Effect of Antisolvent Used to Regenerate Cellulose Treated with Ionic Liquid on Its Properties
Abstract
1. Introduction
2. Results and Discussion
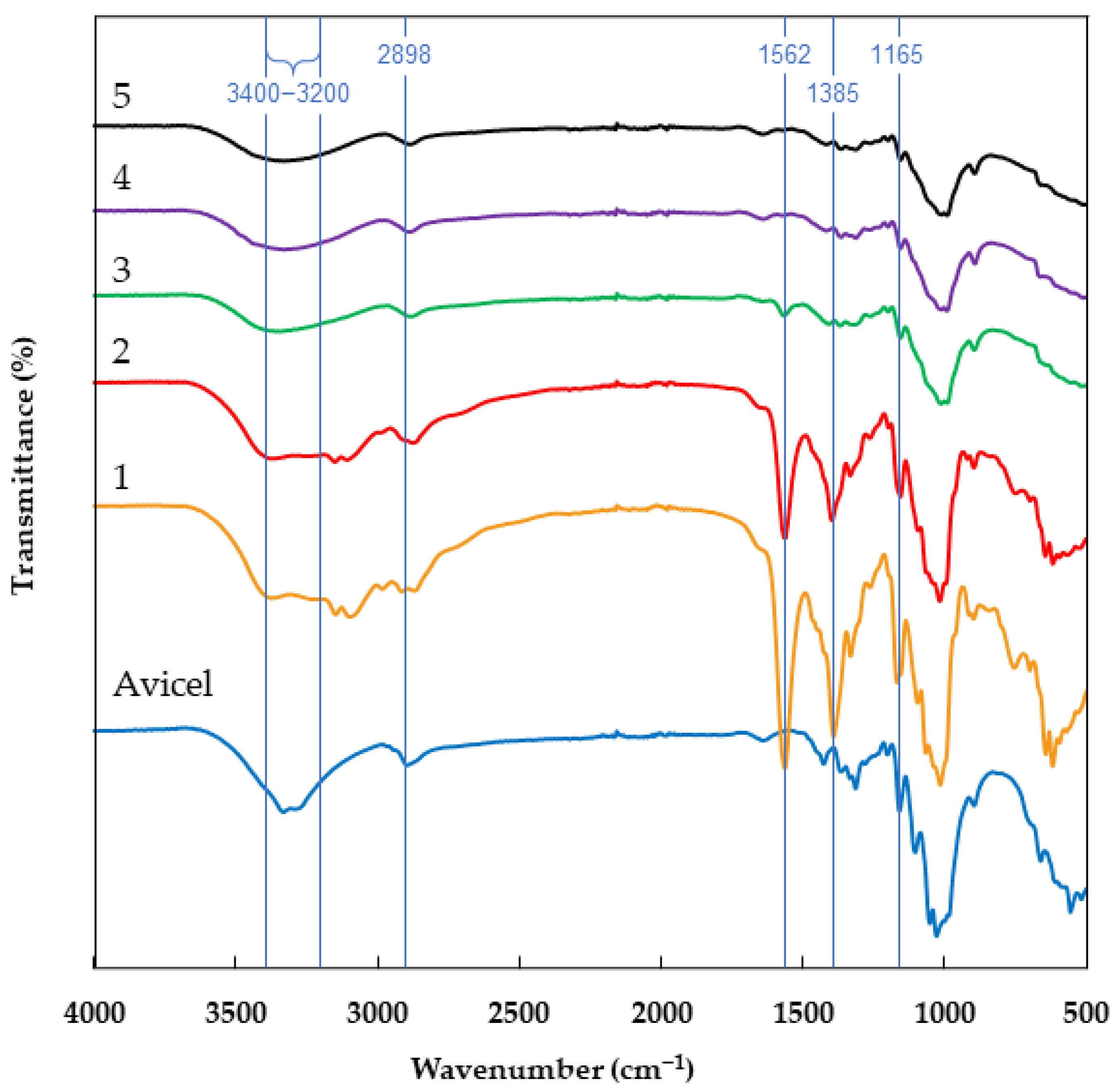
2.1. Infrared Spectroscopy (ATR-FTIR)
2.2. Elemental Analysis (EA)
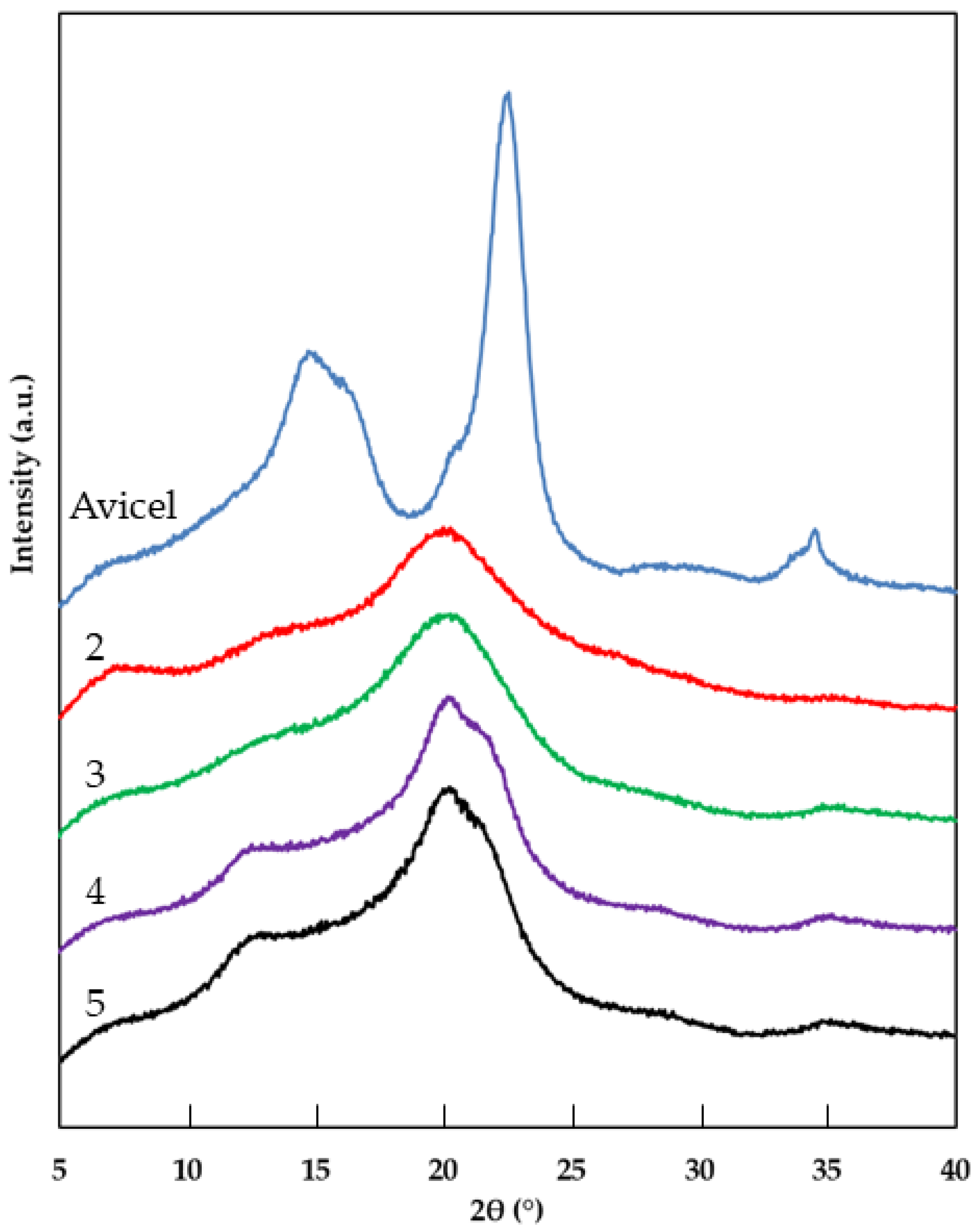
2.3. X-ray Diffraction (XRD)
2.4. Dynamic Light Scattering (DLS)
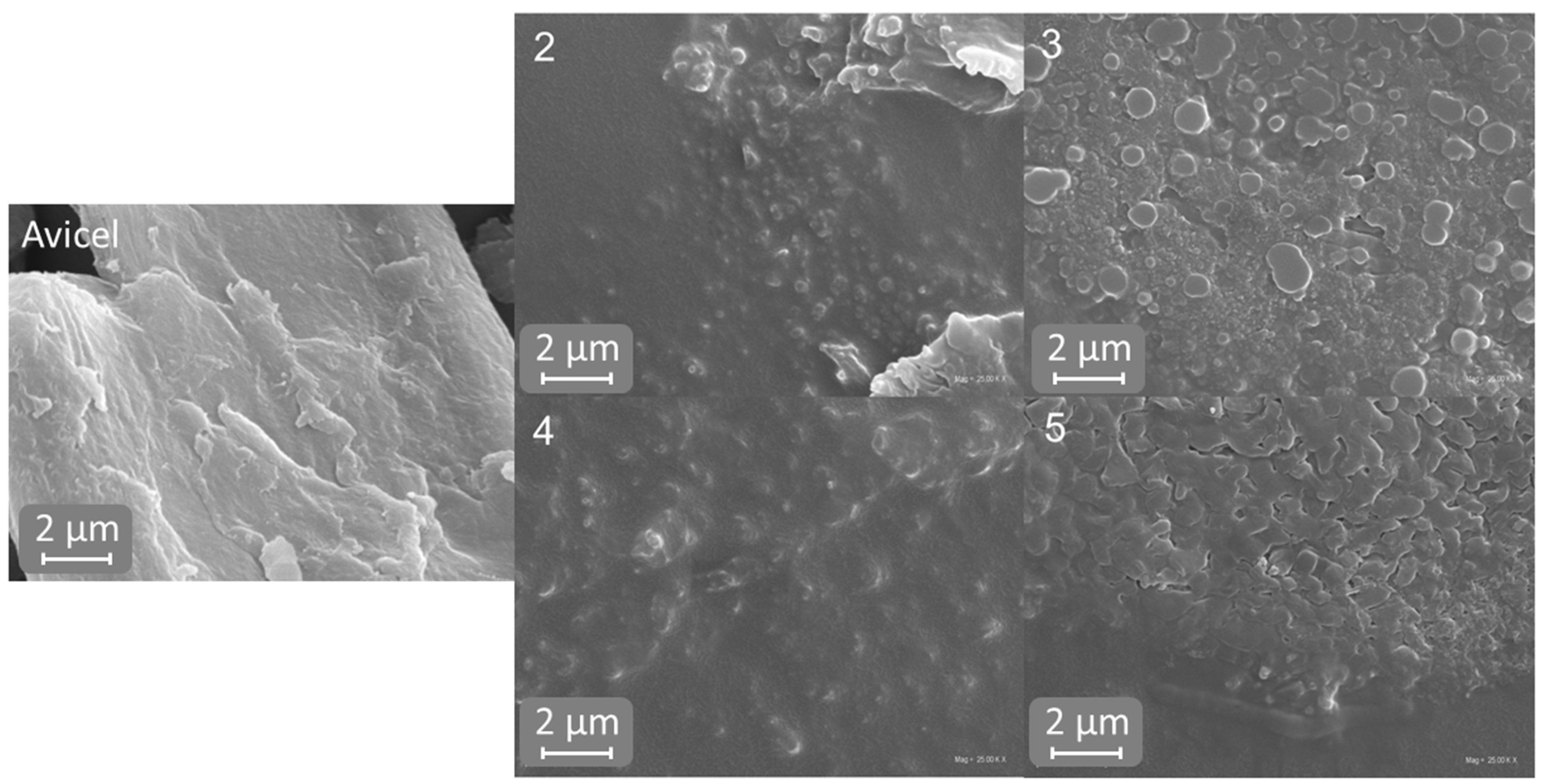
2.5. Scanning Electron Microscopy (SEM)
3. Materials and Methods
3.1. Materials
3.2. Nanocellulose Preparation
3.3. Characterization of Cellulose
3.3.1. Infrared Spectroscopy
3.3.2. Elemental Analysis
3.3.3. X-ray Diffraction (XRD) Technique
3.3.4. Dynamic Light Scattering (DLS) Method
3.3.5. Scanning Electron Microscopy (SEM) Technique
3.3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind. Crops Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose: Preparation methods and applications cellulose-reinforced nanofibre composites: Production, properties and applications. Cellul.-Reinf. Nanofibre Compos. 2017, 261–276. [Google Scholar] [CrossRef]
- Phanthong, P.; Karnjanakom, S.; Reubroycharoen, P.; Hao, X.; Abudula, A.; Guan, G. A facile one-step way for extraction of nanocellulose with high yield by ball milling with ionic liquid. Cellulose 2017, 24, 2083–2093. [Google Scholar] [CrossRef]
- Stefanowska, K.; Bucher, M.; Reichert, C.L.; Sip, A.; Woźniak, M.; Schmid, M.; Dobrucka, R.; Ratajczak, I. Chitosan-based films with nanocellulose and propolis as active packaging materials. Ind. Crops Prod. 2024, 219, 119112. [Google Scholar] [CrossRef]
