Cucurbitacin B and Its Derivatives: A Review of Progress in Biological Activities
Abstract
1. Introduction
2. Pharmacological Activity
2.1. Antioxidant Activity
2.2. Anti-Inflammatory Activity
2.3. Neuroprotection
2.4. Cardiovascular Protection
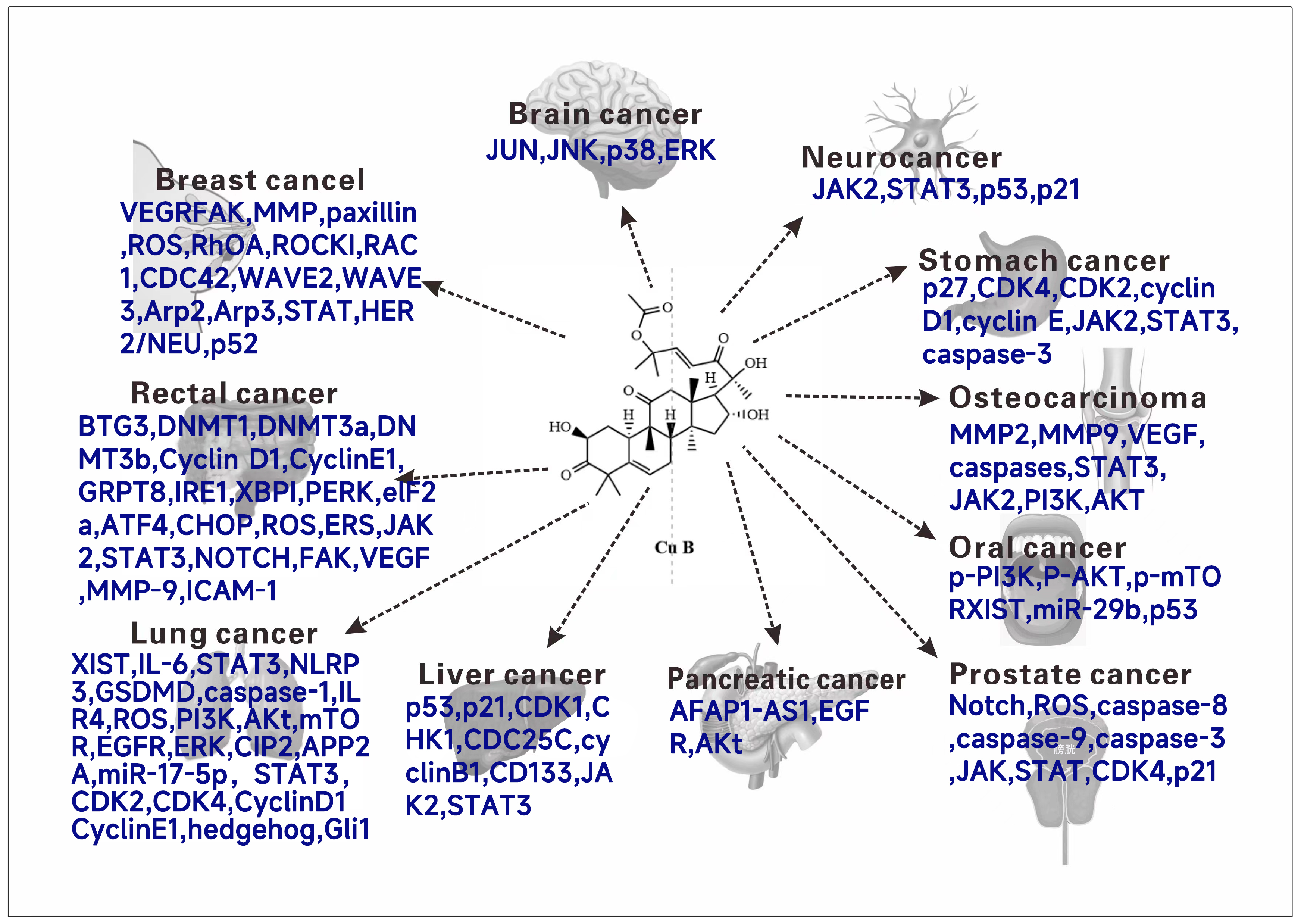
2.5. Antitumor Activity
2.5.1. Breast Cancer
2.5.2. Glial Tumor
2.5.3. Neuroblastoma
2.5.4. Gastric Cancer
2.5.5. Osteosarcoma
2.5.6. Oral Squamous Cancer
2.5.7. Prostate Cancer
2.5.8. Pancreatic Cancer
2.5.9. Hepatocellular Carcinoma
2.5.10. Lung Cancer
2.5.11. Rectal Cancer
2.5.12. Bile Duct Cancer
2.5.13. Bladder Cancer
2.5.14. Lymphoma
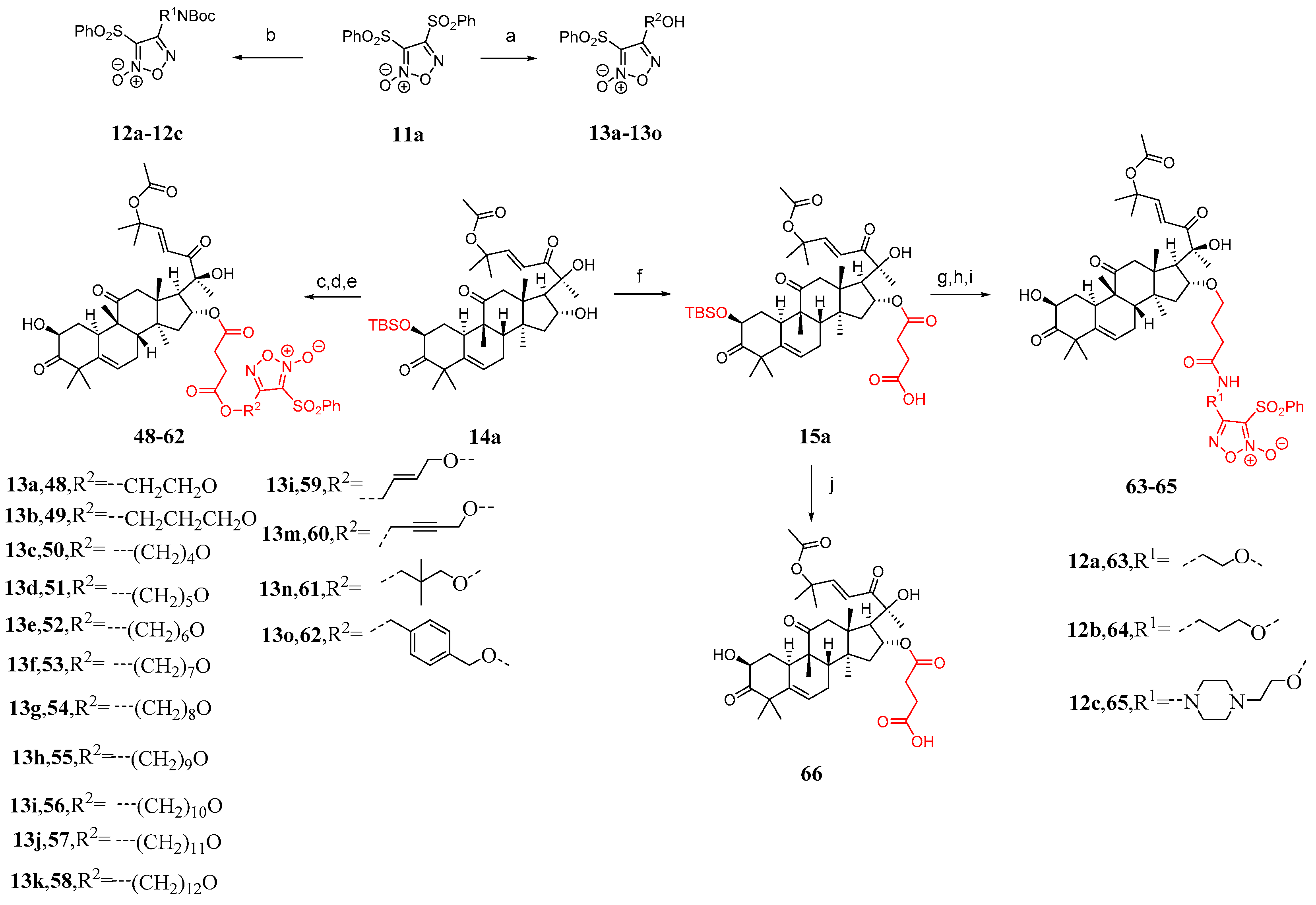
3. Research Progress of Cucurbitacin B Derivatives
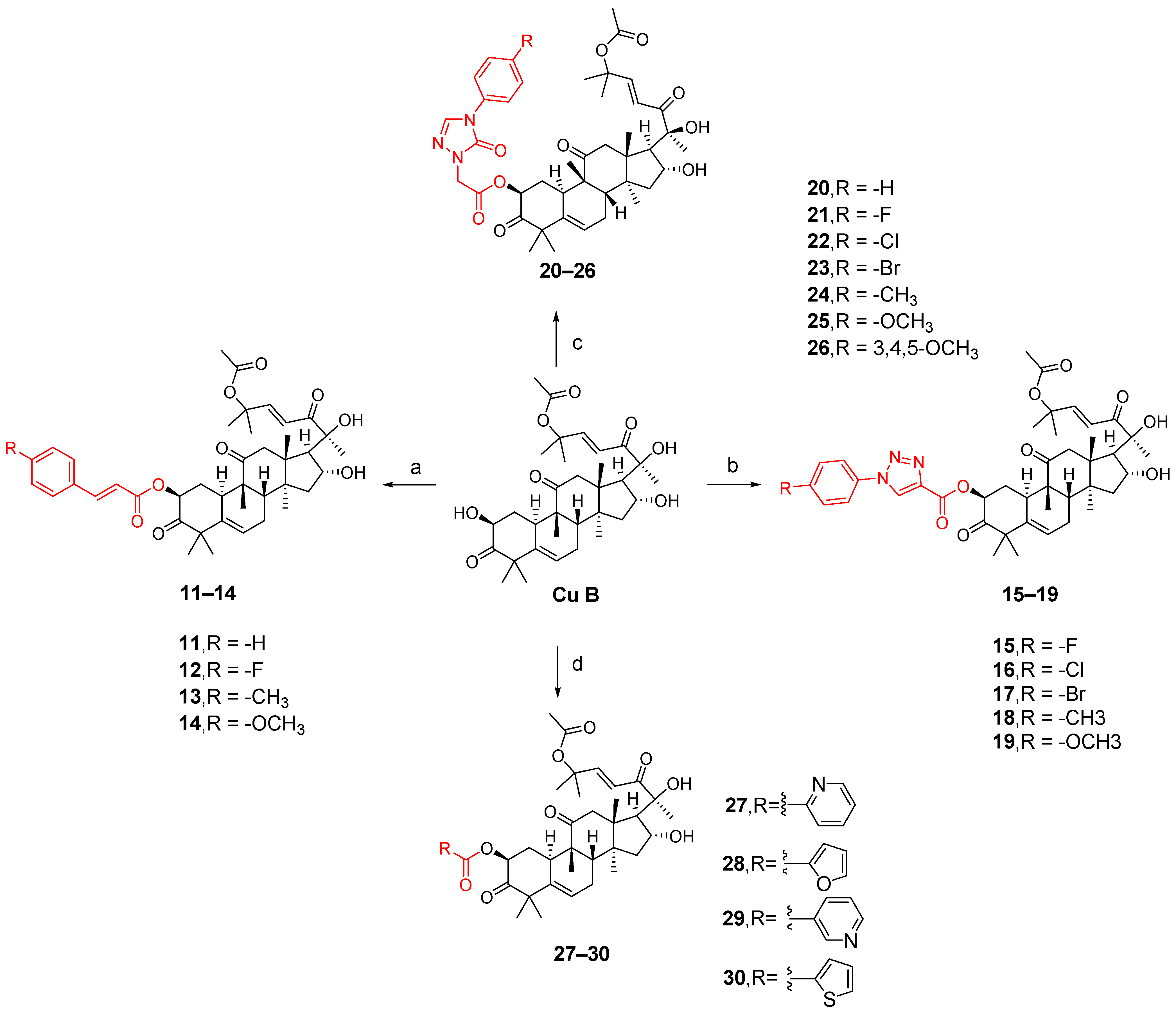
3.1. Modification and Pharmacological Activity of 2-Hydroxyl Group
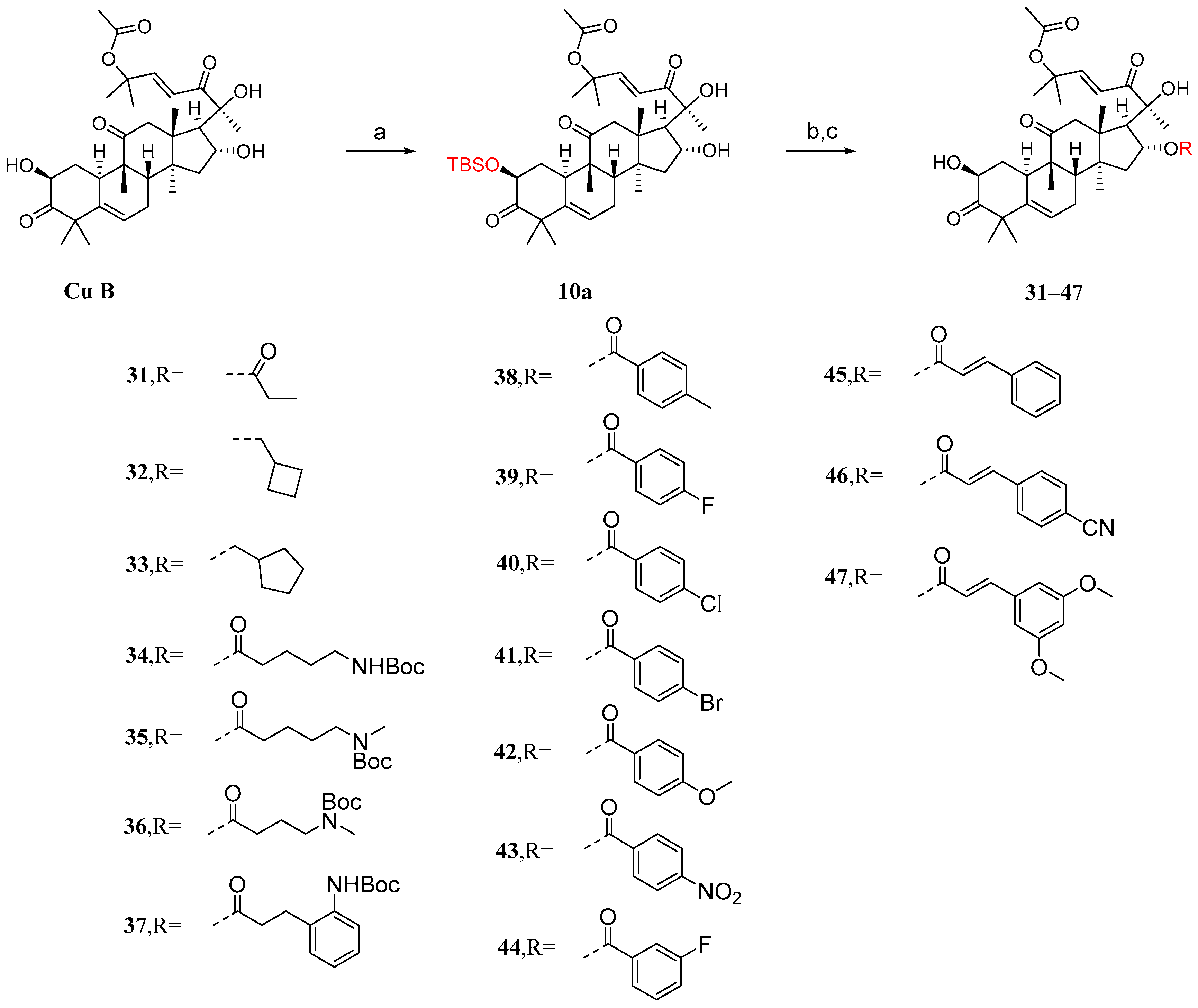
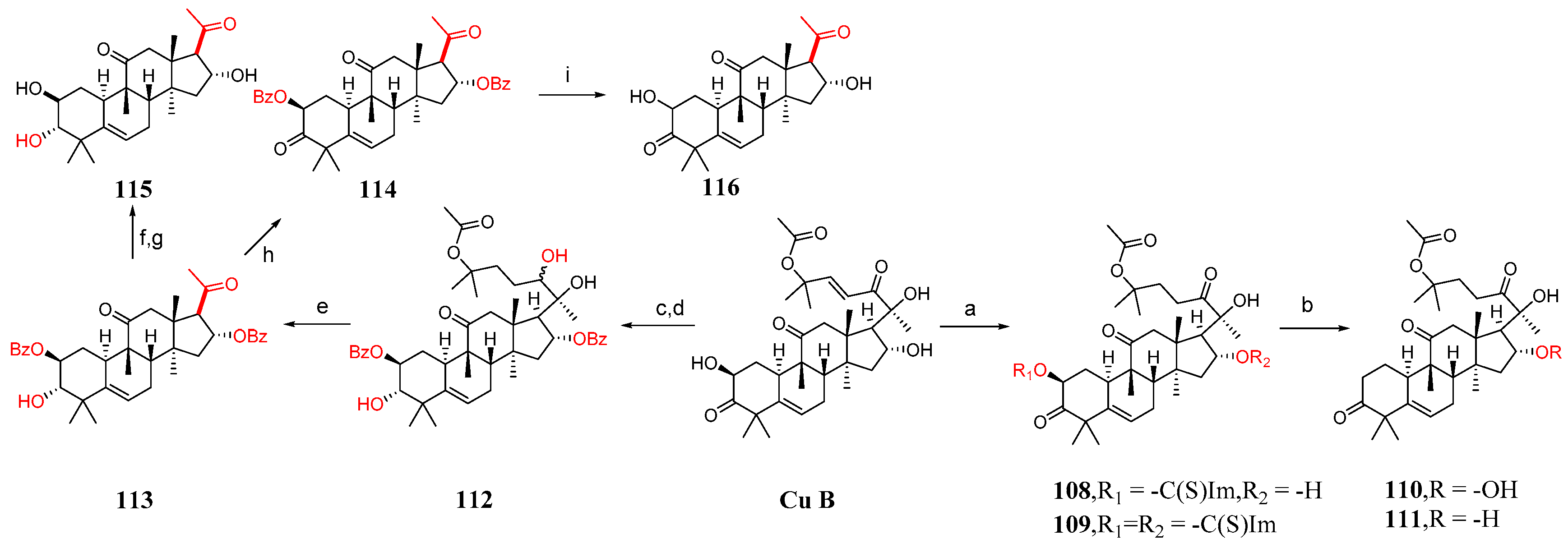
3.2. Modification and Pharmacological Activity of 16-Hydroxyl Group
3.3. Modification and Pharmacological Activity of C-25 Acetyl Group
