Design and Synthesis of an Azo Reductase Responsive Flavonol–Indomethacin Hybrid Used for the Diagnosis and Treatment of Colitis
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
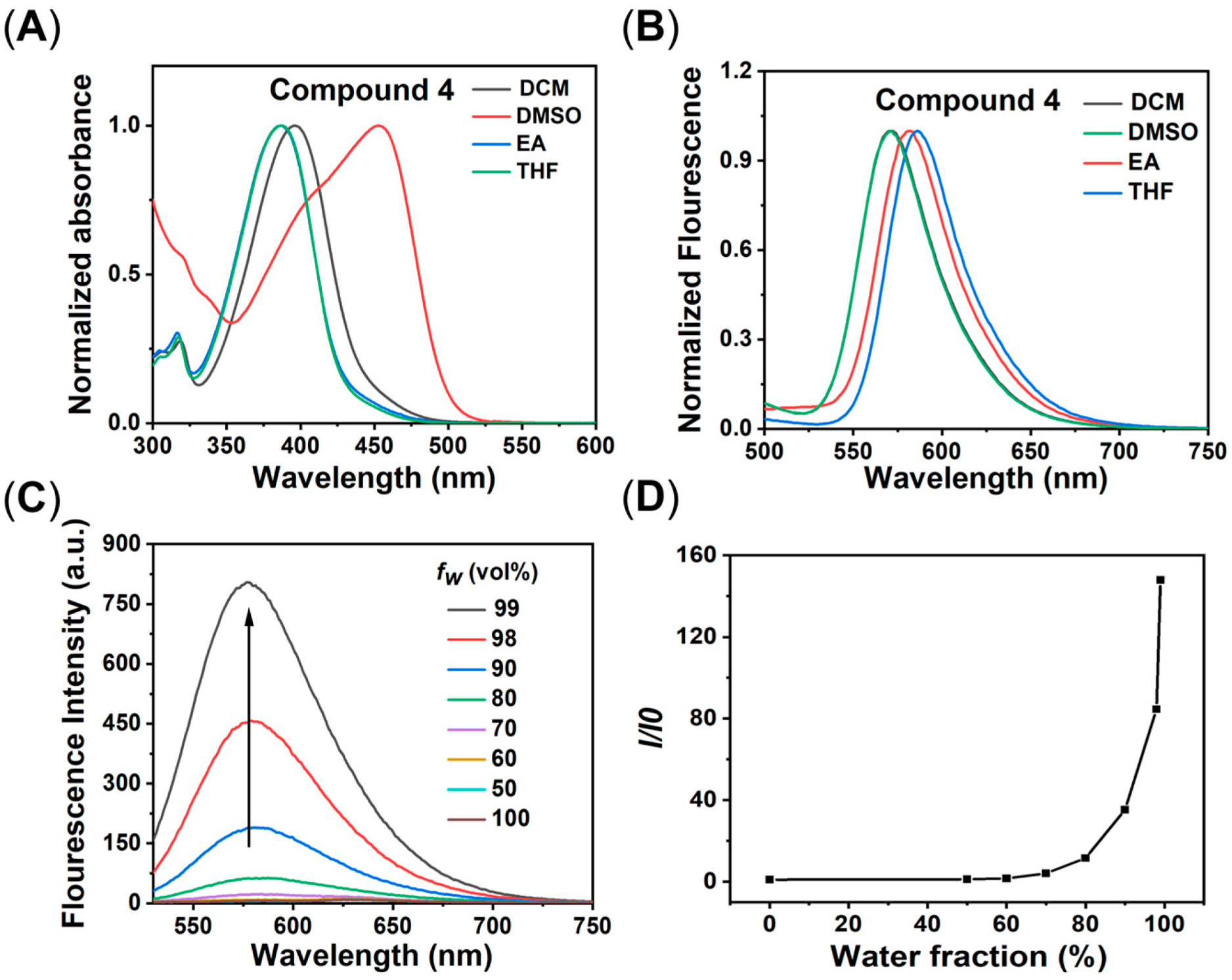
2.2. Photophysical Properties of Compound 4
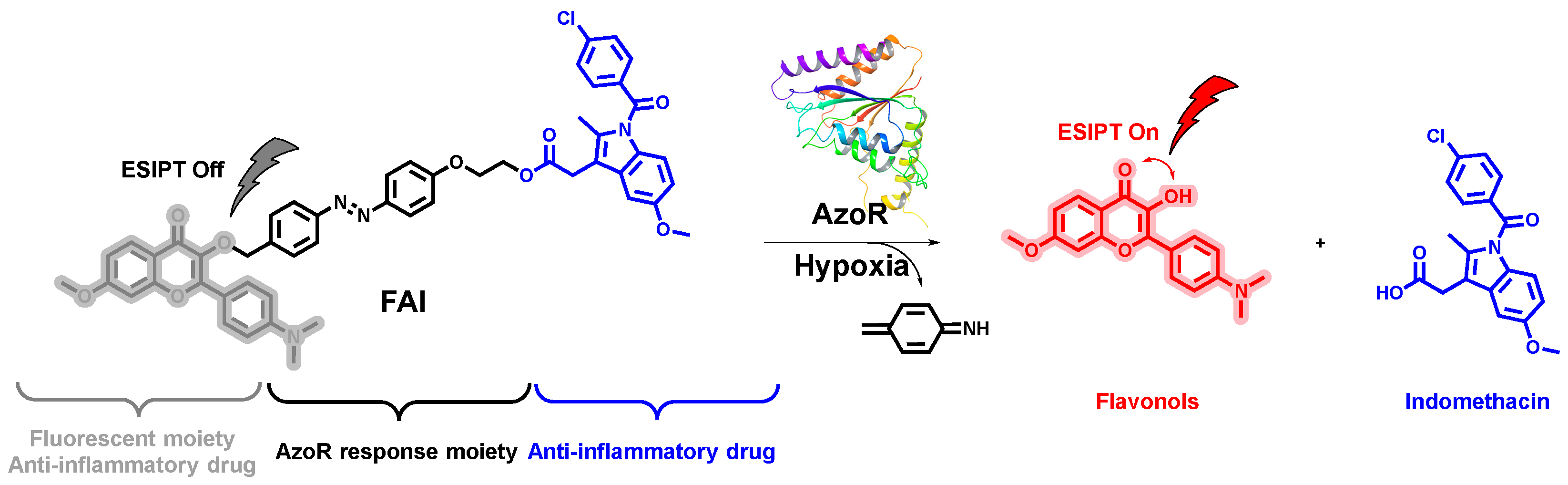
2.3. Release Properties of Compound FAI
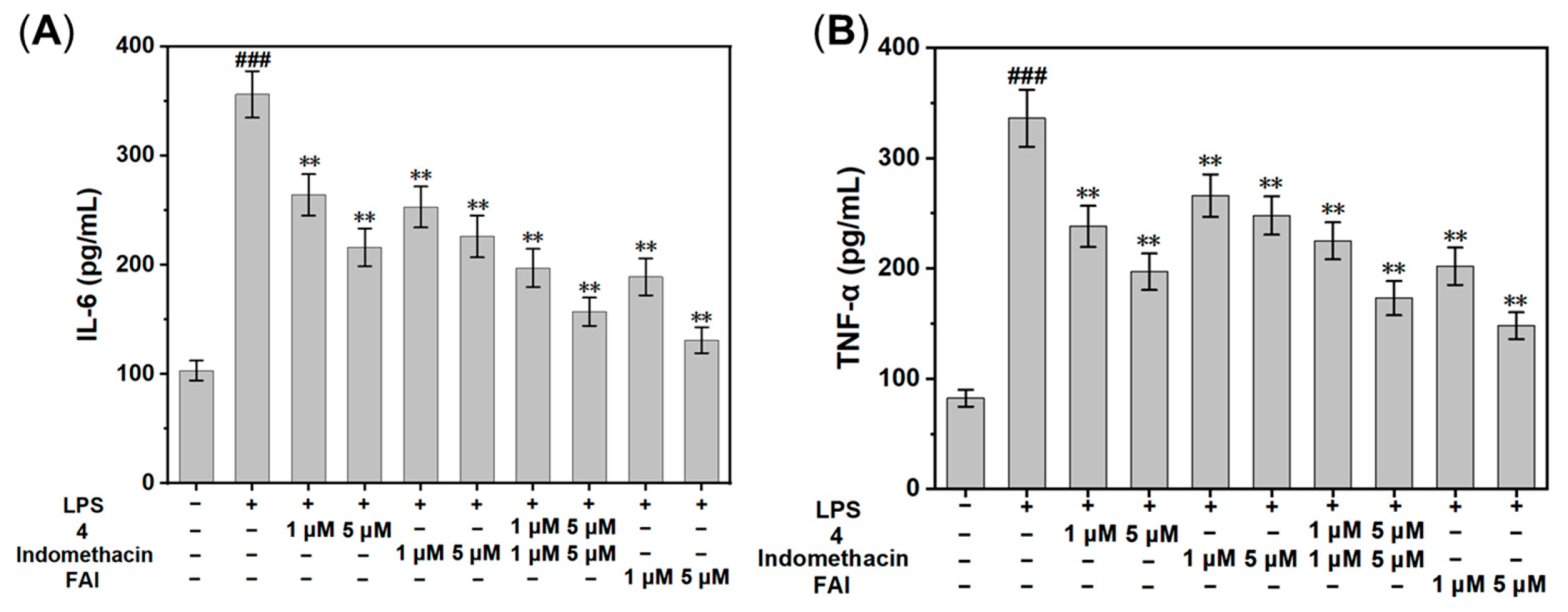
2.4. In Vitro Anti-Inflammatory Activity
2.5. Antioxidant and Anti-Mitochondrial Damage
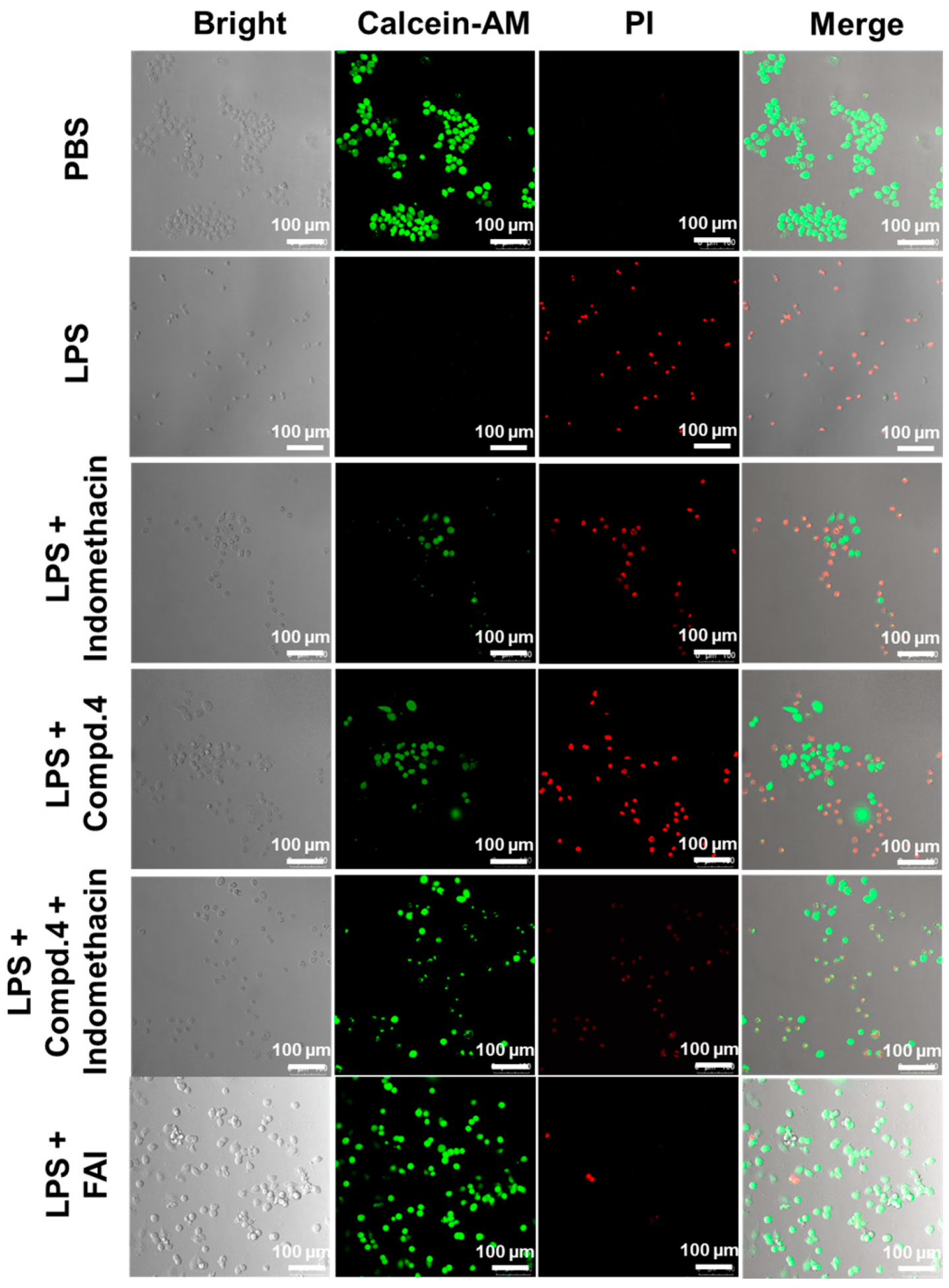
2.6. The Protective Influence of FAI on LPS-Treated RAW264.7 Cells
2.7. Activity and Imaging of FAI In Vivo
2.8. H&E and iNOS Immunofluorescence Staining
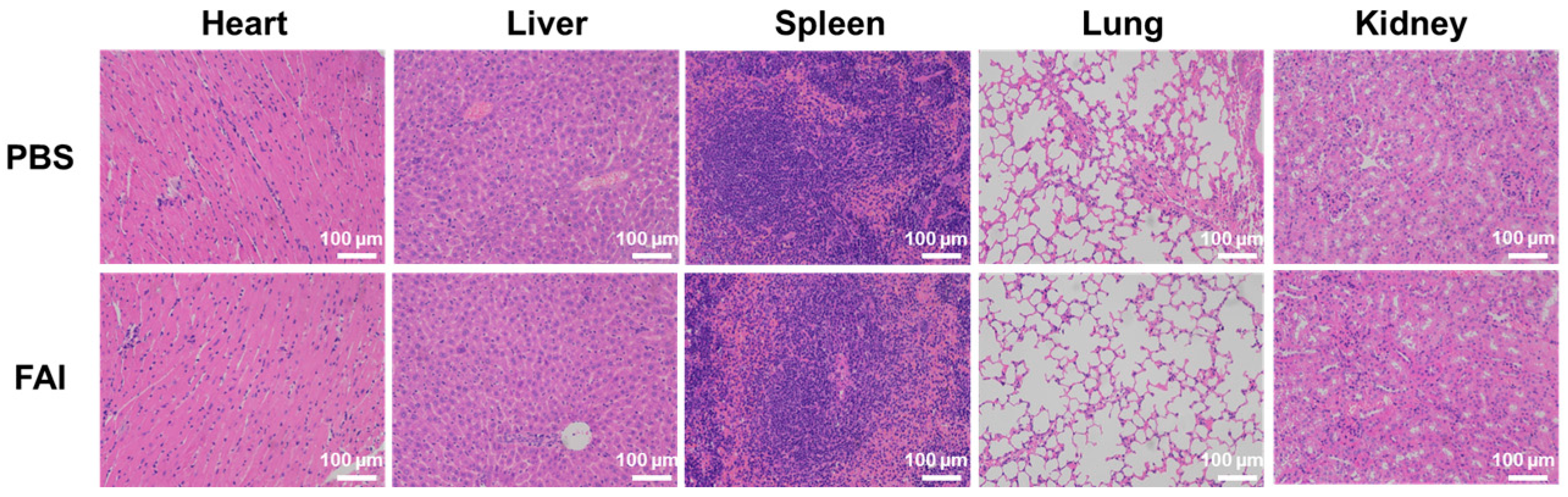
2.9. In Vivo Biosafety Evaluation of FAI
3. Materials and Methods
3.1. Experimental Procedures
3.2. Synthesis Procedure of Compound FAI
3.2.1. Synthesis of Compound 3
3.2.2. Synthesis of Compound 4
3.2.3. Synthesis of Compound 7
3.2.4. Synthesis of Compound 8
3.2.5. Synthesis of Compound 9
3.2.6. Synthesis of Compound 10
3.2.7. Synthesis of FAI
3.3. UV and Fluorescence Spectra
3.4. Characteristics of the Spectral Response of FAI to Azo Reductase
3.5. High-Performance Liquid Chromatography
3.6. Computational Methods of HOMO and LUMO
