, Z.; Mitsi, M.; Zhang, Y.; Vogel, V. Heparin-induced conformational changes of fibronectin within the extracellular matrix promote hMSC osteogenic differentiation. Biomater. Sci. 2015, 3, 73–84. [Google Scholar] [CrossRef]
- Stauber, D.J.; DiGabriele, A.D.; Hendrickson, W.A. Structural interactions of fibroblast growth factor receptor with its ligands. Proc. Natl. Acad. Sci. USA 2000, 97, 49–54. [Google Scholar] [CrossRef]
- Koledova, Z.; Sumbal, J.; Rabata, A.; De La Bourdonnaye, G.; Chaloupkova, R.; Hrdlickova, B.; Damborsky, J.; Stepankova, V. Fibroblast growth factor 2 protein stability provides decreased dependence on heparin for induction of FGFR signaling and alters ERK signaling dynamics. Front. Cell Dev. Biol. 2019, 7, 331. [Google Scholar] [CrossRef]
- Hudalla, G.A.; Murphy, W.L. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv. Funct. Mater. 2011, 21, 1754–1768. [Google Scholar] [CrossRef]
- Mimura, S.; Kimura, N.; Hirata, M.; Tateyama, D.; Hayashida, M.; Umezawa, A.; Kohara, A.; Nikawa, H.; Okamoto, T.; Furue, M.K. Growth factor-defined culture medium for human mesenchymal stem cells. Int. J. Dev. Biol. 2011, 55, 181–187. [Google Scholar] [CrossRef]
- Jung, S.; Panchalingam, K.M.; Rosenberg, L.; Behie, L.A. Ex Vivo Expansion of Human Mesenchymal Stem Cells in Defined Serum-Free Media. Stem Cells Int. 2012, 2012, 123030. [Google Scholar] [CrossRef]
- Ling, L.; Camilleri, E.T.; Helledie, T.; Samsonraj, R.M.; Titmarsh, D.M.; Chua, R.J.; Dreesen, O.; Dombrowski, C.; Rider, D.A.; Galindo, M.; et al. Effect of heparin on the biological properties and molecular signature of human mesenchymal stem cells. Gene 2016, 576, 292–303. [Google Scholar] [CrossRef]
- Hudalla, G.A.; Kouris, N.A.; Koepsel, J.T.; Ogle, B.M.; Murphy, W.L. Harnessing endogenous growth factor activity modulates stem cell behavior. Integr. Biol. 2011, 3, 832–842. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Curtin, S.A.; Freitas, S.C.; Salgueiro, P.; Ratner, B.D.; Barbosa, M.A. Molecularly designed surfaces for blood deheparinization using an immobilized heparin-binding peptide. J. Biomed. Mater. Res. Part A 2008, 88A, 162–173. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. α-Aminoacid-N-Carboxy-Anhydrides and Related Heterocycles; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Mittal, K.L. Contact Angle, Wettability and Adhesion; CRC Press: Boca Raton, FL, USA, 2009; Volume 6. [Google Scholar]
- Parreira, P.; Monteiro, C.; Graça, V.; Gomes, J.; Maia, S.; Gomes, P.; Gonçalves, I.C.; Martins, M.C.L. Surface Grafted MSI-78A Antimicrobial Peptide has High Potential for Gastric Infection Management. Sci. Rep. 2019, 9, 18212. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.; Martins, M.C.L.; Barbosa, M.A. The stability of self-assembled monolayers with time and under biological conditions. J. Biomed. Mater. Res. Part A 2010, 94, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Mousavifar, L.; Parreira, P.; Taponard, A.; Graça, V.C.D.; Martins, M.C.L.; Roy, R. Validation of Selective Capture of Fimbriated Uropathogenic Escherichia coli by a Label-free Engineering Detection System Using Mannosylated Surfaces. ACS Appl. Bio Mater. 2022, 5, 5877–5886. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.C.L.; Ochoa-Mendes, V.; Ferreira, G.; Barbosa, J.N.; Curtin, S.A.; Ratner, B.D.; Barbosa, M.A. Interactions of leukocytes and platelets with poly(lysine/leucine) immobilized on tetraethylene glycol-terminated self-assembled monolayers. Acta Biomater. 2011, 7, 1949–1955. [Google Scholar] [CrossRef]
- Bae, J.W.; Choi, J.H.; Kim, T.E.; Park, K.D.; Kim, J.Y.; Park, Y.D.; Sun, K. Heparinized Micropatterned Surfaces for the Spatial Control of Human Mesenchymal Stem Cells. J. Bioact. Compat. Polym. 2009, 24, 493–506. [Google Scholar] [CrossRef]