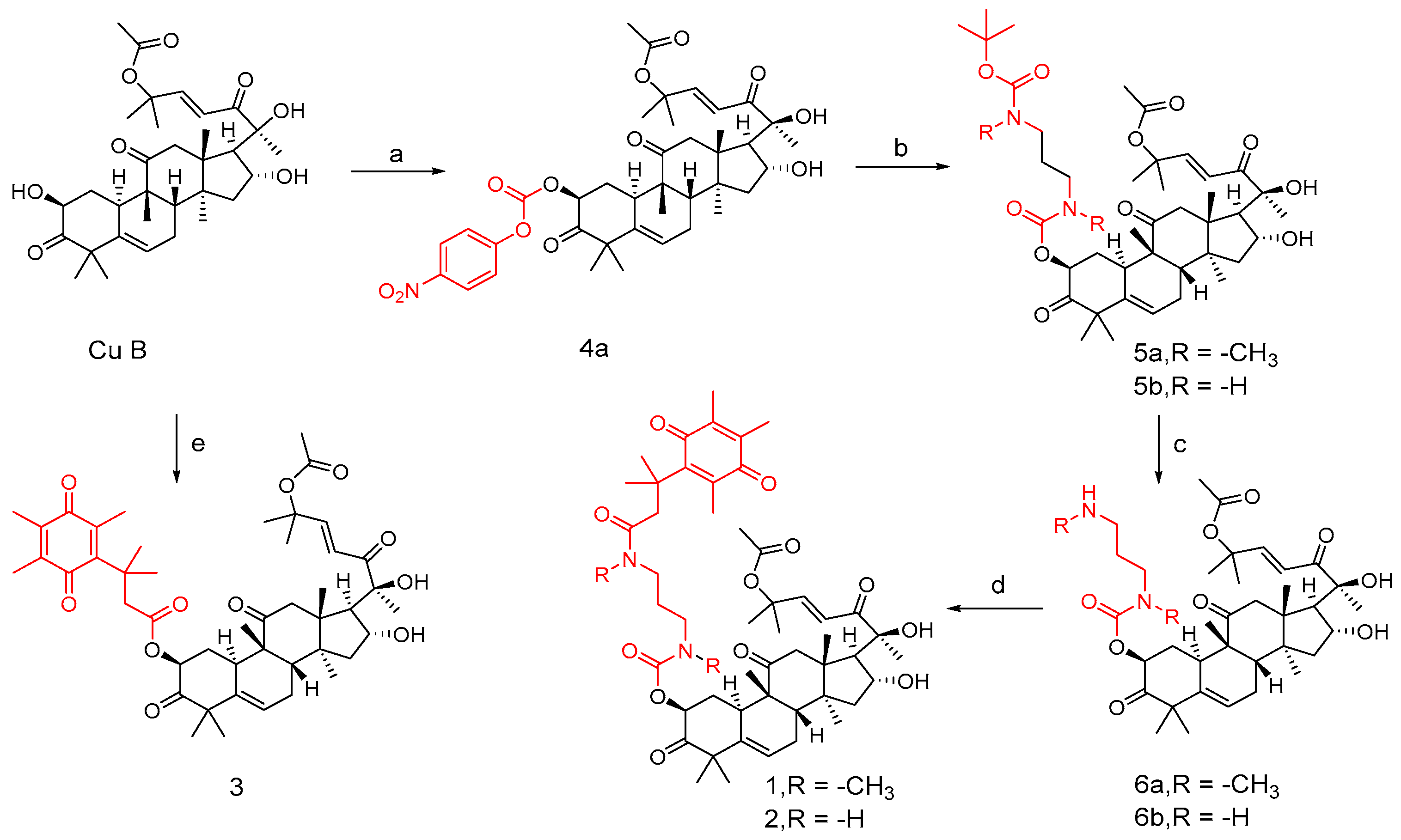
3.4. Co-Modification of 2-OH and 16-OH
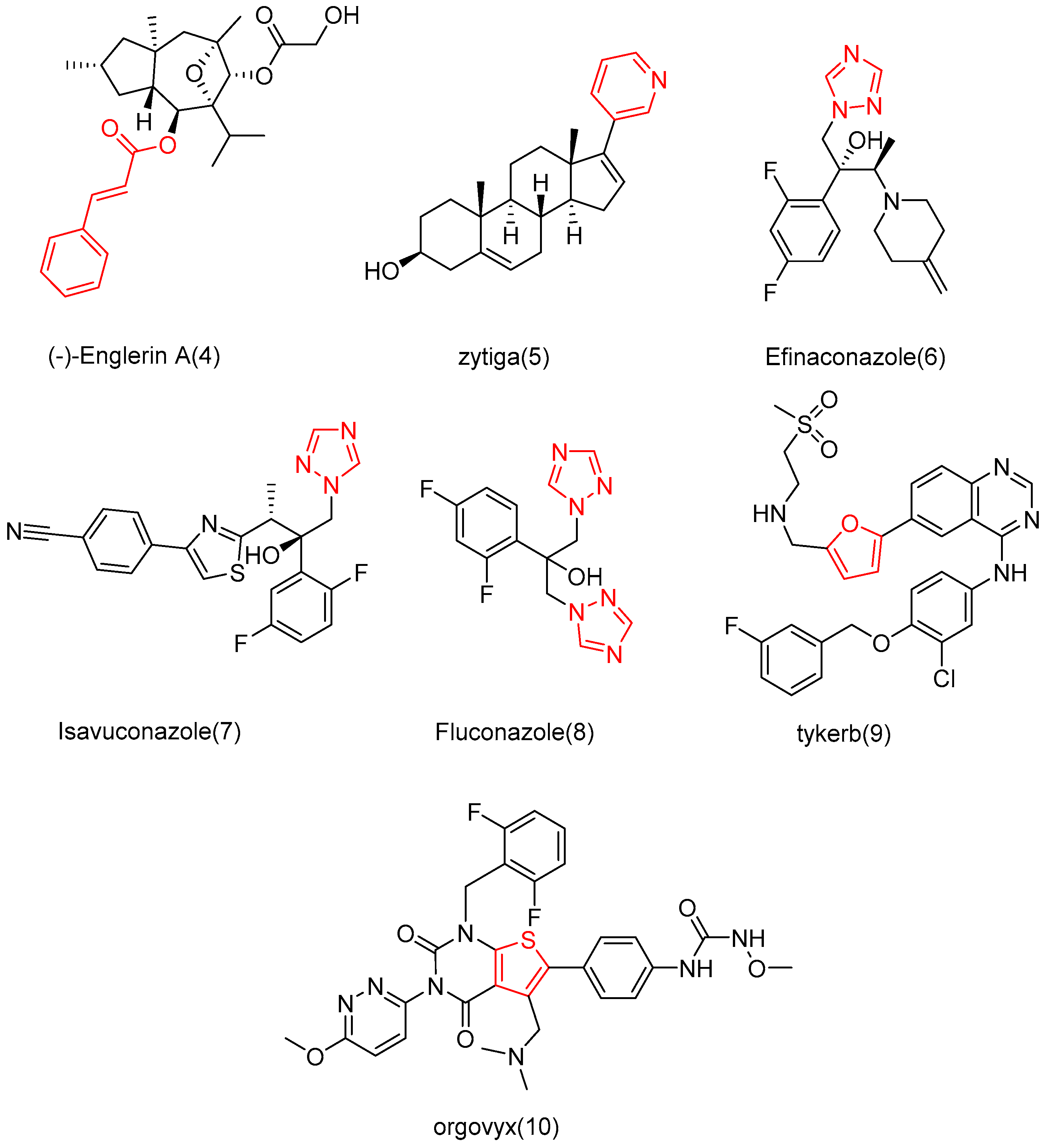
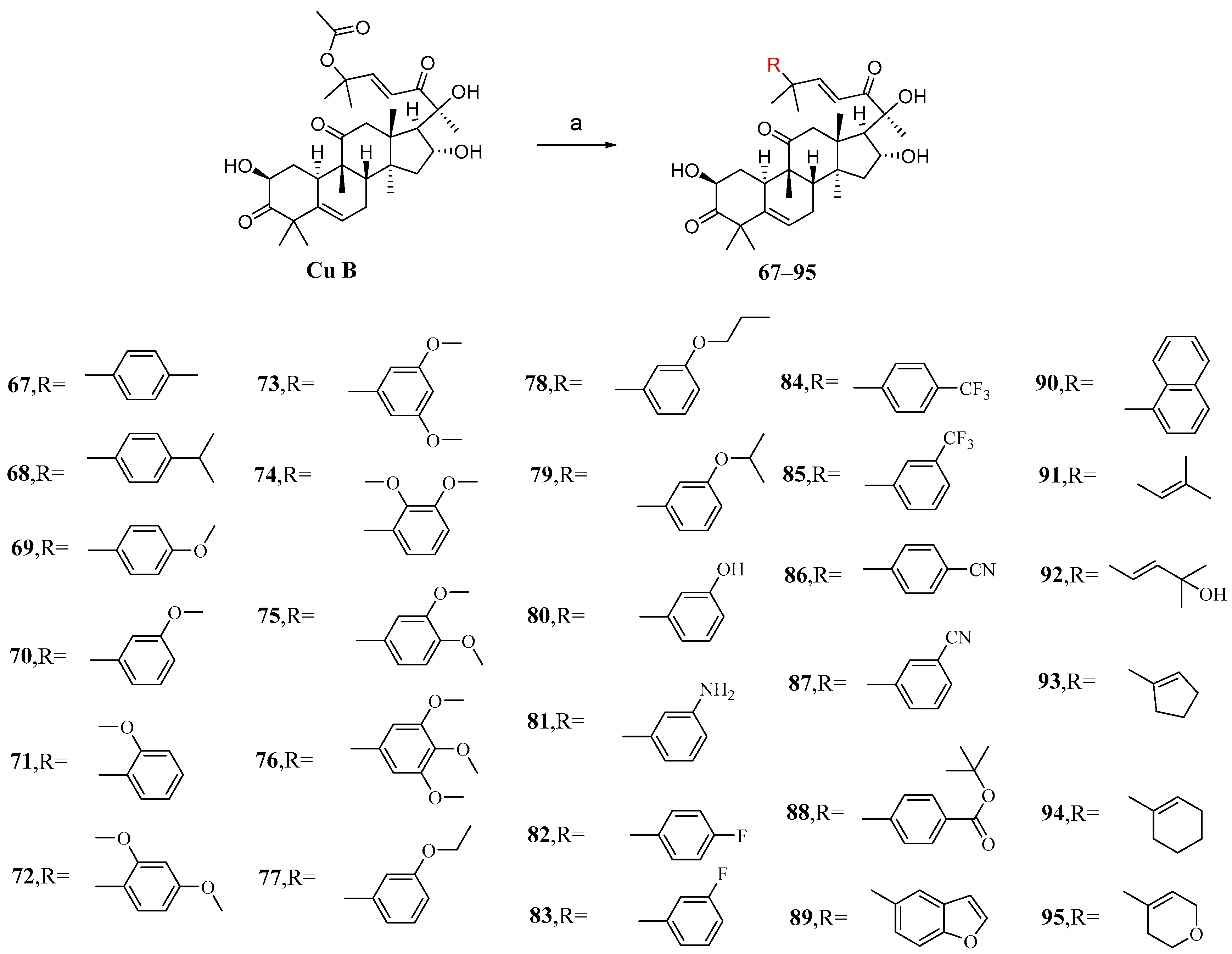
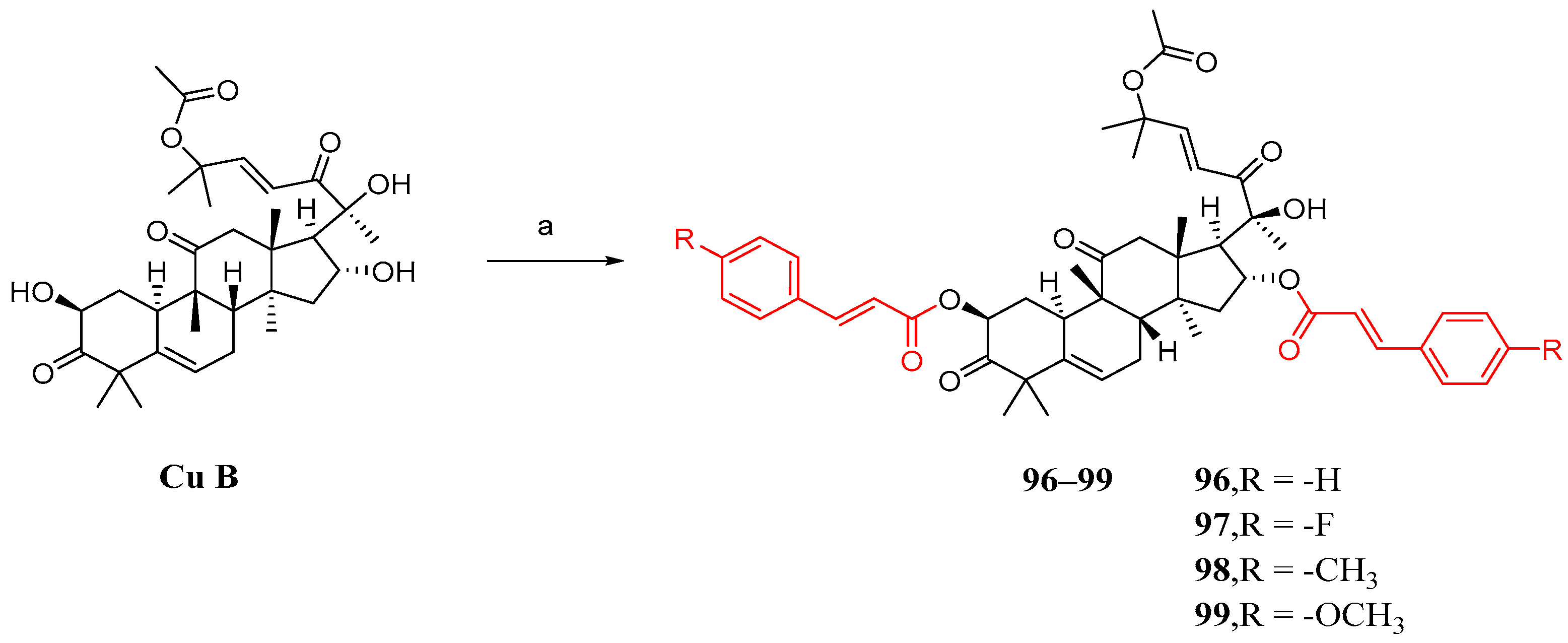
3.5. Study on Modification and Pharmacological Activity of Other Sites
4. Studies on Cucurbitacin B Preparations
5. Research Progress on Cucurbitacin B in Agronomy
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, S.; Das, S.K.; Sinha, K.; Ghosh, B.; Sen, K.; Ghosh, N.; Sil, P.C. The Emerging Role of Natural Products in Cancer Treatment. Arch. Toxicol. 2024, 98, 2353–2391. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Wen, H.; Zhu, J.; Deng, H.; Jiang, X.; Ye, X.-Y.; Wang, L.; Xie, T.; Bai, R. Discovery of plant-derived anti-tumor natural products: Potential leads for anti-tumor drug discovery. Bioorganic Chem. 2024, 142, 106957. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Alsalahi, A.; Imam, M.U.; Ooi, D.J.; Khaza’ai, H.; Aljaberi, M.A.; Shamsudin, M.N.; Idrus, Z. Safety