3.7. Molecular Docking
3.8. Cell Culture
3.9. Cell Cytotoxicity
3.10. NO Assay
3.11. In Vitro ROS-Scavenging Capacity of FAI
3.12. Evaluation of Mitochondrial Membrane Potential
3.13. Live/Dead Cell Staining
3.14. In Vivo Fluorescence Imaging
3.15. Levels of IL-6, TNF-α, and Calprotectin
3.16. H&E Staining
3.17. Immunofluorescence Staining
3.18. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Y.Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tang, X.D.; Zhang, L. Huangqin-Tang and Ingredients in Modulating the Pathogenesis of Ulcerative Colitis. Evid. Based Compl. Alt. 2017, 2017, 7016468. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A. Azoreductases in drug metabolism. Brit. J. Pharmacol. 2017, 174, 2161–2173. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Y.F.; Jiang, W.L.; Zhou, D.Y.; Fei, J.J.; Li, C.Y. In-Situ Imaging of Azoreductase Activity in the Acute and Chronic Ulcerative Colitis Mice by a Near-Infrared Fluorescent Probe. Anal. Chem. 2019, 91, 10901–10907. [Google Scholar] [CrossRef]
- Li, S.J.; Li, C.Y.; Li, Y.F.; Fei, J.J.; Wu, P.; Yang, B.; Ou-Yang, J.; Nie, S.X. Facile and Sensitive Near-Infrared Fluorescence Probe for the Detection of Endogenous Alkaline Phosphatase Activity In Vivo. Anal. Chem. 2017, 89, 6854–6860. [Google Scholar] [CrossRef] [PubMed]