- Sobolewski, P.; Murthy, N.S.; Kohn, J.; El Fray, M. Adsorption of Fibrinogen and Fibronectin on Elastomeric Poly(butylene succinate) Copolyesters. Langmuir 2019, 35, 8850–8859. [Google Scholar] [CrossRef]
- Molino, P.J.; Higgins, M.; Innis, P.; Kapsa, R.; Wallace, G. Fibronectin and bovine serum albumin adsorption and conformational dynamics on inherently conducting polymers: A QCM-D study. Langmuir 2012, 28, 8433–8445. [Google Scholar] [CrossRef]
- Hayman, E.G.; Ruoslahti, E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J. Cell Biol. 1979, 83, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Barkalow, F.J.B.; Schwarzbauer, J.E. Localization of the major heparin-binding site in fibronectin. J. Biol. Chem. 1991, 266, 7812–7818. [Google Scholar] [CrossRef]
- Hubbard, B.; Buczek-Thomas, J.A.; Nugent, M.A.; Smith, M.L. Heparin-dependent regulation of fibronectin matrix conformation. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 34, 124–131. [Google Scholar] [CrossRef]
- Uygun, B.E.; Stojsih, S.E.; Matthew, H.W. Effects of Immobilized Glycosaminoglycans on the Proliferation and Differentiation of Mesenchymal Stem Cells. Tissue Eng. Part A 2009, 15, 3499–3512. [Google Scholar] [CrossRef] [PubMed]
- Cimino, M.; Goncalves, R.; Barrias, C.; Martins, M.C.L. Xeno-Free Strategies for Safe Human Mesenchymal Stem/Stromal Cell Expansion: Supplements and Coatings. Stem Cells Int. 2017, 2017, 6597815. [Google Scholar] [CrossRef]
- Cimino, M.; Parreira, P.; Bidarra, S.J.; Gonçalves, R.M.; Barrias, C.C.; Martins, M.C.L. Effect of surface chemistry on hMSC growth under xeno-free conditions. Colloids Surf. B Biointerfaces 2020, 189, 110836. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Ratner, B.D.; Barbosa, M.A. Protein adsorption on mixtures of hydroxyl- and methyl-terminated alkanethiols self-assembled monolayers. J. Biomed. Mater. Res. Part A 2003, 67, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Easley, A.D.; Ma, T.; Eneh, C.I.; Yun, J.; Thakur, R.M.; Lutkenhaus, J.L. A practical guide to quartz crystal microbalance with dissipation monitoring of thin polymer films. J. Polym. Sci. 2022, 60, 1090–1107. [Google Scholar] [CrossRef]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average protein density is a molecular-weight-dependent function. Protein Sci. 2004, 13, 2825–2828. [Google Scholar] [CrossRef]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 17374. [Google Scholar] [CrossRef] [PubMed]
- Page, B.; Page, M.; Noel, C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimino, M.; Parreira, P.; Leiro, V.; Sousa, A.; Gonçalves, R.M.; Barrias, C.C.; Martins, M.C.L. Enhancement of hMSC In Vitro Proliferation by Surface Immobilization of a Heparin-Binding Peptide. Molecules 2023, 28, 3422. https://doi.org/10.3390/molecules28083422
Cimino M, Parreira P, Leiro V, Sousa A, Gonçalves RM, Barrias CC, Martins MCL. Enhancement of hMSC In Vitro Proliferation by Surface Immobilization of a Heparin-Binding Peptide. Molecules. 2023; 28(8):3422. https://doi.org/10.3390/molecules28083422
Chicago/Turabian StyleCimino, Maura, Paula Parreira, Victoria Leiro, Aureliana Sousa, Raquel M. Gonçalves, Cristina C. Barrias, and M. Cristina L. Martins. 2023. "Enhancement of hMSC In Vitro Proliferation by Surface Immobilization of a Heparin-Binding Peptide" Molecules 28, no. 8: 3422. https://doi.org/10.3390/molecules28083422
APA StyleCimino, M., Parreira, P., Leiro, V., Sousa, A., Gonçalves, R. M., Barrias, C. C., & Martins, M. C. L. (2023). Enhancement of hMSC In Vitro Proliferation by Surface Immobilization of a Heparin-Binding Peptide. Molecules, 28(8), 3422. https://doi.org/10.3390/molecules28083422