and Neuroprotective Efficacy of Palm oil and Tocotrienol-Rich Fraction from Palm oil: A Systematic Review. Nutrients 2020, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.-R.; Jia, L.-Q.; Lei, M.; Gao, D.; Zhang, N.; Sha, L.; Liu, X.-H.; Liu, Y.-D. Natural products as pharmacological modulators of mitochondrial dysfunctions for the treatment of diabetes and its complications: An update since 2010. Pharmacol. Res. 2024, 200, 107054. [Google Scholar] [CrossRef]
- Huang, X.; Deng, H.; Shen, Q.-K.; Quan, Z.-S. Tanshinone IIA: Pharmacology, Total Synthesis, and Progress in Structure-modifications. Curr. Med. Chem. 2022, 29, 1959–1989. [Google Scholar] [CrossRef]
- Luo, Z.; Yin, F.; Wang, X.; Kong, L. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195–211. [Google Scholar] [CrossRef]
- Nabi, N.; Singh, S.; Saffeullah, P. An updated review on distribution, biosynthesis and pharmacological effects of artemisinin: A wonder drug. Phytochemistry 2023, 214, 113798. [Google Scholar] [CrossRef]
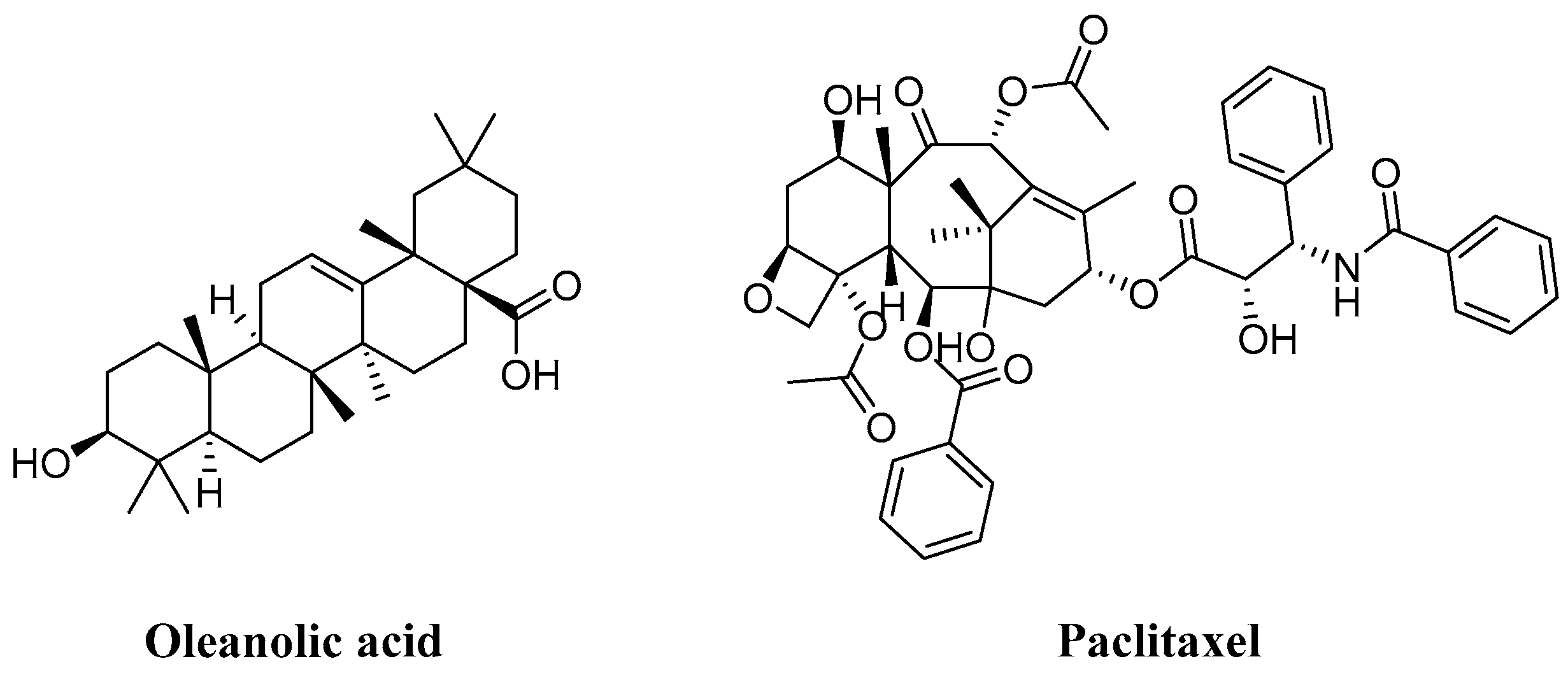
- Ghiulai, R.; Rosca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macasoi, I.; Olariu, T.; Dehelean, C.; Cretu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef]
- Fernandez-Aparicio, A.; Correa-Rodriguez, M.; Castellano, J.M.; Schmidt-RioValle, J.; Perona, J.S.; Gonzalez-Jimenez, E. Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1517. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxidative Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef] [PubMed]
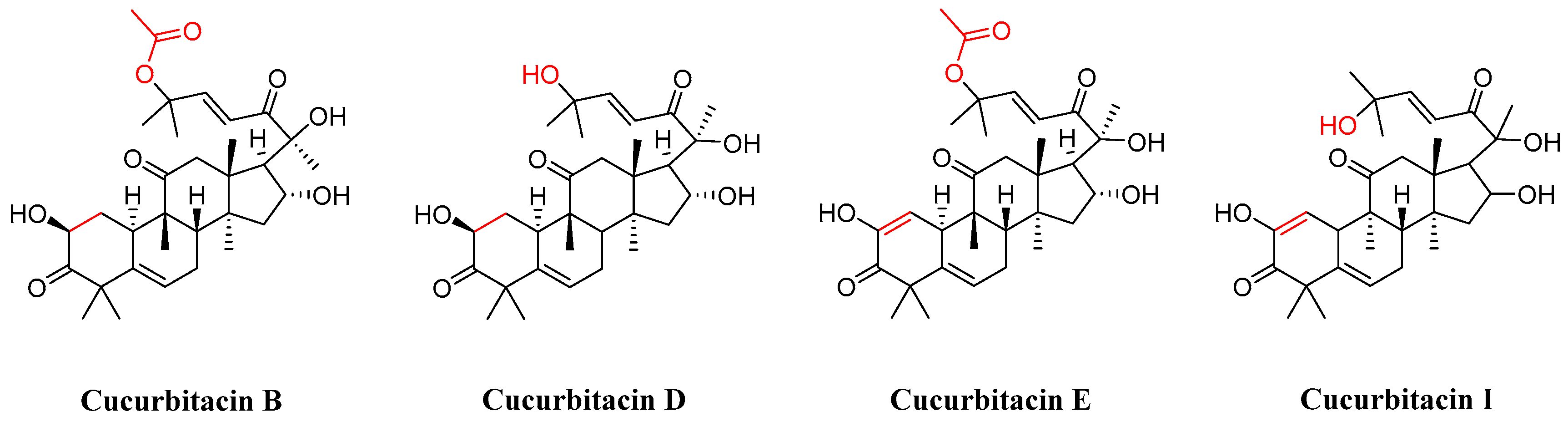
- Cai, Y.; Fang, X.; He, C.; Li, P.; Xiao, F.; Wang, Y.; Chen, M. Cucurbitacins: A Systematic Review of the Phytochemistry and Anticancer Activity. Am. J. Chin. Med. 2015, 43, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Yao, Y.; Li, H.; Gao, C.; Sun, C.; Zhuang, J. Potential of cucurbitacin as an anticancer drug. Biomed. Pharmacother. 2023, 168, 115707. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, R.-L.; Xie, C.; Lin, Y.