- Juan, O.Y.; Li, Y.F.; Jiang, W.L.; He, S.Y.; Liu, H.W.; Li, C.Y. Fluorescence-Guided Cancer Diagnosis and Surgery by a Zero Cross Talk Ratiometric Near-Infrared γ-Glutamyltranspeptidase Fluorescent Probe. Anal. Chem. 2019, 91, 1056–1063. [Google Scholar]
- Kiyose, K.; Hanaoka, K.; Oushiki, D.; Nakamura, T.; Kajimura, M.; Suematsu, M.; Nishimatsu, H.; Yamane, T.; Terai, T.; Hirata, Y.; et al. Hypoxia-Sensitive Fluorescent Probes for Real-Time Fluorescence Imaging of Acute Ischemia. J. Am. Chem. Soc. 2010, 132, 15846–15848. [Google Scholar] [CrossRef]
- Cai, Q.; Yu, T.; Zhu, W.P.; Xu, Y.F.; Qian, X.H. A turn-on fluorescent probe for tumor hypoxia imaging in living cells. Chem. Commun. 2015, 51, 14739–14741. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, S.P.; Huang, J.H.; Cui, L.; Hu, J.; Tan, S.Y. Novel designed azo substituted semi-cyanine fluorescent probe for cytochrome P450 reductase detection and hypoxia imaging in cancer cells. RSC Adv. 2019, 9, 21572–21577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Baumann, R.P.; Penketh, P.G.; Shyam, K.; Sartorelli, A.C. Hypoxia-Selective-Alkylguanine-DNA Alkyltransferase Inhibitors: Design, Synthesis, and Evaluation of 6-(Benzyloxy)-2-(aryldiazenyl)-9-purines