- Favatela, F.; Horst, M.F.; Bracone, M.; Gonzalez, J.; Alvarez, V.; Lassalle, V. GELATIN/CELLULOSE nanowhiskers hydrogels intended for the administration of drugs in dental treatments: Study of lidocaine as model case. J. Drug Deliv. Sci. Technol. 2021, 61, 101886. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Garbarczyk, J.; Borysiak, S. Polypropylene—Cellulose fibres composites. Part I. Influence of conditions of extrusion and injection processes on the structure of polypropylene matrix. Polimery/Polymers 2004, 49, 541–546. [Google Scholar] [CrossRef]
- Nyamayaro, K.; Keyvani, P.; D’Acierno, F.; Poisson, J.; Hudson, Z.M.; Michal, C.A.; Madden, J.D.W.; Madden, S.G.; Mehrkhodavandi, P. Toward Biodegradable Electronics: Ionic Diodes Based on a Cellulose Nanocrystal–Agarose Hydrogel. ACS Appl. Mater. Interfaces 2020, 12, 52182–55219. [Google Scholar] [CrossRef]
- Ghahari, S.; Assi, L.N.; Alsalman, A.; Alyamaç, K.E. Fracture Properties Evaluation of Cellulose Nanocrystals Cement Paste. Materials 2020, 13, 2507. [Google Scholar] [CrossRef] [PubMed]
- Haron, G.A.S.; Noh, H.B.; Moniruzzaman, M. Ionic liquid-assisted nanocellulose preparation from microcrystalline cellulose. J. Phys. Conf. Ser. 2021, 1793, 012046. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 7272, 1391–1398. [Google Scholar] [CrossRef]
- Sashina, E.S.; Novoselov, N.P.; Kuz’mina, O.G.; Troshenkova, S.V. Ionic liquids as new solvents of natural polymers. Fibre Chem. 2008, 40, 270–277. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Bartkowiak, M.; Peplińska, B.; Waliszewska, H.; Borysiak, S.; Ratajczak, I. Miscanthus and Sorghum as sustainable biomass sources for nanocellulose production. Ind. Crops Prod. 2022, 186, 115177. [Google Scholar] [CrossRef]
