Density Functional Theory Prediction of Laser Dyes–Cucurbit[7]uril Binding Affinities
Abstract
1. Introduction
2. Results
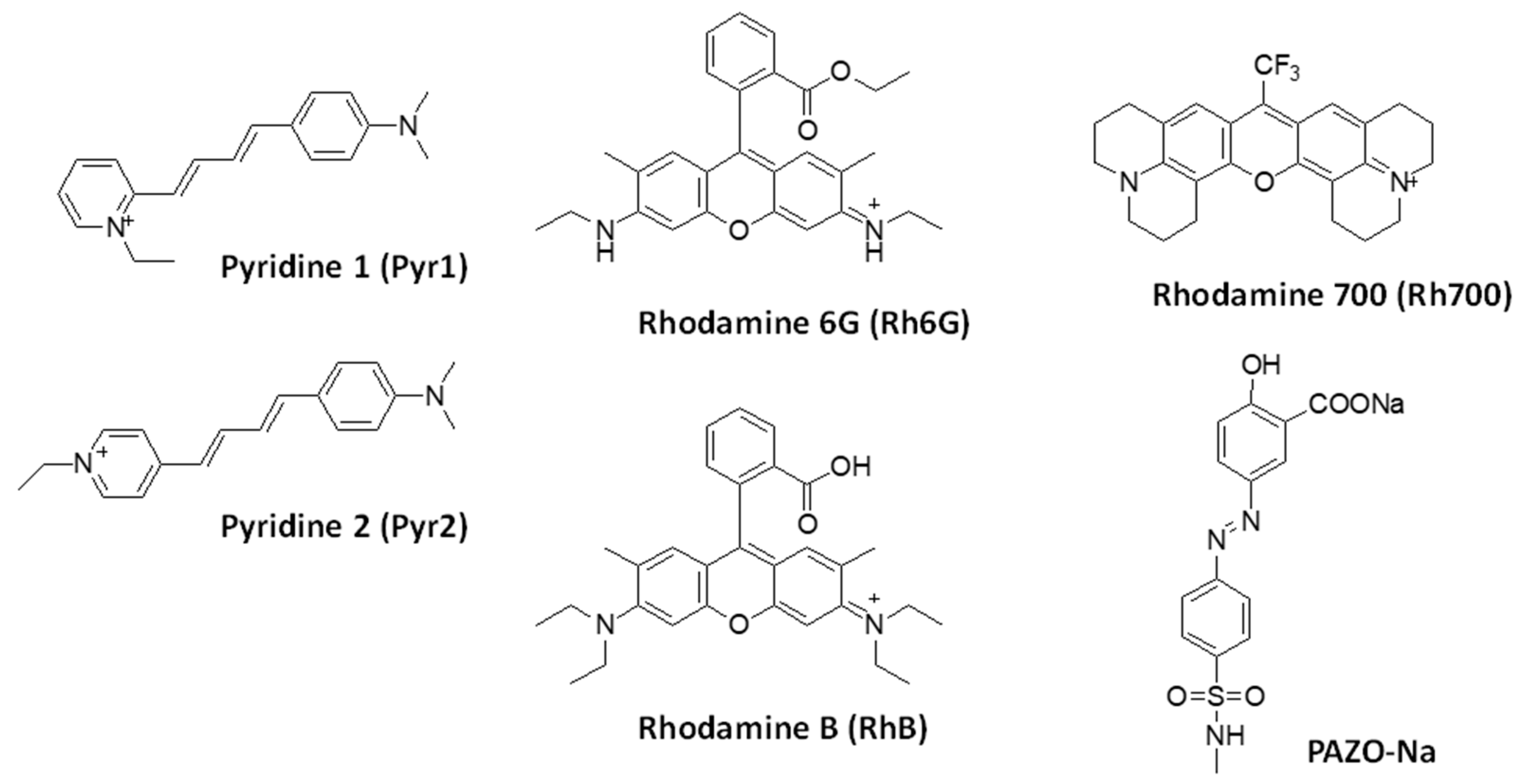
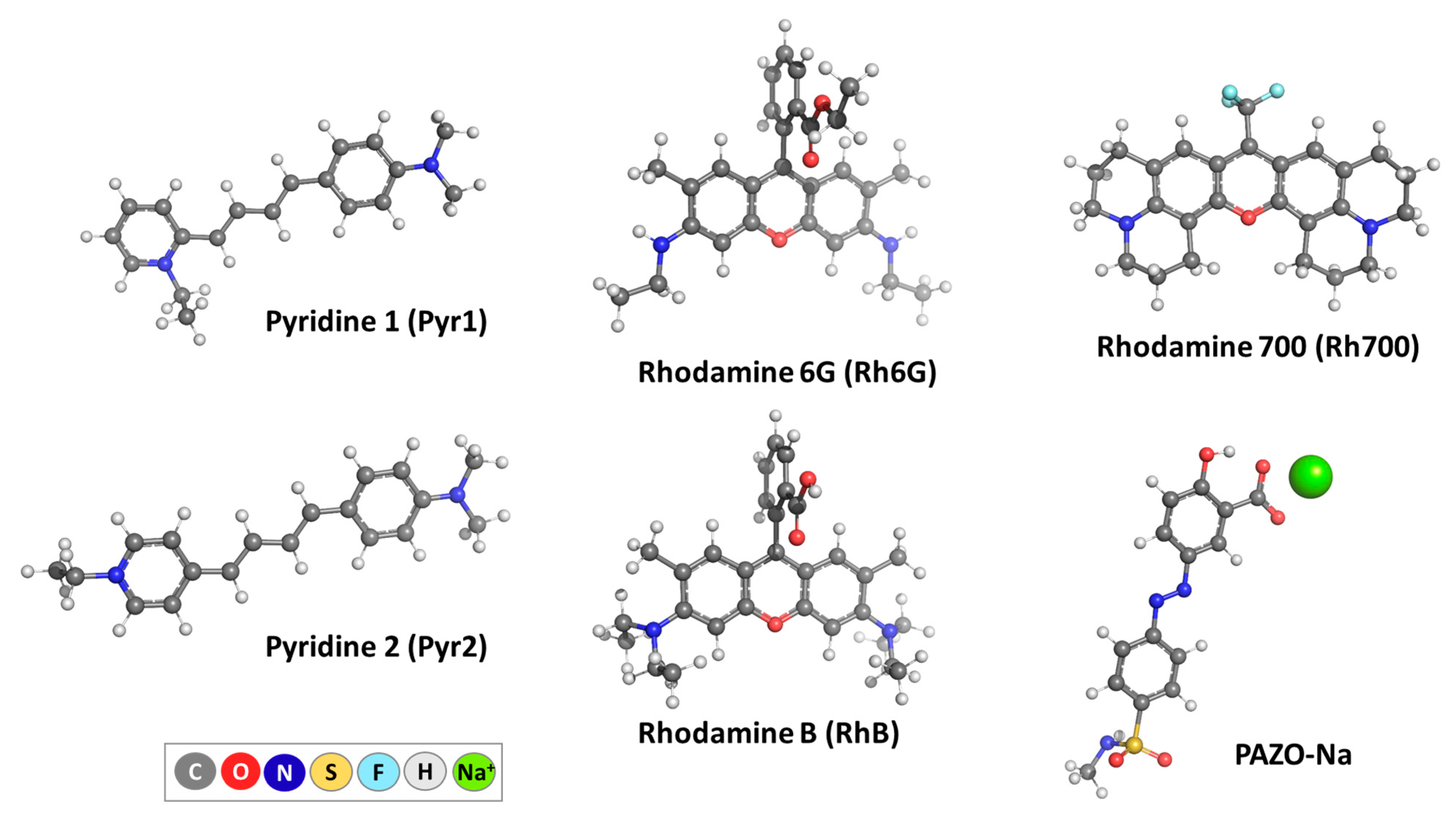
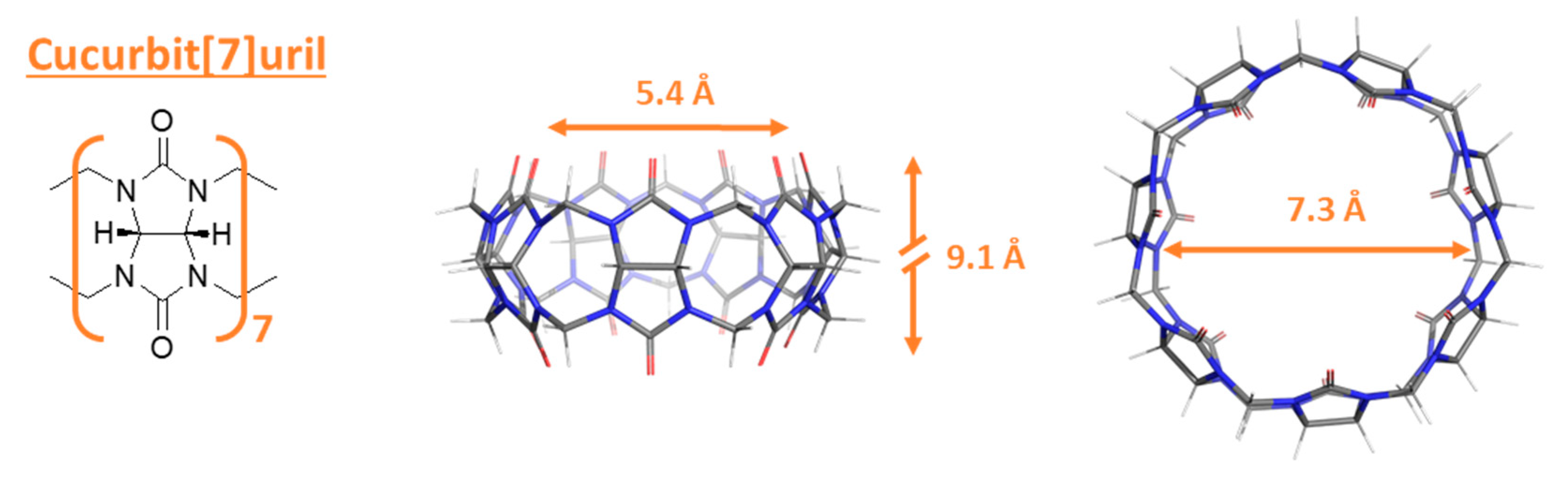
2.1. Guest and Host Molecules
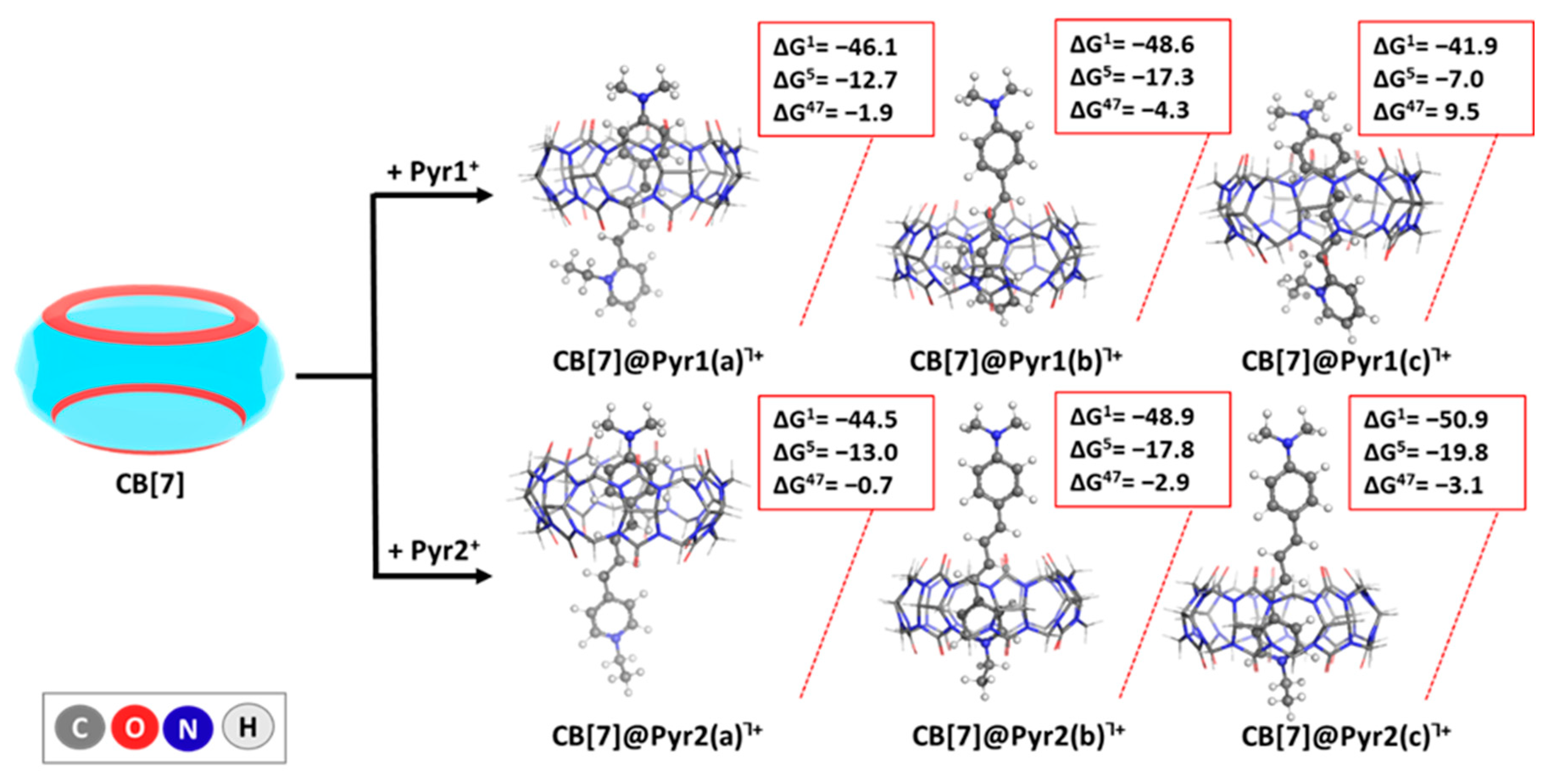
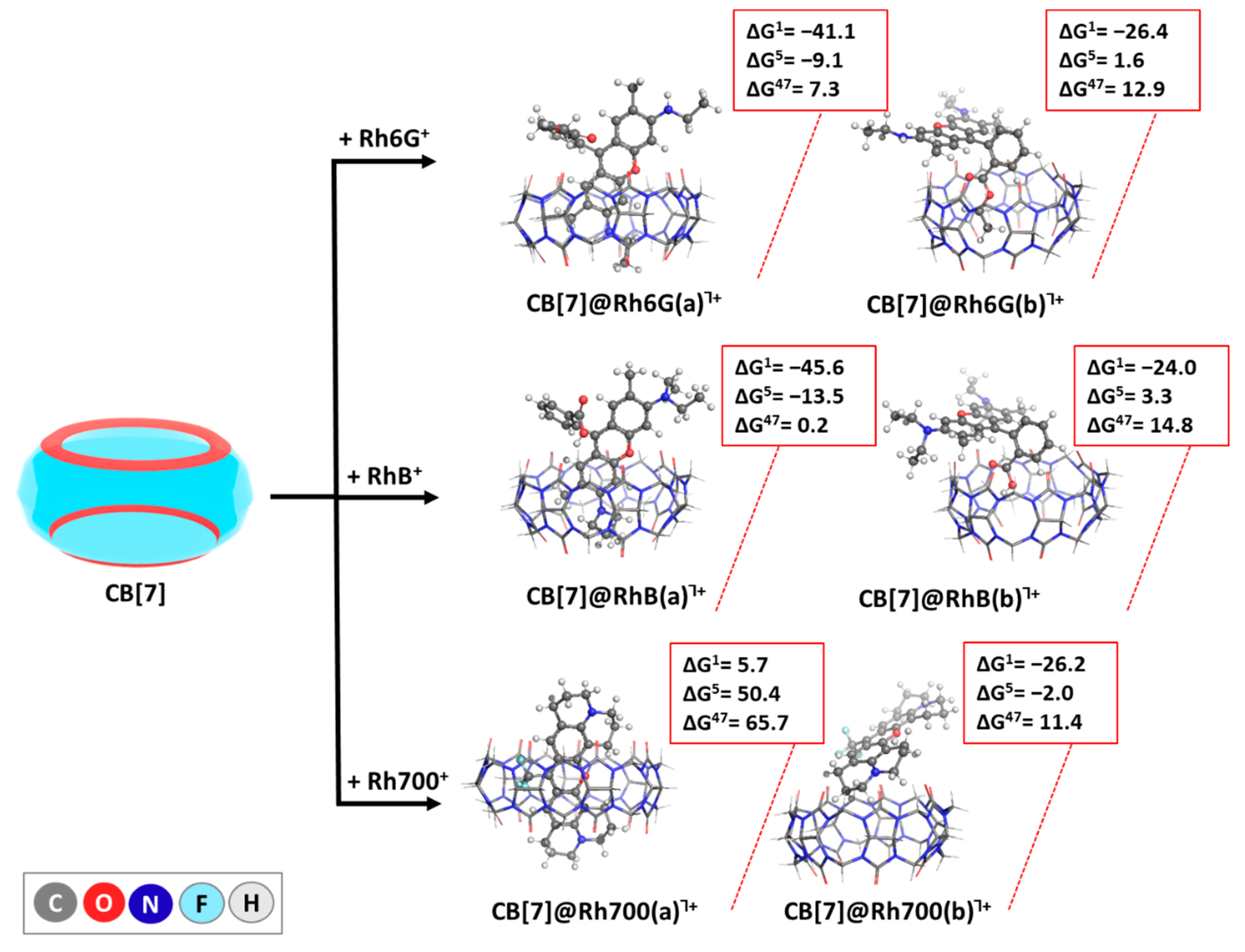
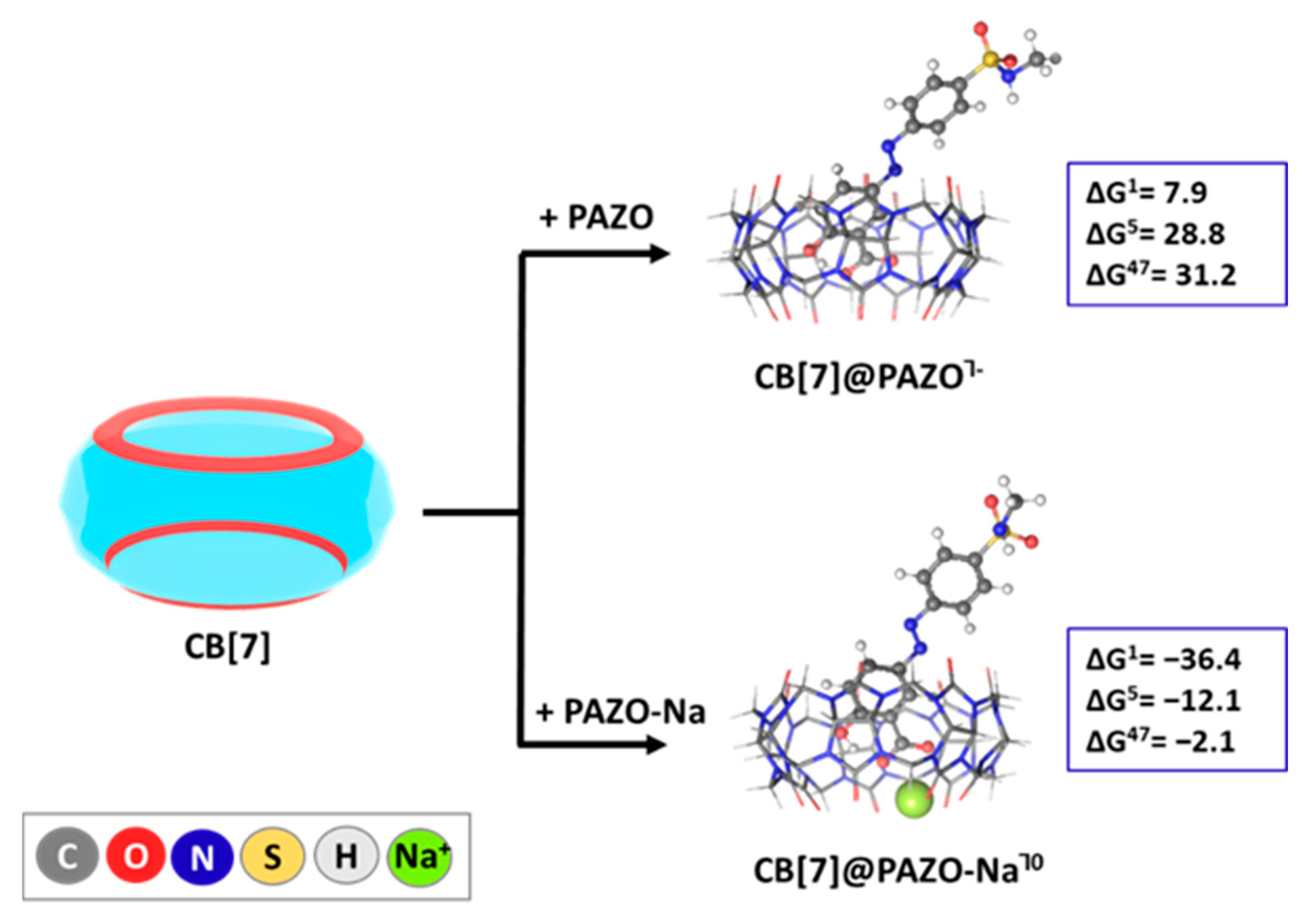
2.2. Reactions Modeled
| CB[7] + dye+ → CB[7]@dye┐+ | Reaction (1) |
| CB[7] + PAZO−/PAZO-Na0 → CB[7]@PAZO/PAZO-Na┐−1/0 | Reaction (2) |
2.3. Analysis of Thermodynamic Data for the Guests (Laser Dyes and PAZO) Encapsulation in CB[7]
2.4. Influence of Diverse Factors on the Host–Guest Recognition
2.5. Implications for Photophysical Properties Modulation
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; Wiley: Chichester, UK, 2009; ISBN 9780470512333. [Google Scholar]
- Davis, A.V.; Yeh, R.M.; Raymond, K.N. Supramolecular Assembly Dynamics. Proc. Natl. Acad. Sci. USA 2002, 99, 4793–4796. [Google Scholar] [CrossRef] [PubMed]
- Wenz, G. An Overview of Host-Guest Chemistry and Its Application to Nonsteroidal Anti-Inflammatory Drugs. Clin. Drug Investig. 2000, 19, 21–25. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives–A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Bukhzam, A.; Bader, N. Crown Ethers: Their Complexes and Analytical Applications. J. Appl. Chem. 2014, 3, 237–244. [Google Scholar]
- Kralj, M.; Tušek-Božić, L.; Frkanec, L. Biomedical Potentials of Crown Ethers: Prospective Antitumor Agents. ChemMedChem 2008, 3, 1478–1492. [Google Scholar] [CrossRef]
- Homden, D.M.; Redshaw, C. The Use of Calixarenes in Metal-Based Catalysis. Chem. Rev. 2008, 108, 5086–5130. [Google Scholar] [CrossRef]
- Vicens, J.; Böhmer, V. Calixarenes: A Versatile Class of Macrocyclic Compounds; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Mejuto, J.C.; Simal-Gandara, J. Host–Guest Complexes. Int. J. Mol. Sci. 2022, 23, 15730. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; Del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The Cucurbit[n]Uril Family. Angew. Chem.-Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Ling, X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X. Cucurbituril Chemistry: A Tale of Supramolecular Success. RSC Adv. 2012, 2, 1213–1247. [Google Scholar] [CrossRef]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.J.; Kim, K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc. Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef]
- Kim, K. (Ed.) Cucurbiturils and Related Macrocycles; The Royal Society of Chemistry: London, UK, 2019; ISBN 978-1-78801-500-4. [Google Scholar]
- El-Sheshtawy, H.S.; Chatterjee, S.; Assaf, K.I.; Shinde, M.N.; Nau, W.M.; Mohanty, J. A Supramolecular Approach for Enhanced Antibacterial Activity and Extended Shelf-Life of Fluoroquinolone Drugs with Cucurbit[7]Uril. Sci. Rep. 2018, 8, 13925. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Lin, J.X.; Cao, M.N.; Cao, R. Cucurbituril: A Promising Organic Building Block for the Design of Coordination Compounds and Beyond. Coord. Chem. Rev. 2013, 257, 1334–1356. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: From Synthesis to High-Affinity Binding and Catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [PubMed]
- Nau, W.M. Supramolecular Capsules: Under Control. Nat. Chem. 2010, 2, 248–250. [Google Scholar] [CrossRef]
- Kircheva, N.; Petkova, V.; Dobrev, S.; Nikolova, V.; Angelova, S.; Dudev, T. N-Methyl- and N-Phenylpiperazine Functionalized Styryl Dyes Inside Cucurbiturils: Theoretical Assessment of the Factors Governing the Host–Guest Recognition. Molecules 2023, 28, 8130. [Google Scholar] [CrossRef]
- Nau, W.M.; Florea, M.; Assaf, K.I. Deep inside Cucurbiturils: Physical Properties and Volumes of Their Inner Cavity Determine the Hydrophobic Driving Force for Host-Guest Complexation. Isr. J. Chem. 2011, 51, 559–577. [Google Scholar] [CrossRef]
- Jeon, W.S.; Moon, K.; Park, S.H.; Chun, H.; Ko, Y.H.; Lee, J.Y.; Lee, E.S.; Samal, S.; Selvapalam, N.; Rekharsky, M.V.; et al. Complexation of Ferrocene Derivatives by the Cucurbit[7]Uril Host: A Comparative Study of the Cucurbituril and Cyclodextrin Host Families. J. Am. Chem. Soc. 2005, 127, 12984–12989. [Google Scholar] [CrossRef]
- Shaikh, M.; Mohanty, J.; Singh, P.K.; Nau, W.M.; Pal, H. Complexation of Acridine Orange by Cucurbit[7]Uril and β-Cyclodextrin: Photophysical Effects and PKa Shifts. Photochem. Photobiol. Sci. 2008, 7, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Pal, H.; Koti, A.S.R.; Sapre, A.V. Photophysical Properties and Rotational Relaxation Dynamics of Neutral Red Bound to β-Cyclodextrin. J. Phys. Chem. A 2004, 108, 1465–1474. [Google Scholar] [CrossRef]
- Mohanty, J. Cucurbiturils, Synthesis and Applications. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–31. ISBN 9780471440260. [Google Scholar]
- Yin, H.; Cheng, Q.; Bardelang, D.; Wang, R. Challenges and Opportunities of Functionalized Cucurbiturils for Biomedical Applications. JACS Au 2023, 3, 2356–2377. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Wei, M.; Shang, L.; Yang, Y.W. Cucurbiturils-Mediated Noble Metal Nanoparticles for Applications in Sensing, SERS, Theranostics, and Catalysis. Adv. Funct. Mater. 2021, 31, 2007277. [Google Scholar] [CrossRef]
- Peveler, W.J.; Jia, H.; Jeen, T.; Rees, K.; Macdonald, T.J.; Xia, Z.; Chio, W.-I.K.; Moorthy, S.; Parkin, I.P.; Carmalt, C.J.; et al. Cucurbituril-Mediated Quantum Dot Aggregates Formed by Aqueous Self-Assembly for Sensing Applications. Chem. Commun. 2019, 55, 5495–5498. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, D.; Miao, R.; Qin, C.; Song, F.; Zhao, W.; Zhao, N.; Liu, H. Carbon Quantum Dots and Cucurbituril Joining Hands to Achieve Luminescence and Self-Healing Performance in a Hydrogel. J. Mater. Sci. 2023, 58, 1739–1751. [Google Scholar] [CrossRef]
- Khaligh, A.; Tuncel, D. Cucurbituril-Assisted Supramolecular Polymeric Hydrogels. In Cucurbituril-based Functional Materials; Tuncel, D., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 120–148. ISBN 978-1-78801-488-5. [Google Scholar]
- Paatelainen, M.; Lahikainen, M.; Berdin, A.; Kuntze, K.; Nau, W.M.; Nonappa; Priimagi, A. Hydrogel Lasers Via Supramolecular Host–Guest Complexation. Adv. Opt. Mater. 2023, 11, 2300232. [Google Scholar] [CrossRef]
- Nedelchev, L.; Mateev, G.; Nikolova, L.; Nazarova, D.; Ivanov, B.; Strijkova, V.; Stoykova, E.; Choi, K.; Park, J. In-Line and off-Axis Polarization-Selective Holographic Lenses Recorded in Azopolymer Thin Films via Polarization Holography and Polarization Multiplexing. Appl. Opt. 2023, 62, D1–D7. [Google Scholar] [CrossRef]
- Blagoeva, B.; Nedelchev, L.; Nazarova, D.; Stoykova, E.; Park, J. Reversible Supramolecular Chiral Structures Induced in Azopolymers by Elliptically Polarized Light: Influence of the Irradiation Wavelength and Intensity. Appl. Opt. 2022, 61, B147–B155. [Google Scholar] [CrossRef]
- Yadavalli, N.S.; Santer, S. In-Situ Atomic Force Microscopy Study of the Mechanism of Surface Relief Grating Formation in Photosensitive Polymer Films. J. Appl. Phys. 2013, 113, 224304. [Google Scholar] [CrossRef]