as Prodrugs of Benzylguanine. J. Med. Chem. 2013, 56, 1355–1359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piao, W.; Hanaoka, K.; Fujisawa, T.; Takeuchi, S.; Komatsu, T.; Ueno, T.; Terai, T.; Tahara, T.; Nagano, T.; Urano, Y. Development of an Azo-Based Photosensitizer Activated under Mild Hypoxia for Photodynamic Therapy. J. Am. Chem. Soc. 2017, 139, 13713–13719. [Google Scholar] [CrossRef] [PubMed]
- Verwilst, P.; Han, J.; Lee, J.; Mun, S.; Kang, H.G.; Kim, J.S. Reconsidering azobenzene as a component of small-molecule hypoxia-mediated cancer drugs: A theranostic case study. Biomaterials 2017, 115, 104–114. [Google Scholar] [CrossRef]
- Hu, M.X.; Yang, C.; Luo, Y.; Chen, F.; Yang, F.F.; Yang, S.P.; Chen, H.; Cheng, Z.Q.; Li, K.; Xie, Y.M. A ypoxia-specific and mitochondria-targeted anticancer theranostic agent with high selectivity for cancer cells. J. Mater. Chem. B 2018, 6, 4385. [Google Scholar] [CrossRef]
- Zhou, Y.; Maiti, M.; Sharma, A.; Won, M.; Yu, L.; Miao, L.X.; Shin, J.; Podder, A.; Bobba, K.N.; Han, J.; et al. Azo-based small molecular hypoxia responsive theranostic for tumor-specific imaging and therapy. J. Control. Release 2018, 288, 14–22. [Google Scholar] [CrossRef]
- van Brakel, R.; Vulders, R.C.M.; Bokdam, R.J.; Grüll, H.; Robillard, M.S. A doxorubicin prodrug activated by the staudinger reaction. Bioconjug. Chem. 2008, 19, 714–718. [Google Scholar] [CrossRef]
- Li, S.Y.; Jiang, X.Y.; Zheng, R.R.; Zuo, S.J.; Zhao, L.P.; Fan, G.L.; Fan, J.H.; Liao, Y.H.; Yu, X.Y.; Cheng, H. An azobenzene-based heteromeric prodrug for hypoxia-activated chemotherapy by regulating subcellular localization. Chem. Commun. 2018, 54, 7983–7986. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.L.; Zeng, F.; Wu, S.Z. An Activatable Near-Infrared Chromophore for Multispectral Optoacoustic Imaging of Tumor Hypoxia and for Tumor Inhibition. Theranostics 2019, 9, 7313–7324. [Google Scholar] [CrossRef]
- Lu, Y.X.; Xu, J.J.; Jia, Z.Y.; Kong, S.Y.; Qiao, Y.M.; Li, L.; Wu, Q.; Zhou, Y. A near-infrared multifunctional fluorescent probe for hypoxia monitoring and tumor-targeted therapy. Chin. Chem. Lett. 2022, 33, 1589–1594. [Google Scholar] [CrossRef]
- Guisán-Ceinos, S.; Rivero, A.R.; Romeo-Gella, F.; Simón-Fuente, S.; Gómez-Pastor, S.; Calvo, N.; Orrego, A.H.; Guisan, J.M.; Corral, I.; Sanz-Rodriguez, F.; et al. Turn-on Fluorescent Biosensors for Imaging Hypoxia-like Conditions in Living Cells. J. Am. Chem. Soc. 2022, 144, 8185–8193. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Kai, H.; Obuchi, M.; Yoshida, H.; Watanabe, W.; Tsutsumi, S.; Park, Y.K.; Matsuno, K.; Yasukawa, K.; Kurokawa, M. In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08). J. Funct. Foods 2014, 8, 214–223. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell B 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- El-Deeb, O.S.; Elesawy, R.O.; Eltokhy, A.K.; Al-Shenawy, H.A.; Ghanem, H.B.; Rizk, F.H.; Barhoma, R.A.; Shalaby, R.H.; Abdelsattar, A.M.; Mashal, S.S.; et al. Moderating Gut Microbiome/Mitochondrial Axis in Oxazolone Induced Ulcerative Colitis: The Evolving Role of β-Glucan and/or, Aldose Reductase Inhibitor, Fidarestat. Int. J. Mol. Sci. 2023, 24, 2711. [Google Scholar] [CrossRef]
- Sosnovski, K.E.; Braun, T.; Amir, A.; Moshel, D.; BenShoshan, M.; VanDussen, K.L.; Levhar, N.; Abbas-Egbariya, H.; Beider, K.; Ben-Yishay, R.; et al. GATA6-AS1 Regulates Intestinal Epithelial Mitochondrial Functions, and its Reduced Expression is Linked to Intestinal Inflammation and Less Favourable Disease Course in Ulcerative Colitis. J. Crohn’s Colitis 2023, 17, 960–971. [Google Scholar] [CrossRef] [PubMed]





| Compd. | IC 50 ± SD (μM) | |
|---|---|---|
| Cytotoxicity a | NO Production b | |
| 4 | 897 ± 25.8 | 12.8 ± 1.08 |