- Grząbka-Zasadzińska, A.; Skrzypczak, A.; Borysiak, S. The influence of the cation type of ionic liquid on the production of nanocrystalline cellulose and mechanical properties of chitosan-based biocomposites. Cellulose 2019, 26, 4827–4840. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.; Alam, M.Z.; Moniruzzaman, M. Ionic Liquids as a Sustainable Platform for Nanocellulose Processing from Bioresources: Overview and Current Status. ACS Sustain. Chem. Eng. 2021, 9, 1008–1034. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Jamil, S.N.A.; Abdan, K. Nanocellulose extraction using ionic liquids: Syntheses, processes, and properties. Front. Mater. 2022, 9, 919918. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Influence of residual ionic liquid on the thermal stability and electromechanical behavior of cellulose regenerated from 1-ethyl-3-methylimidazolium acetate. Fibers Polym. 2012, 13, 289–294. [Google Scholar] [CrossRef]
- Elhi, F.; Aid, T.; Koel, M. Ionic liquids as solvents for making composite materials from cellulose. Proc. Est. Acad. Sci. 2016, 65, 255. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Cellulose regeneration from a cellulose/ionic liquid mixture: The role of anti-solvents. RSC Adv. 2013, 3, 12794. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Lee, Y.Y.; Gupta, R.B. Cellulose pretreatment in subcritical water: Effect of temperature on molecular structure and enzymatic reactivity. Bioresour. Technol. 2010, 101, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Szentner, K.; Bartkowiak, M.; Peplińska, B.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Nanocellulose production using ionic liquids with enzymatic pretreatment. Materials 2021, 14, 3264. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yashchenko, O.V.; Vasylieva, O.A. Preparation and properties of nanocellulose from Miscanthus x giganteus. J. Nanomater. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Panahian, P.; Salami-Kalajahi, M.; Hosseini, M.S. Synthesis of dual thermoresponsive and pH-sensitive hollow nanospheres by atom transfer radical polymerization. J. Polym. Res. 2014, 21, 455. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 10th Joint Conference on Chemistry, Solo, Indonesia, 8–9 September 2015; IOP Publishing: Bristol, UK, 2016; Volume 107, p. 012045. [Google Scholar]
- Kawalerczyk, J.; Walkiewicz, J.; Dziurka, D.; Mirski, R.; Brózdowski, J. APTES-Modified nanocellulose as the formaldehyde scavenger for UF adhesive-bonded particleboard and strawboard. Polymers 2022, 14, 5037. [Google Scholar] [CrossRef]
- Williams, M.L.; Holahan, S.P.; McCorkill, M.E.; Dickmann, J.S.; Kiran, E. Thermal and spectral characterization and stability of mixtures of ionic liquids [EMIM]Ac and [BMIM]Ac with ethanol, methanol, and water at ambient conditions and at elevated temperatures and pressures. Thermochim. Acta 2018, 669, 126–139. [Google Scholar] [CrossRef]
- Castro, J.M.; Montalbán, M.G.; Domene-López, D.; Martín-Gullón, I.; García-Quesada, J.C. Study of the plasticization effect of 1-ethyl-3-methylimidazolium acetate in TPS/PVA biodegradable blends produced by melt-mixing. Polymers 2023, 15, 1788. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Xie, F.; Li, X.; Truss, R.W.; Halley, P.J.; Shamshina, J.L.; Rogers, R.D.; McNally, T. Understanding the structural disorganization of starch in water–ionic liquid solutions. Phys. Chem. Chem. Phys. 2015, 17, 13860–13871. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Kumar, M.P.; Gupta, S.; Sunder, M.S.; Mohana Rao, K.V.; Jagadeesh, B.; Swapna, V.; Kamal, A. Isolation and characterization of cellulose from sweet Sorghum bagasse. Sugar Tech 2014, 17, 395–403. [Google Scholar] [CrossRef]
- Borysiak, S. Influence of cellulose polymorphs on the polypropylene crystallization. J. Therm. Anal. Calorim. 2013, 113, 281–289. [Google Scholar] [CrossRef][Green Version]
- Cheng, G.; Varanasi, P.; Li, C.; Liu, H.; Melnichenko, Y.B.; Simmons, B.A.; Kent, M.S.; Singh, S. Transition of Cellulose Crystalline Structure and Surface Morphology of Biomass as a Function of Ionic Liquid Pretreatment and Its Relation to Enzymatic Hydrolysis. Biomacromolecules 2011, 12, 933–941. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, F.; Wang, Q.; Zhang, Y.; Kong, L. Homogeneous isolation of nanocellulose from eucalyptus pulp by high pressure homogenization. Ind. Crops Prod. 2017, 104, 237–241. [Google Scholar] [CrossRef]
- Lazko, J.; Sénéchal, T.; Bouchut, A.; Paint, Y.; Dangreau, L.; Fradet, A.; Tessier, M.; Raquez, J.M.; Dubois, P. Acid-free extraction of cellulose type I nanocrystals using Brønsted acid-type ionic liquids. Nanocomposites 2016, 2, 65–75. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Guo, W.; Ma, F.; Sun, S.; Zhou, Q. Crystallinity of regenerated cellulose from [Bmim]Cl dependent on the hydrogen bond acidity/basicity of anti-solvents. RSC Adv. 2017, 7, 41004–41010. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Preparation of nanocellulose using ionic liquids: 1-propyl-3- methylimidazolium chloride and 1-ethyl-3-methylimidazolium chloride. Molecules 2020, 25, 1544. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; French, A.D.; Han, G.; Wu, Q. Characterization of cellulose II nanoparticles regenerated from 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2013, 94, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, X.; Hao, M.; Huang, C.; Xue, Z.; Mu, T. Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr. Polym. 2015, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hindeleh, A.M.; Johnson, D.J. The resolution of multipeak data in fibre science. J. Phys. D Appl. Physic. 1971, 4, 259–263. [Google Scholar] [CrossRef]
- Rabiej, S. A comparison of two X-ray diffraction procedures for crystallinity determination. Eur. Polym. J. 1991, 27, 947–954. [Google Scholar] [CrossRef]

| Symbol | N (%) |
|---|---|
| Avicel | nd |
| 1 (acetone) | 7.759 a ± 0.041 |
| 2 (acetonitrile) | 4.520 b ± 0.245 |
| 3 (ethanol) | 0.722 c ± 0.118 |
| 4 (water) | 0.248 c ± 0.039 |
| 5 (acetone/water) | 0.278 c ± 0.053 |
| Symbol | Xc (%) |
|---|---|
| Avicel | 66 |
| 2 (acetonitrile) | 29 |
| 3 (ethanol) | 33 |
| 4 (water) | 38 |
| 5 (acetone/water) | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloch, M.; Woźniak, M.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Effect of Antisolvent Used to Regenerate Cellulose Treated with Ionic Liquid on Its Properties. Molecules 2024, 29, 4227. https://doi.org/10.3390/molecules29174227
Bloch M, Woźniak M, Dwiecki K, Borysiak S, Ratajczak I. Effect of Antisolvent Used to Regenerate Cellulose Treated with Ionic Liquid on Its Properties. Molecules. 2024; 29(17):4227. https://doi.org/10.3390/molecules29174227
Chicago/Turabian StyleBloch, Marta, Magdalena Woźniak, Krzysztof Dwiecki, Sławomir Borysiak, and Izabela Ratajczak. 2024. "Effect of Antisolvent Used to Regenerate Cellulose Treated with Ionic Liquid on Its Properties" Molecules 29, no. 17: 4227. https://doi.org/10.3390/molecules29174227
APA StyleBloch, M., Woźniak, M., Dwiecki, K., Borysiak, S., & Ratajczak, I. (2024). Effect of Antisolvent Used to Regenerate Cellulose Treated with Ionic Liquid on Its Properties. Molecules, 29(17), 4227. https://doi.org/10.3390/molecules29174227