-L.; Zhang, L.; Qi, Y. Pharmacological basis for the medicinal use of muskmelon base (Pedicellus melo) for abdominal distention and constipation. J. Ethnopharmacol. 2012, 142, 129–135. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Q.; Yang, H.; Zhang, X.-W.; Feng, N.; Wang, J.-K.; Liu, T.-T.; Zeng, K.-W.; Tu, P.-F. Allosteric Regulation of IGF2BP1 as a Novel Strategy for the Activation of Tumor Immune Microenvironment. Acs Cent. Sci. 2022, 8, 1102–1115. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, W.; Hao, W.; Ren, G.; Lu, J.; Chen, X. Cucurbitacin B Induces DNA Damage, G2/M Phase Arrest, and Apoptosis Mediated by Reactive Oxygen Species (ROS) in Leukemia K562 Cells. Anti-Cancer Agents Med. Chem. 2014, 14, 1146–1153. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.Y.; Jin, M.L.; Park, G.; Son, H.-J. Cucurbitacin B inhibits immunomodulatory function and the inflammatory response in macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Park, G. Cucurbitacins attenuate microglial activation and protect from neuroinflammatory injury through Nrf2/ARE activation and STAT/NF-κB inhibition. Neurosci. Lett. 2015, 609, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef]
