Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite
Abstract
1. Introduction
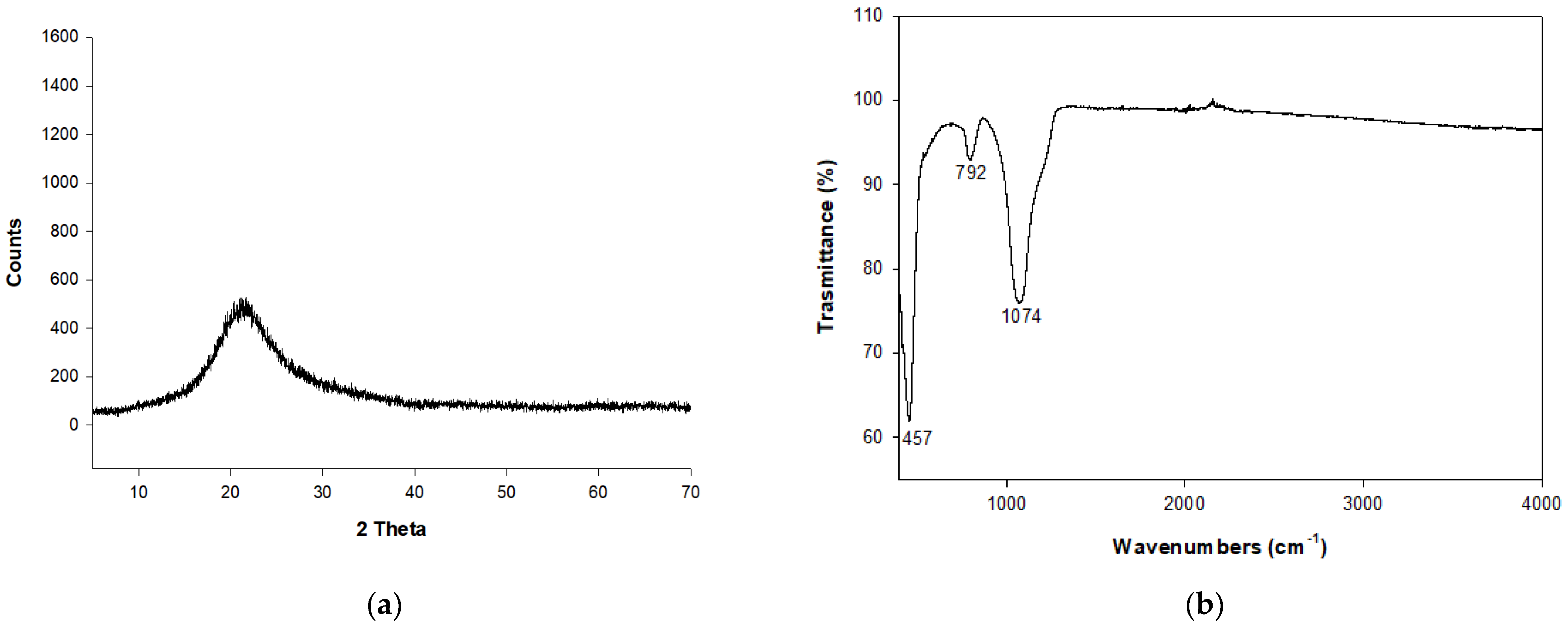
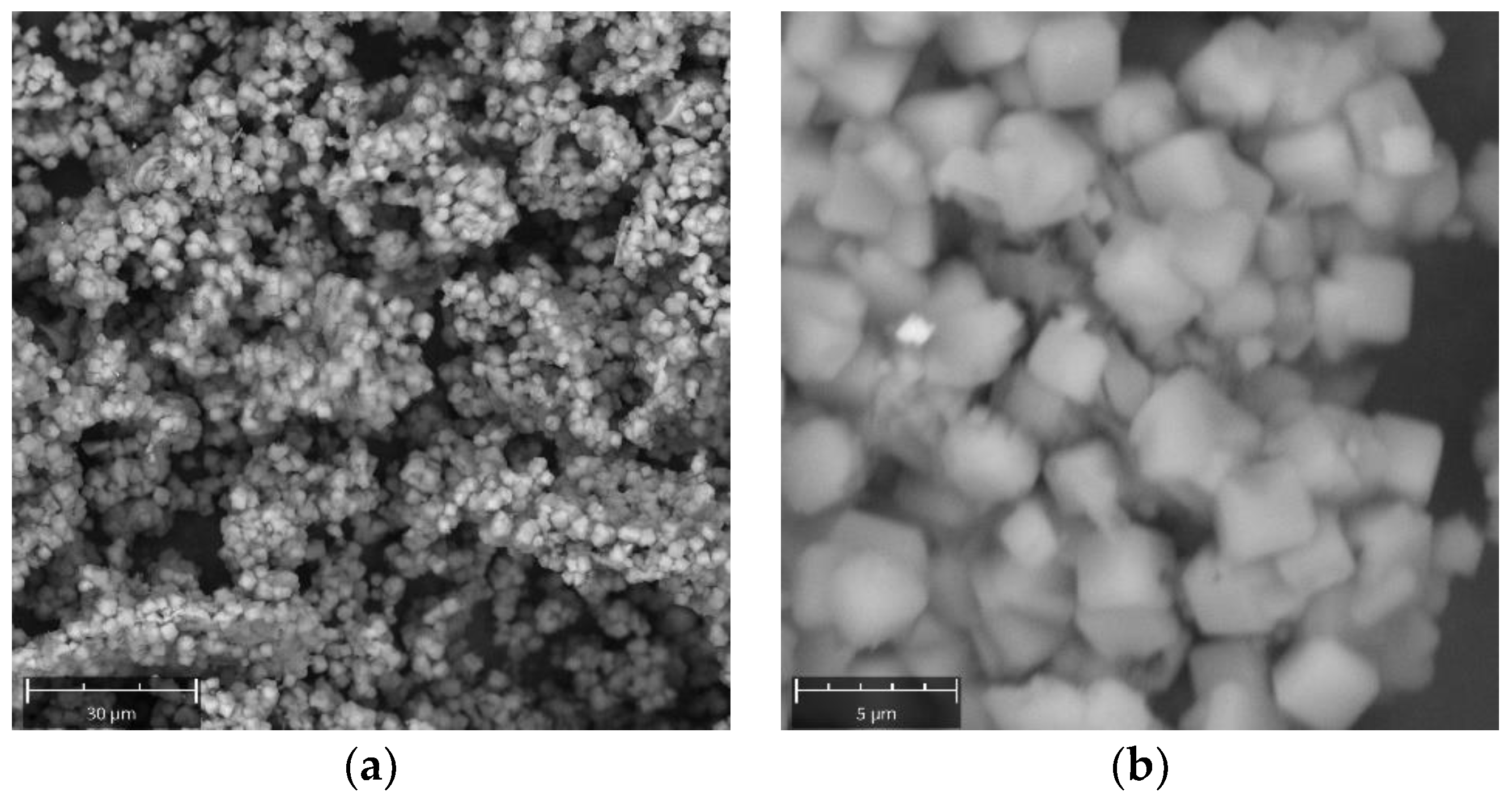
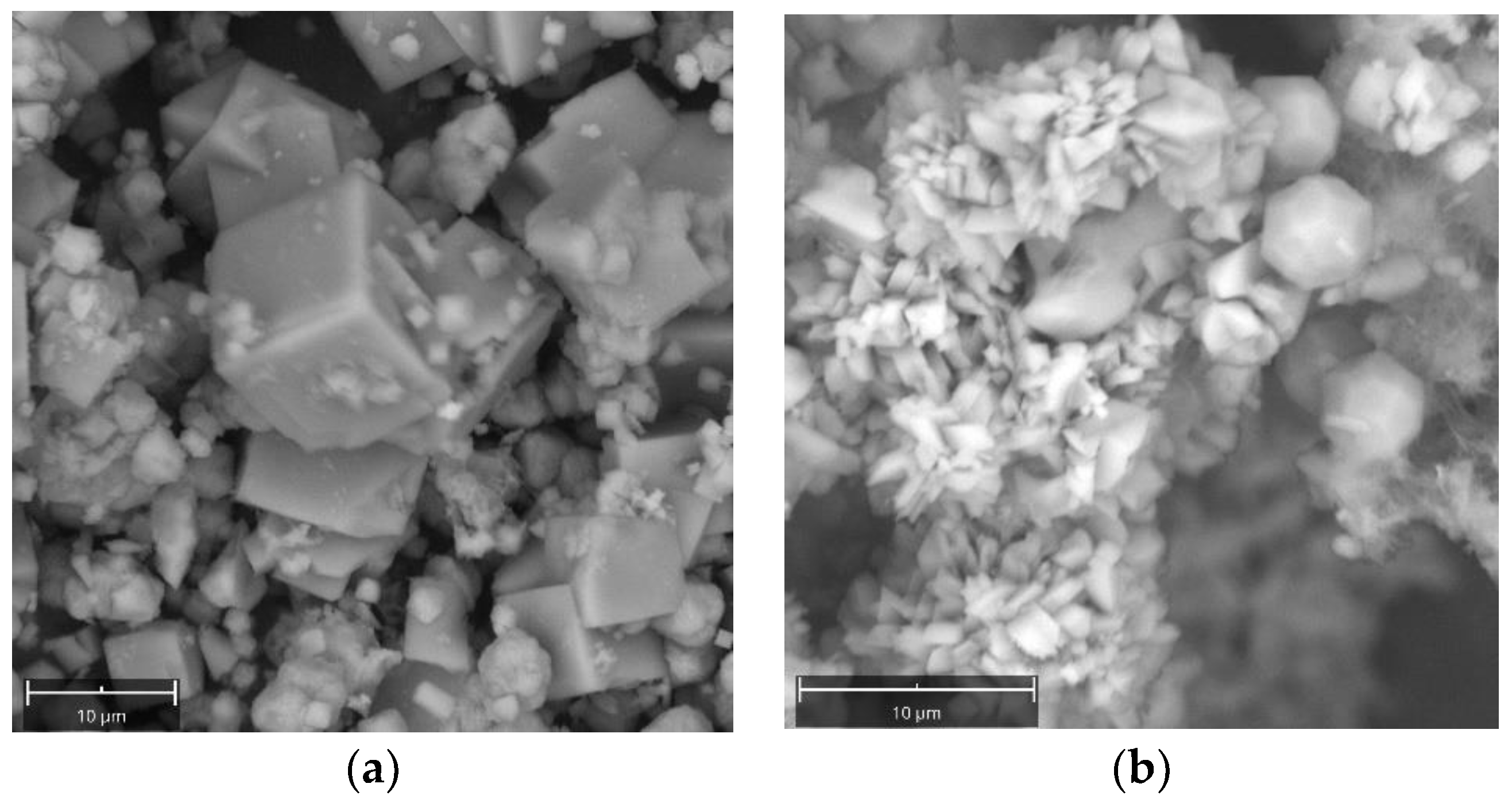
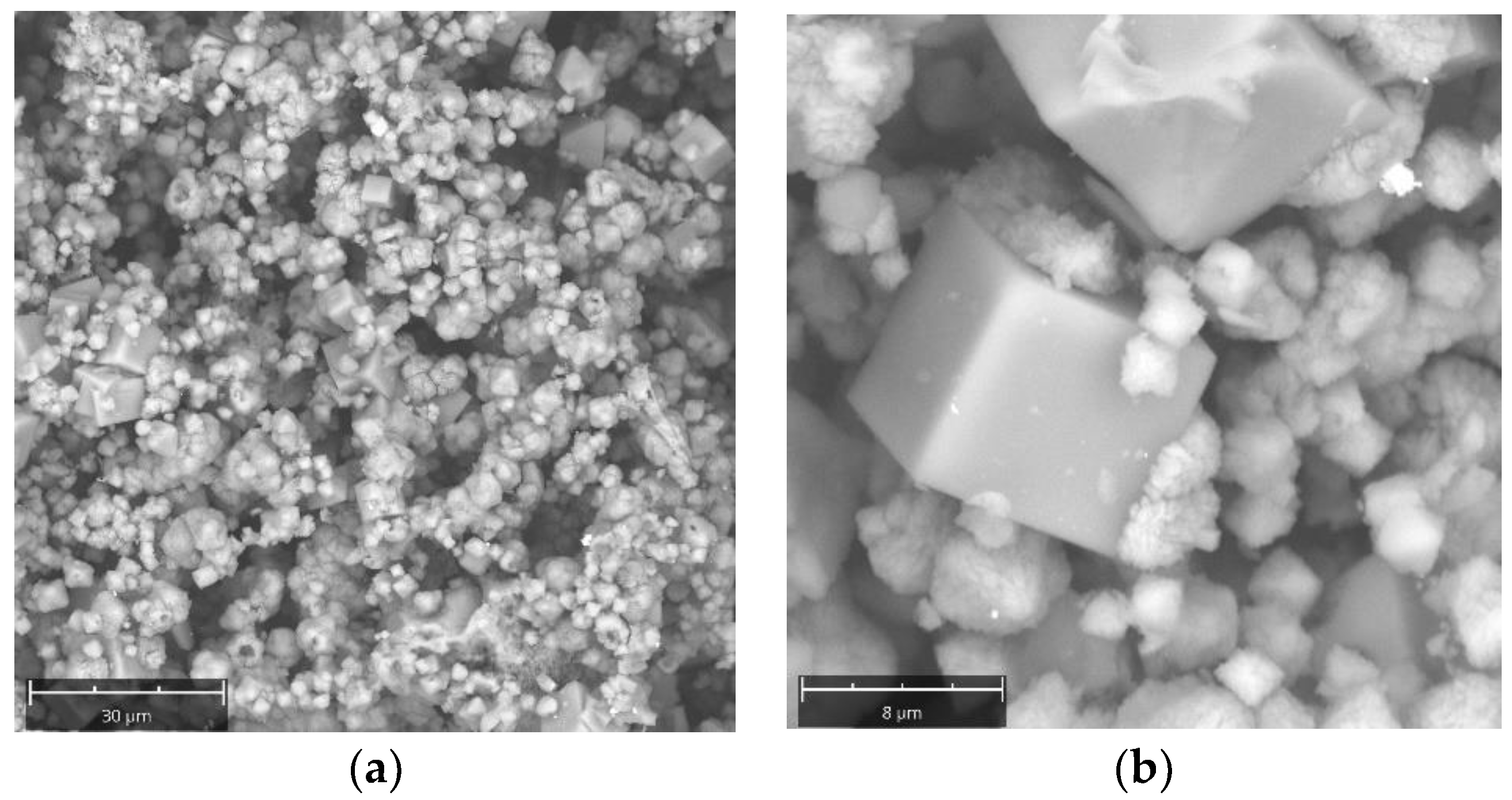
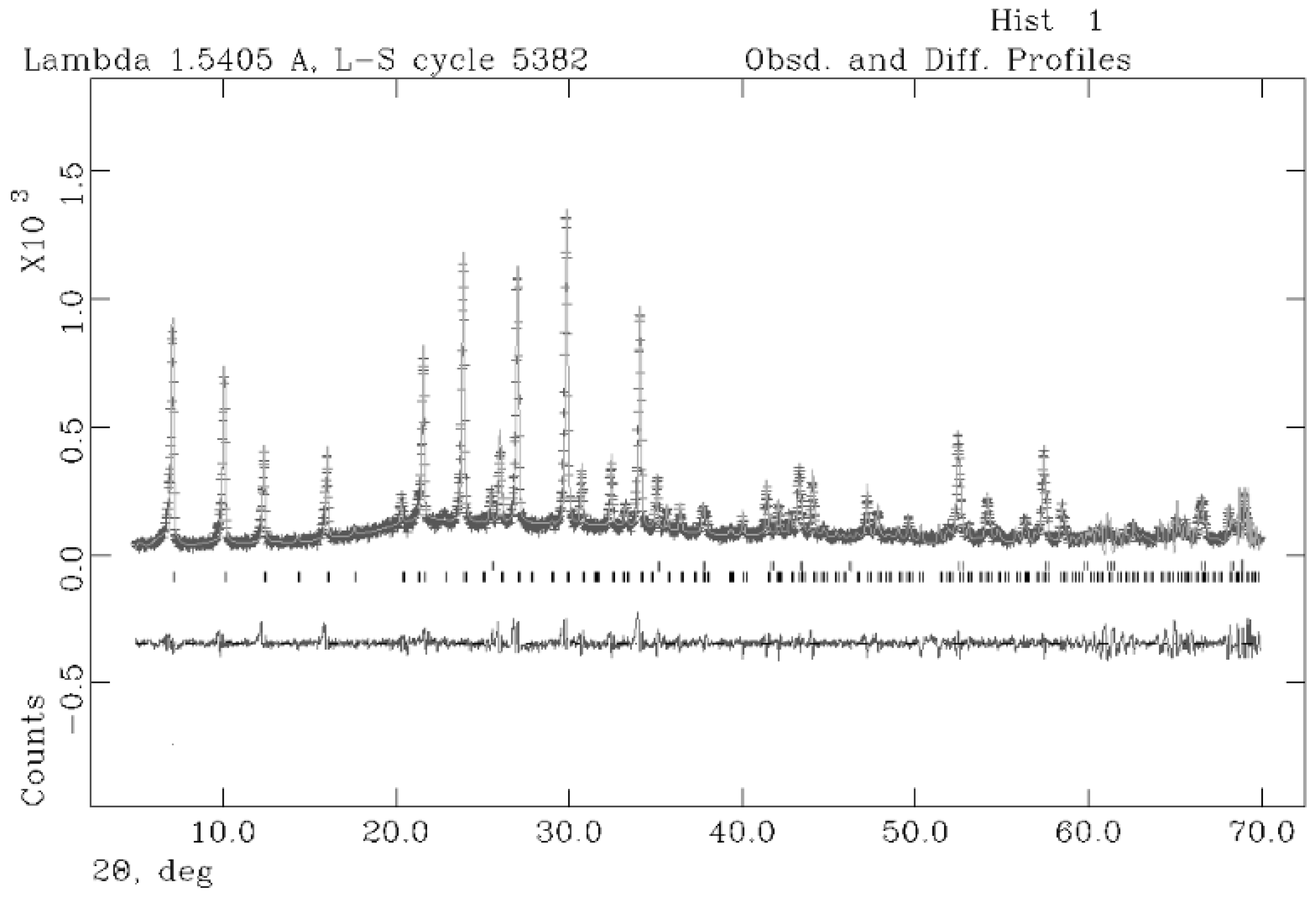
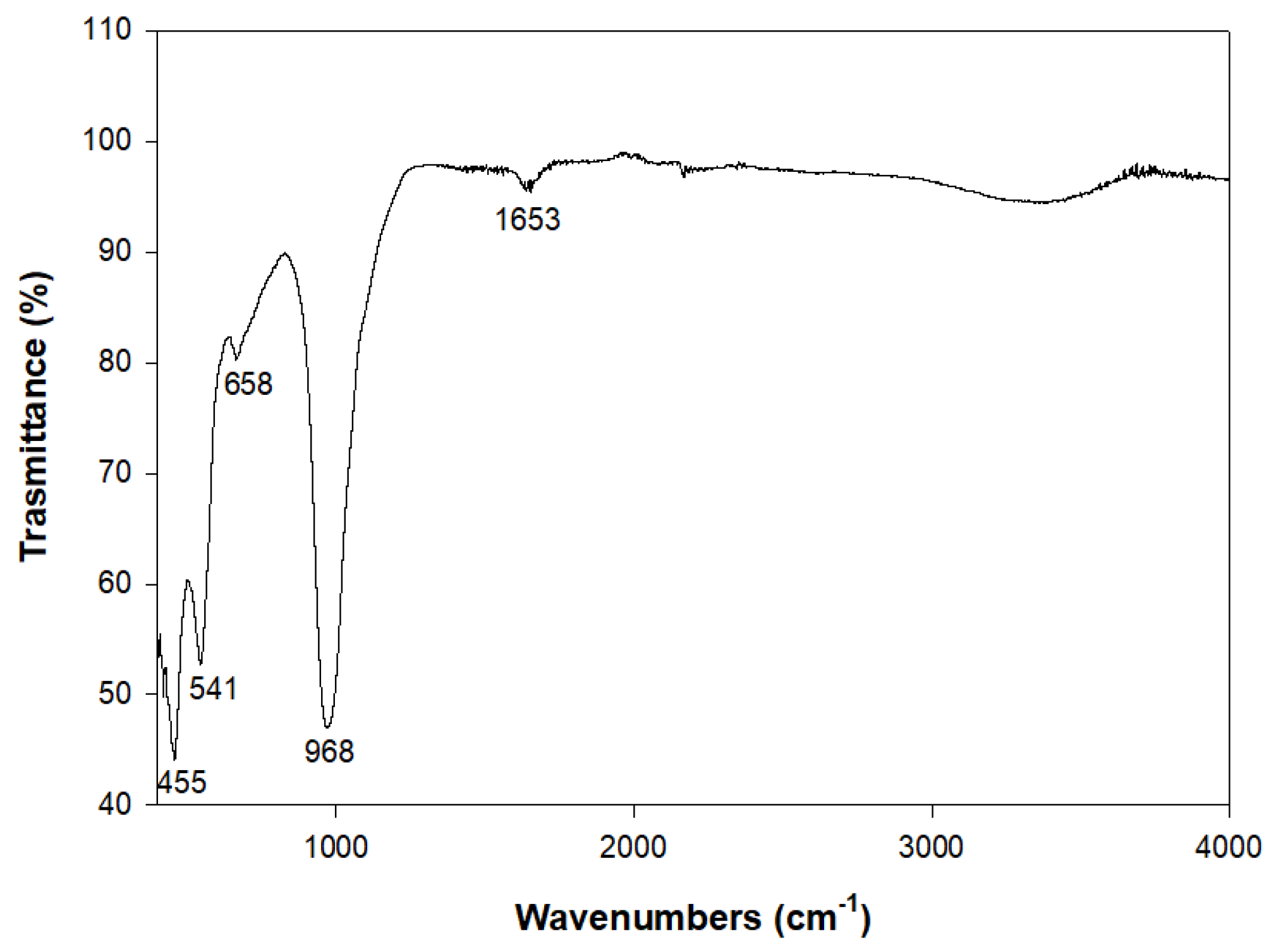
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, P.K. Rice Husk Ash–A Unique Supplementary Cementing Material. Adv. Concr. Technol. 1992, 2, 407–431. [Google Scholar]
- Maiti, S.; Dey, S.; Purakayastha, S.; Ghosh, B. Physical and thermochemical characterization of rice husk char as a potential biomass energy source. Bioresour. Technol. 2006, 97, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.J.; Yoon, K.P.; Young, S.K.; Ji, Y.L.; Bhagiyalakshmi, M. Highly siliceous MCM-48 from rice husk ash for CO2 adsorption. Int. J. Greenh. Gas Con. 2009, 3, 545–549. [Google Scholar]
- Bhavornthanayod, C.; Rungrojchaipon, P. Synthesis of zeolite A membrane from rice husk ash. J. Met. Mater. Miner. 2009, 19, 79–83. [Google Scholar]
- Mohamed, R.M.; Mkhalid, I.A.; Barakat, M.A. Rice husk ash as a renewable source for the production of zeolite NaY and its characterization. Arab. J. Chem. 2015, 8, 48–53. [Google Scholar] [CrossRef]
- Petkowicz, D.I.; Rigo, R.T.; Radtke, C.; Pergher, S.B.; dos Santos, J.H.Z. Zeolite NaA from Brazilian Chrysotile and rice husk. Micropor. Mesopor. Mat. 2008, 116, 548–554. [Google Scholar] [CrossRef]
- Arcos, C.A.; Macíaz Pintob, D.; and Rodríguez Páez, J.E. La cascarilla de arroz como fuente de SiO2 Husk of rice as source of SiO2. Rev. Fac. Ing. Univ. Antioquia. 2007, 41, 7–20. [Google Scholar]
- Barthey, B.R.; Gretella, M.C. Solar grade silicon. J. Mater. Sci. 1982, 17, 3077–3096. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Dalai, A.K.; Rao, M.S. Synthesis of NaX Zeolite Using Silica from Rice Husk Ash. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 465–468. [Google Scholar] [CrossRef]
- Gookhale, K.V.G.K.; Dalai, A.K.; Rao, M.S. Thermal characteristics of synthetic sodium zeolites prepared with silica from rice-husk ash. J. Therm. Anal. 1986, 31, 33–39. [Google Scholar] [CrossRef]
- Kongkachuichay, P.; Lohsoontorn, P. Phase diagram of zeolite synthesized from perlite and rice husk ash. ScienceAsia 2006, 32, 13–16. [Google Scholar] [CrossRef]
- Wittayakun, J.; Khemthong, P.; Prayoonkarach, S. Synthesis and characterization of zeolite NaY from rice husk silica. Korean J. Chem. Eng. 2008, 25, 861–864. [Google Scholar] [CrossRef]
- Khabuanchalead, S.; Khemthong, P.; Prayoonpokarack, S.; Wittayakun, J. Transformation of zeolite NaY synthesized from rice husk silica to NaP during hydrothermal synthesis. J. Sci. Technol. 2008, 15, 225–231. [Google Scholar]
- Rahman, M.M.; Hasnidab, N.; Wan Nik, W.B. Preparation of Zeolite Y Using Local Raw Material Rice Husk as a Silica Source. J. Sci. Res. 2009, 1, 285–291. [Google Scholar] [CrossRef]
- Klunk, M.A.; Das, M.; Dasgupta, S.; Impiombato, A.N.; Caetano, N.R.; Wander, P.R.; Mendes Moraes, C.A. Comparative study using different external sources of aluminum on the zeolites synthesis from rice husk ash. Mater. Res. Express 2020, 7, 015023. [Google Scholar] [CrossRef]
- Solihat, I.; Sulistiawaty, L.; Syaifie, P.H.; Taufiq, A. Removal of Cu Metals from Wastewater by Adsorption using Synthetic Zeolites from Rice Husk and Corncob. Molekul 2020, 15, 105–113. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Alsohaimi, I.H.; Dahan, T.E.; Chen, Q.; Younes, A.A.; El-Gammal, B.; Melhi, S. Photocatalytic Degradation of Methylene Blue and Antibacterial Activity of Mesoporous TiO2-SBA-15 Nanocomposite Based on Rice Husk. Adsorpt. Sci. Technol. 2021, 2021, 9290644. [Google Scholar] [CrossRef]
- Ji, F.; Zhang, C.; Mi, Y. Study on the effect of inorganic aluminum sources on ZSM-5 zeolite synthesis by solvent-free method from rice husk ash. Chem. Pap. 2024, 78, 1217–1226. [Google Scholar] [CrossRef]
- Sherry, H.S. Ion-exchange properties of the natural zeolite erionite. Clay. Clay Miner. 1979, 27, 231–237. [Google Scholar] [CrossRef]
- Novembre, D.; Di Sabatino, B.; Gimeno, D.; Garcia Valles, M.; Martinez-Manent, S. Synthesis of Na-X zeolites from tripolaceous deposits (Crotone, Italy) and volcanic zeolitized rocks (Vico Volcano, Italy). Micropor. Mesopor. Mat. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- The Database of Zeolite Structures 1996–2016, The IZA-SC. 2016. Available online: https://www.iza-structure.org/databases/ (accessed on 1 July 2024).
- Collins, F.; Rozhkovskayaa, A.; Outramb, J.G.; Millar, J. A critical review of waste resources, synthesis, and applications for Zeolite LTA. Microp. Mesop. Mat. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Duchemin, J.; Magoarou, P.; Premazzi, G. Criteria for the identification of freshwaters subject to eutrophication. Their use for the implementation of the ‘Nitrates’ and Urban Waste Treatment directives. Eur. Comm. Environ. Qual. Life Ser. 2001, 19810, 90. [Google Scholar]
- Ghoufi, A.; Gaberova, L.; Rouquerol, J.; Vincent, D.; Llewellyn, P.L.; Maurin, G. Adsorption of CO2, CH4 and their binary mixture in Faujasite NaY: A combination of molecular simulations with gravimetry–manometry and microcalorimetry measurements. Microporous Mesoporous Mater. 2009, 119, 117–128. [Google Scholar] [CrossRef]
- Montanari, T.; Busca, G. On the mechanism of adsorption and separation of CO2 on LTA zeolites: An IR investigation. Vib. Spectrosc. 2008, 46, 45–51. [Google Scholar] [CrossRef]
- Sharma, V.K.; Gautam, S.; Mitra, S.; Mukhopadhyay, R. Dynamics of propylene adsorbed in Na–Y and Na–ZSM5 zeolites: A QENS and MD simulation study. Z. Phys. Chem. 2010, 224, 133–152. [Google Scholar] [CrossRef]
- Bernier-Oviedo, D.J.; Rincón-Moreno, J.A.; Solanilla-Duqué, J.F.; MuñozHernández, J.A.; Váquiro-Herrera, H.A. Comparison of two pretreatments methods to produce second-generation bioethanol resulting from sugarcane bagasse. Ind. Crops Prod. 2018, 122, 414–421. [Google Scholar] [CrossRef]
- Cowan, M.M.; Abshire, K.Z.; Houk, S.L.; Evans, S.M. Antimicrobial efficacy of a silver-zeolite matrix coating on stainless steel, J. Ind. Microbiol. Biotechnol. 2003, 30, 102–106. [Google Scholar] [CrossRef]
- Breck, D. Zeolite Molecular Sieves. John Wiley & Sons: New York, NY, USA, 1984; 771p. [Google Scholar]
- Coombs, D.S.; Ellis, A.J.; Fyfe, W.S.; Taylor, A.M. The zeolite facies, with comments on the interpretation of hydrothermal syntheses. Geochim. Cosmochim. Acta 1959, 17, 53–107. [Google Scholar] [CrossRef]
- Novembre, D.; Di Sabatino, B.; Gimeno, D.; Pace, C. Synthesis and characterization of Na-X, Na-A and Na-P zeolites and hydroxysodalite from metakaolinite. Clay Miner. 2011, 46, 336–354. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Del Vecchio, A. Improvement in the synthesis conditions and studying the physicochemical properties of the zeolite Li-A(BW) obtained from a kaolinitic rock. Sci. Rep. 2020, 10, 5715–5723. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D. Synthesis and characterization of analcime (ANA) zeolite using a kaolinitic rock. Sci. Rep. 2021, 11, 13373–13382. [Google Scholar] [CrossRef] [PubMed]
- Novembre, D.; Gimeno, D.; Del Vecchio, A. Synthesis and characterization of Na-P1 (GIS) zeolite using a kaolinitic rock. Sci. Rep. 2021, 11, 4872–4883. [Google Scholar] [CrossRef] [PubMed]
- De Lucas, A.; Uguina, M.A.; Covian, I.; Rodriguez, L. Synthesis of 13X zeolite from calcined kaolin 352 D. Novembre et al. and sodium silicates for use in detergents. Ind. Eng. Chem. Res. 1992, 31, 2134–2140. [Google Scholar] [CrossRef]
- Covian, I. Sintesis de Zeolite 13X para su Uso en Detergentes. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1991. [Google Scholar]
- Abdmeziem-Hamoudi, K.; Siffert, B. Synthesis of molecular sieve zeolites from a smectite- type clay material. App. Clay Sci. 1989, 4, 1–9. [Google Scholar] [CrossRef]
- Lee, M.J.; Cho, J.; Huh, H.; Choi, B. Studies on synthesis of X-type zeolite from the natural mordenite. J. Korean Ceram. Soc. 1994, 31, 1570–1576. [Google Scholar]
- Gualtieri, A.F. Synthesis of sodium zeolites from a natural halloysite. Phys. Chem. Miner. 2001, 28, 719–728. [Google Scholar] [CrossRef]
- Novembre, D.; Di Sabatino, B.; Gimeno, D. Synthesis of Na-A zeolite from 10 Å halloysite and a new crystallization kinetic model for the transformation of Na-A into HS zeolite. Clay. Clay Miner. 2005, 53, 28–36. [Google Scholar] [CrossRef]
- Boukadir, D.; Bettahar, N.; Derriche, Z. Etude de la synthese des zeolites 4A et HS a partir de produits naturels. Ann. Chim. Sci. Mat. 2002, 27, 1–13. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakai, Y.; Hino, R. Formation of Na-A and Na-X zeolites from waste solutions in conversion of coal fly ash to zeolites. Mater. Res. Bull. 2002, 37, 1873–1884. [Google Scholar] [CrossRef]
- Fotovat, F.; Kazemian, H.; Kazemeini, M. Synthesis of Na-A and faujasitic zeolites from high silicon fly ash. Mater. Res. Bull. 2009, 44, 913–917. [Google Scholar] [CrossRef]
- Maia, A.A.B.; Angélica, R.S.; Neves, R.F. Use of industrial kaolin waste from the Brazilian Amazon region for synthesis of zeolite A. Clay Miner. 2011, 46, 127–136. [Google Scholar] [CrossRef]
- Nur, H. Direct synthesis of NaA zeolite from rice husk and carbonaceous rice husk ash. Indones. J. Agric. Sci. 2001, 1, 40–45. [Google Scholar]
- Yusof, A.M.; Malek, N.A.N.N.; Rashid, N.A.A. Hydrothermal conversion of rice husk ash to faujasite-types and NaA-type of zeolites. J. Porous. Mater. 2010, 17, 39–47. [Google Scholar] [CrossRef]
- Azizi, S.N.; Maryam, Y. Synthesis of Zeolites NaA and Analcime Using Rice Husk Ash as Silica Source without Using Organic Template. J. Mat. Sci. 2010, 45, 5692–5697. [Google Scholar] [CrossRef]
- Tan, W.C.; Yap, S.Y.; Matsumoto, A.; Othman, R.; Yeoh, F.Y. Synthesis and characterization of zeolites NaA and NaY from rice husk ash. Adsorption 2011, 17, 863–868. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H. Preparation and characterization of nanozeolite NaA from rice husk at room temperature without organic additives. J. Nanomater. 2011, 2011, 858961. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H. Preparation of Free-Template Nanometer-Sized Na–A and –X Zeolites From Rice Husk Ash. Waste Biomass Valor. 2012, 3, 61–74. [Google Scholar] [CrossRef]
- Tepamat, T.; Mongkolkachit, C.; Wasanapiarnpong, T. Synthesis of zeolite NaA and activated carbon composite from rice husk. Suranaree J. Sci. Technol. 2014, 21, 119–123. [Google Scholar]
- Ahmedzeki, N.S.; Abbas, M.N.; Joodee, A.M.; Jaed, Y.M. Waste resources utilization for zeolite a synthesis. J. Chem. Technol. Metall. 2018, 53, 239–244. [Google Scholar]
- Wang, Y.; Du, T.; Jia, H.; Qiu, Z.; Song, Y. Synthesis, characterization and CO2 adsorption of NaA, NaX and NaZSM-5 from rice husk ash. Solid State Sci. 2018, 86, 24–33. [Google Scholar] [CrossRef]
- Madhu, J.; Santhanam, A.; Natarajan, M.; Velauthapillai, D. CO2 adsorption performance of template free zeolite A and X synthesized from rice husk ash as silicon source. RSC Adv. 2022, 12, 23221. [Google Scholar] [CrossRef] [PubMed]
- Treacy, M.M.J.; Higgins, J.B. Linde type A, hydrated. In Collection of Simulated XRD Power Patterns for Zeolites, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 252–253. [Google Scholar]
- Gramlich, V.; Meier, W.M. The crystal structure of hydrated NaA: A detailed refinement of a pseudosymmetric zeolite structure. Z. Kristallogr. 1971, 133, 134–149. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Khatami, H.; Szymanski, H.A. Infrared Structural Studies of Zeolite Frameworks. In Molecular Sieve Zeolites, Advances in Chemistry 101; Flanigen, E.M., Sand, L.B., Eds.; American Chemical Society: Washington DC, USA, 1971; pp. 201–229. [Google Scholar]
- No, K.T.; Bae, D.H.; Jhon, M.S. Lattice vibrational calculation of A-type zeolite using the pseudolattice method. J. Phys. Chem. 1986, 90, 1772. [Google Scholar] [CrossRef]
- Dutta, P.K.; Shieh, D.C.; Puri, M. Raman Spectroscopic Study of the Synthesis of Zeolite YJ. J. Phys. Chem. 1987, 91, 2332–2336. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, G.; Li, C.; Xiao, F.S. Characterization of aluminosilicate zeolites by UV Raman spectroscopy. Microp. Mesopor. Mat. 2008, 46, 23–34. [Google Scholar] [CrossRef]
- Gimeno, D.; Puges, M. Caracterización química de la vidriera histórica de Sant Pere i Sant Jaume (Monestir de Pedralbes, Barcelona). Bol. Soc. Esp. Ceram. Vidr. 2002, 41, 13–20. [Google Scholar] [CrossRef]
- Aulinas, M.; Gimeno, D.; Fernandez-Turiel, J.L.; Perez-Torrado, F.J.; Rodriguez-Gonzalez, A.; Gasperini, D. The Plio-Quaternary magmatic feeding system beneath Gran Canaria (Canary Islands, Spain): Constraints from thermobarometric studies. J. Geol. Soc. 2010, 167, 785–801. [Google Scholar] [CrossRef]
- Aulinas, M.; Civetta, L.; Di Vito, M.; Orsi, G.; Gimeno, D.; Fernandez Turiel, J.L. The Plinian Mercato eruption of Somma Vesuvius: Magma chamber processes and eruption dynamics. Bull. Volcanol. 2008, 70, 825–840. [Google Scholar] [CrossRef]
- Gisbert, G.; Gimeno, D. Ignimbrite correlation using whole-rock geochemistry: An example from the Sulcis (SW Sardinia, Italy). Geol. Mag. 2017, 154, 740–756. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; d’Alessandro, N.; Tonucci, L. Hydrothermal synthesis and characterization of kalsilite by using a kaolinitic rock from Sardinia, Italy, and its application in the production of biodiesel. Mineral. Mag. 2018, 82, 961–973. [Google Scholar] [CrossRef]
- Novembre, D.; Pace, C.; Gimeno, D. Synthesis and characterization of wollastonite-2M by using a diatomite precursor. Miner. Mag. 2018, 82, 95–110. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. GSAS: General Structure Analysis System. Document Laur 86-748. Los Alamos Natl. Lab 1997, 121–124. [Google Scholar]
- Toby, B.H. EXPGUI, a Graphical User Interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D. The solid-state conversion of kaolin to KAlSiO4 minerals: The effects of time and temperature. Clays Clay Miner. 2017, 65, 355–366. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Poe, B. Synthesis and Characterization of Leucite Using a Diatomite Precursor. Sci. Rep. 2019, 9, 10051–10061. [Google Scholar] [CrossRef]
- Novembre, D.; Pace, C.; Gimeno, D. Syntheses and characterization of zeolites K-F and W type using a diatomite precursor. Mineral. Mag. 2014, 78, 1209–1225. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Cappelletti, P.; Graziano, S.F. A case study of zeolitization process: “tufo Rosso a Scorie Nere” (Vico Volcano, Italy): Inferences for a general model. Eur. J. Mineral. 2021, 33, 315–328. [Google Scholar] [CrossRef]
- Ciulla, M.; Canale, V.; Wolicki, R.D.; Pilato, S.; Bruni, P.; Ferrari, S.; Siani, G.; Fontana, A.; Di Profio, P. Enhanced CO2 Capture by Sorption on Electrospun Poly (Methyl Methacrylate). Separations 2023, 10, 505–521. [Google Scholar] [CrossRef]




| SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | MnO | TiO2 | MgO | P2O5 |
|---|---|---|---|---|---|---|---|---|
| 98.50 | 0.26 | 0.18 | 0.13 | 0.20 | 0.14 | 0.02 | 0.14 | 0.11 |
| Sample + 10% Corundum Nist 676a | 60 °C–8 h |
|---|---|
| Rwp | 0.18 |
| Rp | 0.15 |
| CHI2 | 2.41 |
| space group NaA | Fm-3c |
| a (Å) | 24.5932 (0.0035) |
| % amorphous | 8.6 (14) |
| NaA | 91.4 (18) |
| SiO2/Al2O3 Ratio | Na2O/SiO2 Ratio | H2O/Na2O Ratio | T (°C) | |
|---|---|---|---|---|
| 1 | 1.75 | 3.1 | 63.9 | 40 |
| 2 | 1.75 | 3.1 | 63.9 | 60 |
| 3 | 1.75 | 3.1 | 63.9 | 85 |
| 4 | 3.50 | 2.19 | 69.7 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novembre, D.; Gimeno, D.; Marinangeli, L.; Tangari, A.C.; Rosatelli, G.; Ciulla, M.; di Profio, P. Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite. Molecules 2024, 29, 4396. https://doi.org/10.3390/molecules29184396
Novembre D, Gimeno D, Marinangeli L, Tangari AC, Rosatelli G, Ciulla M, di Profio P. Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite. Molecules. 2024; 29(18):4396. https://doi.org/10.3390/molecules29184396
Chicago/Turabian StyleNovembre, Daniela, Domingo Gimeno, Lucia Marinangeli, Anna Chiara Tangari, Gianluigi Rosatelli, Michele Ciulla, and Pietro di Profio. 2024. "Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite" Molecules 29, no. 18: 4396. https://doi.org/10.3390/molecules29184396
APA StyleNovembre, D., Gimeno, D., Marinangeli, L., Tangari, A. C., Rosatelli, G., Ciulla, M., & di Profio, P. (2024). Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite. Molecules, 29(18), 4396. https://doi.org/10.3390/molecules29184396