| Indomethacin | 369 ± 20.3 | 10.3 ± 0.82 |
| 4 + Indomethacin | 404 ± 19.8 | 5.9 ± 0.56 |
| FAI | 887 ± 29.7 | 5.1 ± 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Yang, R.; Chen, J.; Fan, Y.; Xie, W.; Wu, H.; Ding, J. Design and Synthesis of an Azo Reductase Responsive Flavonol–Indomethacin Hybrid Used for the Diagnosis and Treatment of Colitis. Molecules 2024, 29, 4244. https://doi.org/10.3390/molecules29174244
Gu Y, Yang R, Chen J, Fan Y, Xie W, Wu H, Ding J. Design and Synthesis of an Azo Reductase Responsive Flavonol–Indomethacin Hybrid Used for the Diagnosis and Treatment of Colitis. Molecules. 2024; 29(17):4244. https://doi.org/10.3390/molecules29174244
Chicago/Turabian StyleGu, Yaqin, Rui Yang, Jine Chen, Yu Fan, Wenna Xie, Hongyan Wu, and Jinfeng Ding. 2024. "Design and Synthesis of an Azo Reductase Responsive Flavonol–Indomethacin Hybrid Used for the Diagnosis and Treatment of Colitis" Molecules 29, no. 17: 4244. https://doi.org/10.3390/molecules29174244
APA StyleGu, Y., Yang, R., Chen, J., Fan, Y., Xie, W., Wu, H., & Ding, J. (2024). Design and Synthesis of an Azo Reductase Responsive Flavonol–Indomethacin Hybrid Used for the Diagnosis and Treatment of Colitis. Molecules, 29(17), 4244. https://doi.org/10.3390/molecules29174244