- Sallam, A.M.; Esmat, A.; Abdel-Naim, A.B. Cucurbitacin-B attenuates CCl4-induced hepatic fibrosis in mice through inhibition of STAT-3. Chem. Biol. Drug Des. 2018, 91, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, H.; Zhong, Y.; Zhang, X.; Zeng, T.; Li, L.; Xu, G.; Li, M.; Liu, J.; Yang, T. Identification and characterization of the Cucurbitacins, a novel class of small-molecule inhibitors of Tropomyosin receptor kinase a. BMC Complement. Altern. Med. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, X.; Cheng, C.; Yan, W.; Guo, R.; Wang, Y.; Zhang, H.; Chai, J.; Cheng, Y.; Zhang, F. Cucurbitacin B induces ferroptosis in oral leukoplakia via the SLC7A11/mitochondrial oxidative stress pathway. Phytomedicine 2024, 129, 155548. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem. Biophys. Res. Commun. 2007, 364, 181–186. [Google Scholar] [CrossRef]
- Low, A.; Mak, E.; Rowe, J.B.; Markus, H.S.; O’Brien, J.T. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res. Rev. 2019, 53, 100916. [Google Scholar] [CrossRef]
- Andrusiow, S.; Pawlak, Z.; Stanczykiewicz, B.; Bogunia-Kubik, K.; Koszewicz, M. Chronic inflammatory demyelinating polyradiculoneuropathy in patients with diabetes mellitus-treatment with intravenous immunoglobulins: A systematic review. Biomed. Pharmacother. 2023, 164, 114974. [Google Scholar] [CrossRef]
- Chen, B.; Collen, L.V.; Mowat, C.; Isaacs, K.L.; Singh, S.; Kane, S.V.; Farraye, F.A.; Snapper, S.; Jneid, H.; Lavie, C.J.; et al. Inflammatory Bowel Disease and Cardiovascular Diseases. Am. J. Med. 2022, 135, 1453–1460. [Google Scholar] [CrossRef]
- Masson-Lecomte, A.; Rava, M.; Real, F.X.; Hartmann, A.; Allory, Y.; Malats, N. Inflammatory Biomarkers and Bladder Cancer Prognosis: A Systematic Review. Eur. Urol. 2014, 66, 1078–1091. [Google Scholar] [CrossRef]
- Temmerman, J.; Engelborghs, S.; Bjerke, M.; D’Haeseleer, M. Cerebrospinal fluid inflammatory biomarkers for disease progression in Alzheimer’s disease and multiple sclerosis: A systematic review. Front. Immunol. 2023, 14, 1162340. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, L.; Zhou, Y.; Fang, Q. Cucurbitacin B alleviates cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome-mediated inflammation and reducing oxidative stress. Biosci. Biotechnol. Biochem. 2022, 86, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, R.; Fang, P.; Ye, Z.-Q.; Zhao, Y.; Zhou, Y.; Zhang, K.-Q.; Li, L. NLRP3 inflammasome inhibitor cucurbitacin B suppresses gout arthritis in mice. J. Mol. Endocrinol. 2021, 67, 27–40. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, Y.; Deng, X.; Liu, M.; Luo, W. Cucurbitacin B supplementation reduces inflammatory responses and alveolar bone loss via regulating MPO, COX-2 and RANK/RANKL/OPG signals in a rodent model of ligature -induced periodontitis. J. King Saud Univ. Sci. 2020, 32, 1889–1895. [Google Scholar] [CrossRef]
- Liu, P.; Tan, X.-Y.; Zhang, H.-Q.; Su, K.-L.; