1. Introduction
As a vital C4 platform chemical, 1, 3-Butanediol (1, 3-BDO) is widely used in the field of cosmetics, acting as a solvent, and it is also a key intermediate in the synthesis of restoratives, aromas, and pheromones [
1,
2]. Also, 1, 3-BDO is largely demanded and can be used as an important precursor for the synthesis of butadiene, which is a chemical widely used in the manufacture of synthetic latex, resins, and rubber [
3]. In addition, as a key intermediate, optical (
R)-1, 3-BDO is very important in synthesizing aromatics, insecticides, beta-lactam antibiotics, and pheromones [
3,
4]. At present, the chemical approach from acetaldehyde is the main method to synthesize 1, 3-BDO; the products are racemic mixtures with
R and
S types [
4]. (
R)-1, 3-BDO also can be produced by microbial reduction of 4-hydroxybutanone, which is complex and costly and is not suitable for large-scale production in industry [
4]. Also, (
R)-1, 3-BDO was obtained by microbial metabolism through constructing artificial metabolic pathways in
E. coli [
5,
6]. Despite the fermentative routes for the production of (
R)-1, 3-BDO are green bioprocesses, but the titers, purification, and yields are too low for large-scale application. The production of optically pure (
R)-1, 3-BDO by green bioprocesses using cheap and sustainable biological resources is highly desirable.
In industrial production, chemical and enzymatic methods are the main methods for preparing chiral alcohols. The enzymatic method is often favored by the industry for its advantages of simplicity of procedure, high selectivity, high conversion rate, substrate tolerance, and the eco-friendly nature of the process, compared to the chemical and synthetic biology-based methods. In the field of biocatalysis, the conversion of ketones to chiral alcohols is catalyzed by the ketoreductase (KREDs) [
7] or alcohol dehydrogenase (Alcohol dehydrogenase (ADH) [
8]. Some studies showed that the mutated Rhodococcus phenylacetaldehyde reductase (PAR) or Leifsonia alcohol dehydrogenase (LSADH) [
9] and a short-chain carbonyl reductase (LnRCR) [
10] were applied for the production of (
R)-1, 3-BDO using 4-hydroxy-2-butanone (4H2B) as substrate.
The
ChKRED20 is a NADH-dependent ketoreductase identified from
Chryseobacterium sp.
CA49, using 2-propanol as the ultimate reducing agent [
11,
12]. It has been found that in the presence of the cofactor of NADH,
ChKRED20 or its mutants could asymmetrically reduce COBE to ethyl (
S)-4-chloro-3-hydroxybutanoate ((
S)-CHBE) [
11] and reduce CFPO to (1
S)-2-chloro-1-(3, 4-difluorophenyl) ethanol ((
S)-CFPL) [
7]. So far, no studies have reported that
ChKRED20 or its mutants could be directly used for the synthesis of (
R)-1, 3-BDO using 4-hydroxy-2-butanone (4H2B) as substrate.
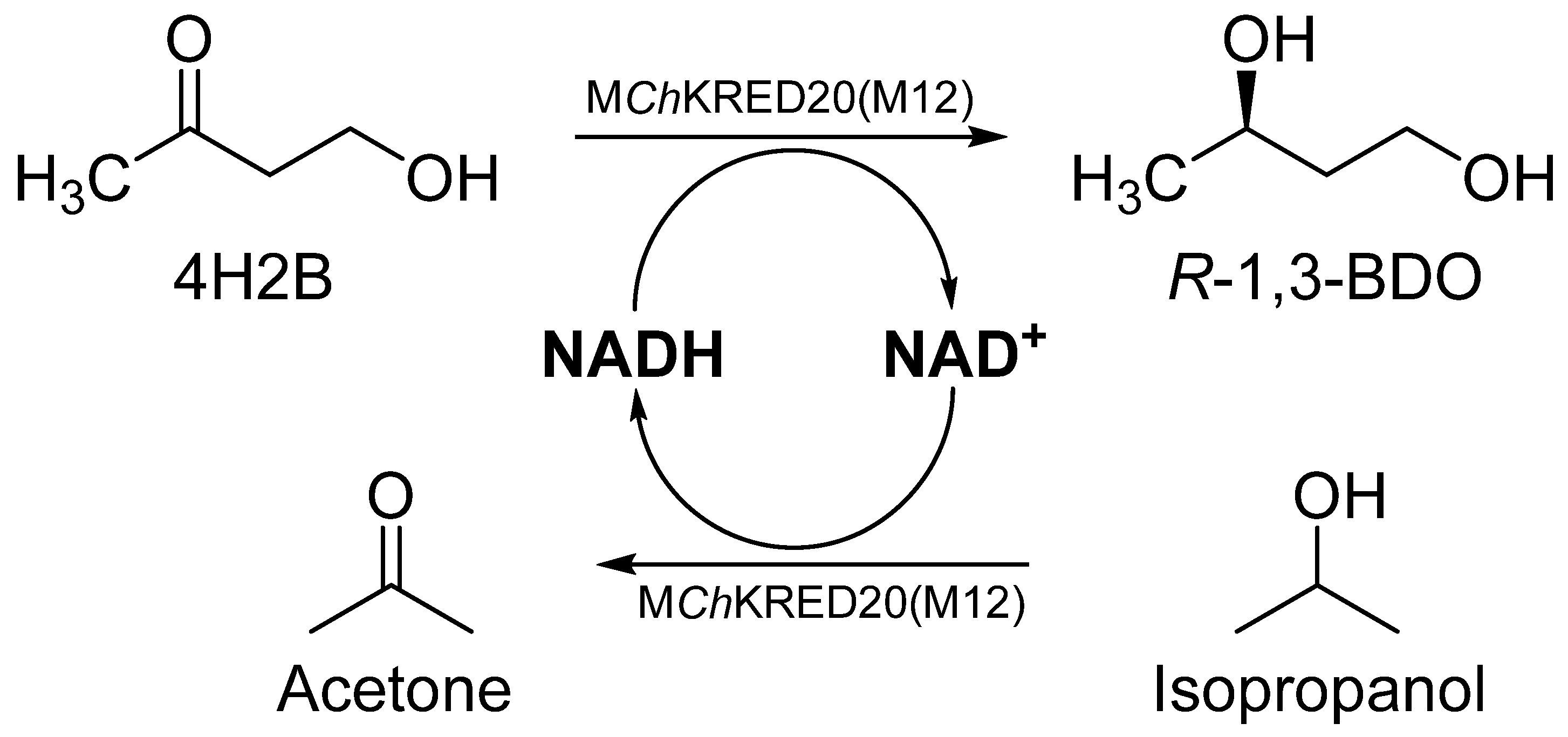
Previous work in our lab had found that
ChKRED20 could serve as a catalyst that can asymmetrically reduce 4H2B to (
R)-1, 3-BDO, but its stability and activity were poor and cannot be used as an industrial enzyme for the synthesis of (
R)-1, 3-BDO, so we performed through directed evolutionary screening of ketoreductase
ChKRED20 and had obtained a mutant (M12) with significantly improved activity and thermal stability, the M12 mutant is a robust NADH-dependent ketoreductase that can asymmetrically reduce 4H2B to (
R)-1, 3-BDO (
Figure 1).
So far, recombinant expression of
ChKRED20 or its mutants have been expressed in intracellular in
E. coli [
11,
13,
14]; the process of purification after intracellular expression is complicated, which leads to high cost, and the system in bacteria cannot undergo shear modification. Due to the large market for ketoreductase, it is critical to develop low-cost methods for the large-scale purification of
ChKRED20 or its mutants.
For the production of heterologous proteins, such as industrial enzymes, currently, the yeast
P. pastoris is regarded as one of the most versatile and popular expression systems [
14,
15]. As a secretory expression system,
P. pastoris can simplify the production of industrial enzymes and simplify the downstream purifying process. The
P. pastoris is suitable for high-density continuous fermentation, producing a high yield of secreted foreign proteins and with small amounts of endogenous proteins; as a single-celled microorganism, its genetic operation is easy, and it grows fast with simple nutrition [
15,
16]. The
P. pastoris expression system has many advantages, such as its well-developed protein processing mechanisms, including signal peptide cleavage, protein folding, intracellular post-translational modification, and secretion of normal functional proteins into the medium [
16,
17,
18]. In addition, production in
P. pastoris usually uses the strong and tightly regulated AOX1 promoter, thus resulting in a heterologous protein that can account for 30% of the total cell protein when growing in methanol [
18].
Previous studies showed that both ChKRED20 and its mutants were expressed in E. coli, and no related studies have reported that ChKRED20 or its mutants were expressed in P. pastoris. In this study, we found that the mutant of ChKRED20 (M12) could asymmetrically reduce 4H2B to (R)-1, 3-BDO. The ChKRED20 mutant M12 was successfully heterologously expressed in Pichia pastoris, and furthermore, multiple copy expression systems and high-density fermentation were used to improve the yield of recombinant protein to 3.5 g/L. The recombinant ChKRED20 mutant M12 enzyme crude extracts were also used to produce (R)-1, 3-BDO, and 98.9% yield was achieved at 4540 mM 4H2B with the high optical purity of the product (ee > 99%) and met the production requirements. In this study, the ChKRED20 mutant was effectively expressed and evaluated its enzymatic characteristics in P. pastoris for the first time and was used for the biotransformation of 4H2B to (R)-1, 3-BDO, which has the potential for large-scale industrial application.
3. Discussion
The
ChKRED20 or its mutants were used for the enzymatic synthesis of chiral alcohols, such as (
R)-3, 5-bis(trifluoromethyl)-1-phenylethanol [
10] and ethyl (
S)-4-chloro-3-hydroxybutanoate [
13], 1, 3-BDO is an important C4 diol that is widely used as a solvent in cosmetics and as a monomer in the polymer industry. At present, three enzymatic methods were reported to be used for the preparation of (
R)-1, 3-BDO [
10], such as the dehydrogenase was used to oxidize the (
S)-1, 3-BDO enantiomer in racemate to (
R)-1, 3-BDO [
26], or the enantio-selective reduction of 4H2B to (
R)-1, 3-BDO [
27], the short-chain carbonyl reductase (LnRCR) mutant was used for the reduction step to produce (
R)-1, 3-BDO [
10].
So far, no studies have reported that ChKRED20 or its mutants can be used in the synthesis of (R)-1, 3-BDO. Previous work in our lab had found that ChKRED20 could serve as a catalyst that can asymmetrically reduce 4H2B to (R)-1, 3-BDO, but its stability and activity were poor and cannot be used as an industrial enzyme for the synthesis of (R)-1, 3-BDO, so we obtained a mutant M12 with significantly improved activity and thermal stability through directed evolution screening. However, the mechanism by which M12 mutant activity was higher than wild-type ChKRED20 is not clear. In this study, we performed the molecular docking of ChKRED20 and the M12 mutant with the 4H2B substrate. The M12 mutant protein shows significantly higher substrate affinity compared to the wild-type protein, and the mutant had a higher binding energy of 40.25 kJ/mol than that of the wild-type protein (30.37 kJ/mol). The energy difference of approximately 10 kJ/mol, close to the average energy of an N–H…O hydrogen bond (around 8 kJ/mol), was primarily due to the hydrogen bond between the substrate’s 4-hydroxyl group and the N-H of the Q150 side chain. Further, we performed the steady-state kinetic parameters of the wild-type ChKRED20 and mutant M12, and the results indicated that the M12 had a better affinity with the substrate, which further increased the catalytic efficiency. The mutant not only improves the enzyme binding to the substrate but also enhances the enzyme catalytic efficiency. In the future, we can further screen mutants with tighter binding and higher affinity to 4H2B to obtain more mutants with better enzymatic activity for the synthesis of (R)-1, 3-BDO. Our study may also provide a way to screen and optimize other enzymes for the high-efficiency mutants.
With the study of the regulation of metabolic capacity [
28], safety, and function (
R)-1, 3-BDO is increasingly used in the food and health products industry. Our study found that the
ChKRED20 mutant M12 was a key enzyme that could be used to produce (
R)-1, 3-BDO from 4H2B in industrial production, and its expression host safety is particularly important. Previously,
ChKRED20 or its mutants were reported to be expressed in intracellular in
E. coli, leading to cumbersome purification steps and high purification costs in the large-scale preparation. Endotoxin was produced in
E. coli, while
P. pastoris is generally considered to be a safe (GRAS) strain with extraordinary protein-secreting capabilities [
18,
29]. The
P. pastoris has been successfully used as a host for the secretory expression of heterologous proteins, and accordingly, it may be suitable for the production of
ChKRED20 or its mutants in yeast rather than in bacteria. In this study, we achieved high-level expression of the
ChKRED20 mutant using the
P. pastoris system and assessed its reduction activity to produce (
R)-1, 3-BDO.
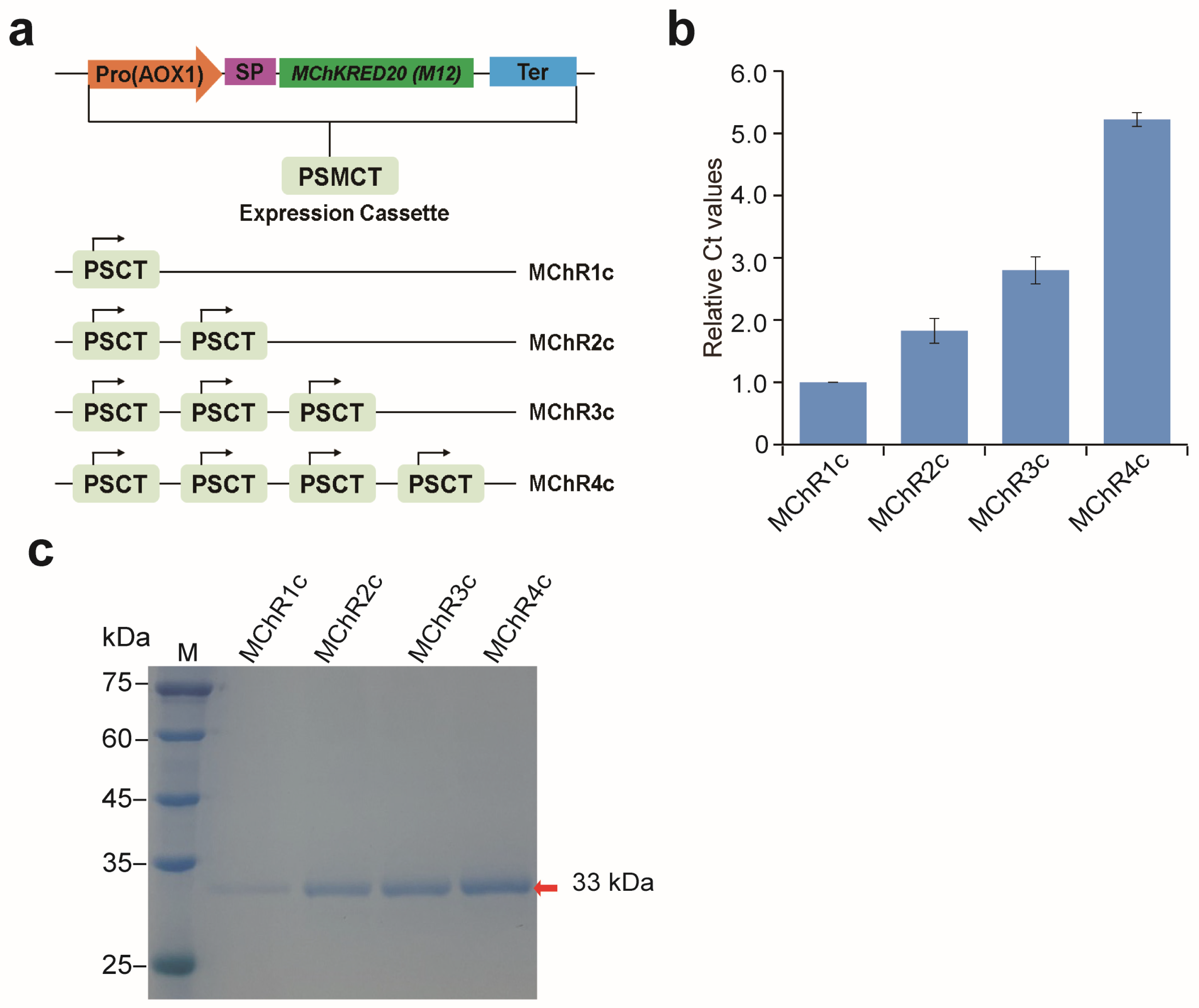
As an industrial enzyme, it is necessary to reduce its production cost and increasing protein expression is one way to reduce production costs. In addition to codon optimization and the use of a strong AOX1 promoter, the copy number of the heterologous gene expression cassette is also an important way to achieve efficient protein expression [
18,
23,
29]. In this study, multi-copy expression was successful at obviously improving the yield of recombinant
ChKRED20 mutant M12. The purification cost is also an important factor to be considered in industrial enzyme production. So far,
ChKRED20 or its mutants were intracellularly expressed in
E. coli, and it needed to break the cell wall and heat treatment to obtain target proteins, which would increase the cost of production, and heat treatment affects the enzyme activity. Large amounts of intrinsic proteins are not secreted in
P. pastoris, while foreign protein is secreted to the medium, which can account for more than 90% of the total in the medium, so it is very easy to isolate the target protein [
16]. Therefore, the crude enzyme can be used directly without purification and simple desalination treatment, which can reduce the cost of production. Moreover, glycosylation modification in
P. pastoris can improve the thermal stability of the enzyme [
25].
In conclusion, we have successfully expressed the
ChKRED20 mutant M12 protein in
P. pastoris and improved the expression level of the target protein by constructing muti-copy strains carrying four copies of the target gene. The protein yield was 302 mg/L by shaking flask fermentation and 3.5 g/L by high-density fermentation. The recombinant
ChKRED20 mutant M12 showed high thermostability, and the crude enzyme extract was efficient in the bioreductive production of (
R)-1, 3-BDO with a high conversion rate (98.9%) and stereoselectivity (ee > 99%). Zheng et al. reported that they used a
C. krusei cell catalyst to convert 45 g/L (510 mM) of 4H2B to (R)-1, 3-BDO with 99% ee, achieving a yield of 83.9% in about 50 h [
27]. Recombinant dehydrogenase was also reported to be used for the transformation of 4H2B to (
R)-1,3-BDO with the 250 g/L (2837 mM) of 4H2B, and the (
R)-1, 3-BDO product yield was 99% with 99% ee when the transformation time was 500 h [
9]. Our results suggested that the recombinant M12 expressed in
P. pastoris had the highest efficiency and conversion rate of (
R)-1, 3-BDO production when using 4H2B as substrate compared to other enzymatic conversion methods reported so far.
Studies found that
ChKRED20 or its mutants were responsible for the enzymatic synthesis of (
R)-3, 5-bis(trifluoromethyl)-1-phenylethanol [
10] and ethyl (
S)-4-chloro-3-hydroxybutanoate [
13], which are key intermediates for the chiral drug. In our study, an efficient and functional expression system for the production of
ChKRED20 mutant will not only facilitate further studies of
ChKRED20 or its mutants but also pave the way for possible large-scale production of ketoreductases in the near future and provide a possibility for the enzymatic synthesis of more chiral alcohols. The X-ray crystal structure of
ChKRED20 was refined, and mutants were designed based on structure, which increased the diversity of enzyme reaction substrates [
11], and some mutants of
ChKRED20 have been obtained to improve some of its properties, including improved thermal stability [
13]. Therefore, our study provides an idea for the expression and application of these
ChKRED20 mutants. In the future, more work on recombinant
ChKRED20 mutants with higher purity and more activity should be studied, and improve the efficiency of (
R) 1, 3-BDO with ketoreductases to further decrease the production cost.
4. Materials and Methods
4.1. Strains, Reagents and Media
E. coli XL10-Gold, BL21(DE3), and
P. pastoris GS115 strains were purchased from Invitrogen (Carlsbad, CA, USA). The pET-23a (+) used as the expression vector was maintained in our laboratory. The expression vector, pHBM905BDM plasmid [
23], was constructed and stored in our laboratory. The
P. pastoris culture media, including minimal dextrose (MD), buffered glycerol-complex (BMGY), and buffered methanol-complex (BMMY), were prepared as described in the
P. pastoris expression manual (Invitrogen, Carlsbad, CA, USA) [
18,
23,
30].
4.2. The Construction of Mutant Library
The Quikchange kit (Agilent, Santa Clara, CA, USA) was used. The sequence design of the mutagenesis primers was performed according to the instructions of the kit. The construction of the site-saturation mutagenesis library was as follows. The PCR reaction consisted of 10 µL of 5× buffer, 1 µL of 10 mM dNTP, 1 µL of plasmid DNA template (50 ng/µL), 0.75 µL (10 µM) each of the upstream and downstream primers, 0.5 µL Phusion and 36 µL of ddH2O, the PCR primer has an NNK codon at the mutation position. The PCR amplification was performed as follows: 98 °C for 3 min and then 25 cycles of 98 °C for 10 s (denaturation), 72 °C for 3 min (annealing and extension), 72 °C for 7 min (extension), cooling to 4 °C. Then the PCR product was digestion at 37 °C for 4 h by adding 2 µL of DpnI to eliminate the plasmid template. The digested PCR product was then transformed into E. coli BL21(DE3) competent cells and plated on LB agar plates with chloramphenicol to obtain a site-saturation mutagenesis library.
4.3. High-Throughput Screening of Ketoreductase ChKRED20 Mutant Library
The expression of the ketoreductase mutant library was performed in 96-well plates. The operation procedure was as follows: mutant colonies were picked and inoculated into LB medium with chloramphenicol in a 96-well shallow plate (150 µL per well), then cultured for 12–18 h at 180 rpm, 30 °C. When OD600 of shallow plate culture reached 2.0, 20 µL of this culture was transferred to a TB medium (containing 400 µL of TB medium and 6 g/L of lactose per well) in a 96-well deep plate as expression culture, and it was shaken overnight for 18 to 20 h at 240 rpm under 30 °C. The expression culture was centrifuged (4000 rpm, 10 min) to collect cell pellets. Next, 200 µL/well of cell lysis buffer (100 mM phosphate buffer, pH 7.5, containing 1 mg/mL lysozyme) was added to the deep well plate, and the plate was sealed, placed on a plate shaker at 700 rpm for 1 h to break the cells. Then, the cell lysate was centrifuged (4000 rpm, 10 min), and 160 µL/well of supernatant enzyme solution was collected into a new plate, which was subsequently sealed and subjected to heat treatment by shaking in a water bath shaker at 72 °C for 2.5 h.
The enzyme solution was centrifuged (4000 rpm,15 min) after the heat treatment, then the supernatant (30 µL) was transferred to a 96-well plate, which was pre-loaded with reaction stock solution (170 µL/well). The reaction plate was then heat-sealed with an aluminum film and placed in a shaker at 45 °C, 200 rpm to start the reaction. After 15 h of reaction, 50 µL of the reaction solution was transferred into a new 96-deep well plate, and the reaction was quenched by adding 1 mL of ethyl acetate. The reaction was shaken on a plate shaker for 30 min (800 rpm), then centrifuged (4000 rpm, 30 min), and the supernatant was subject to GC analysis to determine the conversion.
4.4. Measurement Enzyme kineticsPurified Enzymes Were Used
All measurements were performed in triplicate. To determine the kinetic parameters of the enzyme for 4H2B, WT (0.5 μM)/M12 (0.1 μM) was pre-incubated with 4H2B to a concentration of 1 mM–750 mM in the 100 mM potassium phosphate buffer (pH 7.0) for 90 s at 40 °C. The reaction was then initiated by adding NADH (0.25 mM), bringing the final volume of the assay to 200 µL, and monitored by measuring the decrease in absorbance at 340 nm using a spectrophotometer. Data were fitted to the Michaelis–Menten equation using OriginPro 2021 to generate the estimates of Km, Vmax, and kcat.
4.5. Molecular Docking
The three-dimensional structures of both the wild type (
ChKRED20) and mutant (M12) were generated using the AlphaFold Server (Google DeepMind). Upon providing the peptide sequence, structure prediction was performed under the conditions specifying four protein copies (referencing the homologous structure assembly of 6IXM) and designating the substrate NAD
+ as the ligand. The CB-dock2 software (
https://cadd.labshare.cn/cb-dock2/ accessed on 13 September 2024) [
19] was used to dock the substrate into the active site of the protein
ChKRED20. The interactions between the substrate and the protein were analyzed using the LigPlot
+ software [
20,
21]. Furthermore, the binding energy between the substrate and the protein was calculated using YASARA [
22]. All of the protein structures were visualized and analyzed with PyMOL(TM) 3.0.2 and Discovery studio 2021.
4.6. Construction of pHBM905BDM-MKRED Expression Vectors
The mutant of
ChKRED20 (M12) (gene,
MKRED) was codon optimized based on the
P. pastoris system and was synthesized by Sangon BioTech (Shanghai, China). The
MKRED gene was cloned into the pHBM905BDM vector at the
Cpo I–
Not I site to generate plasmid pHBM905BDM-MKRED1 with the TLTC DNA cloning method [
31]. The multi-copy expression vectors of M
ChKRED20 were constructed by the biobrick assembly method [
30]. The recombinant vectors were verified by sequencing, named pHBM905BDM-MKRED2, pHBM905BDM-MKRED3 and pHBM905BDM-MKRED4.
4.7. Screening of Recombinant Yeast Strains Expressing M12
The recombinant plasmids were linearized with Sal I, purified, and then transformed into competent cells of P. pastoris GS115 through electroporation. The positive transformants were selected on MD plates and further identified by colony PCR with specific primers to amplify the fragment of the MKRED gene.
4.8. Determination of MKRED Gene Copy Numbers
The genomic DNA of yeast was extracted with a Yeast Genomic DNA Isolation Kit (Omega, Rhinebeck, NY, USA). Verification of the gene copy number was carried out as previously described [
30]. The glyceraldehydes-3-phosphate dehydrogenase (
GAP) gene of
P. pastoris was used as an internal reference gene for qPCR. Using
GAP as an internal reference gene, the copy number of
MKRED was determined by the 2
−ΔΔCt method [
30].
4.9. Expression of the Recombinant M12 Using Shake-Flask Fermentation
Recombinant
P. pastoris strains containing different copies of the target gene were cultured in shaking flasks of 100 mL BMGY medium for 48 h at 28 °C; cells were harvested after centrifugation at 4 °C for 5 min (4000×
g), then inoculated in 50 mL BMMY medium, and methanol with a final concentration of 1% (
v/
v) was added every 24 h to induce foreign protein expression. Fermentation was terminated after induction for 144–168 h; supernatants were collected and centrifuged at 4 °C for 5 min (10,000×
g). The different samples were prepared and then detected with 12% (
w/
v) sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie Brilliant Blue G-250. The protein concentrations were determined by the Bradford kit (Beyotime, Shanghai, China) [
23].
4.10. High-Density Fermentation of Recombinant Strain
Fed-batch fermentation was carried out according to the Invitrogen Pichia Fermentation Process Guidelines and previously described methods [
18,
30]. The recombinant strain was cultured in 200 mL YPD medium as the seed medium at 28 °C for 24 h. Then, the seed medium was transferred to a 5 L fermenter containing 2 L of BSM medium. The fermentation was performed at 28 °C, pH 5.8, and 25–30% dissolved oxygen (DO) in the early stages. DO was rapidly increased to 100% when glycerol was exhausted, then 50% (
v/
v) glycerol containing PTM trace salts (12 mL/L) was added at a rate of 12 mL/h/L to continue cell growth. When the OD
600 reached about 300, 1% methanol with PTM trace salts (12 mL/L) was fed at a rate of 3 mL/h/L to induce the expression of foreign protein. In the induction phase of fermentation, the conditions were adjusted to 25 °C and pH 5.0, and 30–35% DO was maintained. Approximately 30 mL cultures were collected per 12 h for protein concentration detection assay until the end of fermentation [
18,
23].
4.11. Glycoprotein Staining and Deglycosylation of the Recombinant Proteins
To perform glycoprotein staining, different samples were treated and separated via SDS-PAGE by 12% (w/v) polyacrylamide gels, then glycoprotein staining was carried out following the method described in the Glycoprotein Staining Kit (Beyotime, Shanghai, China).
Deglycosylation of the glycoproteins was performed as previously described [
32,
33]. Briefly, the fermentation supernatant was collected after 144 h induction with methanol. The target glycoproteins and recombinant Endo H were mixed in a 10:1 concentration ratio and incubated at 37 °C for 2 h, and then the samples were detected by 12% SDS-PAGE.
4.12. Enzymatic Characterization of M12
All experiments were performed in triplicate. The enzyme activity of M12 was measured using 4-hydroxy-2-butanone(4H2B) as the substrate. Purified enzymes were used to determine the enzyme activity. Asymmetric reduction of 4H2B was performed at 40 °C in a two-phase system in which 60% (v/v) potassium phosphate buffer (100 mM, pH 7.0) was dissolved with 0.025 g NAD+/L and 3 g/L purified enzyme, 40% (v/v) of the isopropyl alcohol phase was dissolved with 100 g 4H2B/L (1135 mM) for a total volume of 0.2 mL. The products were extracted with ethyl acetate after reaction for 6 h with shaking at 400 rpm and analyzed with Gas chromatography (GC).
To measure the optimal concentration of the isopropyl alcohol (IPA)in the reaction system, the reaction was performed at a range of IPA concentrations (10% (v/v)–50% (v/v)), and the reaction was performed at 40 °C. To determine the optimal concentration of NAD+ in the reaction, different concentrations of NAD+ (10–100 mg/L) were added to the reaction system, and the reaction was performed at 40 °C.
To measure the optimal temperature of the M12 enzyme, the reaction was performed at a range of temperatures (30–50 °C). To determine the optimal pH of the M12 enzyme, the reaction was performed at 40 °C, as pH 5.0 and pH 9.0 are far from the p
Ka of the phosphate buffer and near the equivalence point [
34], so phosphate buffer may not be suitable for maintaining a constant pH at pH 5.0 and pH 9.0; therefore, different pH buffers were used to regulate the reaction system with sodium acetate (100 mM, pH 5.0–6.0), phosphate buffer (100 mM, pH 7.0) and Tris-HCl (100 mM, pH 8.0–9.0).
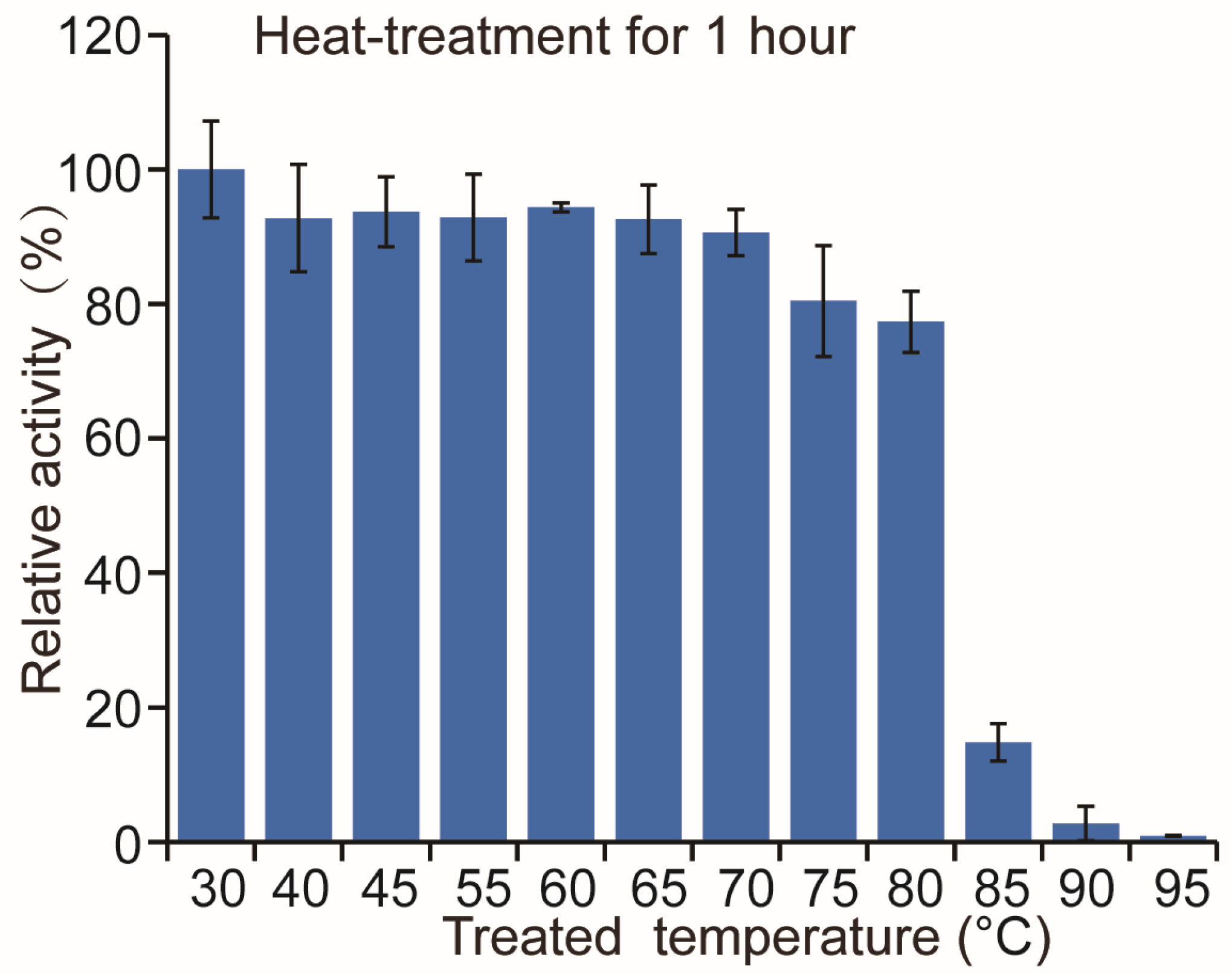
To analyze the thermostability of M12, the purified enzymes were incubated at different temperatures for 1 h, then followed by immediate chilling on ice for 5 min and measured at 40 °C as the method described above; the remaining enzyme activity was measured to indicate thermal stability.
4.13. Bioreduction of 4H2B to (R)-1, 3-BDO Using Crude Enzyme Extracts
Asymmetric reduction of 4H2B to (R)-1, 3-BDO were performed at 40 °C in a two-phase system a total volume of 100 mL, the biphasic system containing 60% (v/v) of the phosphate buffer (100 mM, pH 7.0) dissolving 0.025 g NAD+/L and 3 g/L of the crude enzyme extracts, and 40% (v/v) of the isopropyl alcohol phase dissolved with 400 g/L 4H2B (4540 mM). The reaction was traced over 120 h, and the conversion rate was calculated by detecting the content of the substrate remaining after different reaction times; the products were extracted with ethyl acetate and analyzed with Gas chromatography (GC) to test their purity.