- Madruga, C.; Filho, P.A.; Andrade, M.M.; Gonçalves, M.; Raposo, M.; Ribeiro, P.A. Birefringence Dynamics of Poly{1-[4-(3-Carboxy-4-Hydroxyphenylazo)Benzenesulfonamido]-1,2-Ethanediyl, Sodium Salt} Cast Films. Thin Solid Films 2011, 519, 8191–8196. [Google Scholar] [CrossRef]
- Ferreira, Q.; Gomes, P.J.; Maneira, M.J.P.; Ribeiro, P.A.; Raposo, M. Mechanisms of Adsorption of an Azo-Polyelectrolyte onto Layer-by-Layer Films. Sens. Actuators B Chem. 2007, 126, 311–317. [Google Scholar] [CrossRef]
- Nazarova, D.; Nedelchev, L.; Berberova-Buhova, N.; Mateev, G. Nanocomposite Photoanisotropic Materials for Applications in Polarization Holography and Photonics. Nanomaterials 2023, 13, 2946. [Google Scholar] [CrossRef] [PubMed]
- Falcione, R.; Roldan, M.V.; Pellegri, N.; Goyanes, S.; Ledesma, S.A.; Capeluto, M.G. Increase of SRG Modulation Depth in Azopolymers-Nanoparticles Hybrid Materials. Opt. Mater. 2021, 115, 111015. [Google Scholar] [CrossRef]
- Nedelchev, L.; Mateev, G.; Strijkova, V.; Salgueiriño, V.; Schmool, D.S.; Berberova-Buhova, N.; Stoykova, E.; Nazarova, D. Tunable Polarization and Surface Relief Holographic Gratings in Azopolymer Nanocomposites with Incorporated Goethite (α-FeOOH) Nanorods. Photonics 2021, 8, 306. [Google Scholar] [CrossRef]
- Goldenberg, L.M.; Lisinetskii, V.A.; Schrader, S. Continuously Tunable Laser Based on Polarization Gratings in Azobenzene-Containing Material. Laser Phys. 2014, 24, 35807. [Google Scholar] [CrossRef]
- Goldenberg, L.M.; Lisinetskii, V.; Ryabchun, A.; Bobrovsky, A.; Schrader, S. Influence of the Cation Type on the DFB Lasing Performance of Dye-Doped Azobenzene-Containing Polyelectrolytes. J. Mater. Chem. C 2014, 2, 8546–8553. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kircheva, N.; Dobrev, S.; Dasheva, L.; Nikolova, V.; Angelova, S.; Dudev, T. Metal-Assisted Complexation of Fluorogenic Dyes by Cucurbit[7]Uril and Cucurbit[8]Uril: A DFT Evaluation of the Key Factors Governing the Host–Guest Recognition. Molecules 2023, 28, 1540. [Google Scholar] [CrossRef]
- Choudhury, S.D.; Mohanty, J.; Pal, H.; Bhasikuttan, A.C. Cooperative Metal Ion Binding to a Cucurbit[7]Uril - Thioflavin T Complex: Demonstration of a Stimulus-Responsive Fluorescent Supramolecular Capsule. J. Am. Chem. Soc. 2010, 132, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Škalamera, Đ.; Zavalij, P.Y.; Hostaš, J.; Hobza, P.; Mlinarić-Majerski, K.; Glaser, R.; Isaacs, L. Influence of Hydrophobic Residues on the Binding of CB[7] toward Diammonium Ions of Common Ammonium⋯ammonium Distance. Org. Biomol. Chem. 2015, 13, 6249–6254. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom-Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2013; Available online: https://www.rsc.org/suppdata/c5/sc/c5sc02423d/c5sc02423d1.pdf (accessed on 14 August 2024).
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- The PyMol Graphics System, Version 2.2.3; Schrödinger, LLC.: New York, NY, USA, 2010.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova, V.; Dobrev, S.; Kircheva, N.; Nazarova, D.; Nedelchev, L.; Nikolova, V.; Dudev, T.; Angelova, S. Density Functional Theory Prediction of Laser Dyes–Cucurbit[7]uril Binding Affinities. Molecules 2024, 29, 4394. https://doi.org/10.3390/molecules29184394
Petkova V, Dobrev S, Kircheva N, Nazarova D, Nedelchev L, Nikolova V, Dudev T, Angelova S. Density Functional Theory Prediction of Laser Dyes–Cucurbit[7]uril Binding Affinities. Molecules. 2024; 29(18):4394. https://doi.org/10.3390/molecules29184394
Chicago/Turabian StylePetkova, Vladislava, Stefan Dobrev, Nikoleta Kircheva, Dimana Nazarova, Lian Nedelchev, Valya Nikolova, Todor Dudev, and Silvia Angelova. 2024. "Density Functional Theory Prediction of Laser Dyes–Cucurbit[7]uril Binding Affinities" Molecules 29, no. 18: 4394. https://doi.org/10.3390/molecules29184394
APA StylePetkova, V., Dobrev, S., Kircheva, N., Nazarova, D., Nedelchev, L., Nikolova, V., Dudev, T., & Angelova, S. (2024). Density Functional Theory Prediction of Laser Dyes–Cucurbit[7]uril Binding Affinities. Molecules, 29(18), 4394. https://doi.org/10.3390/molecules29184394









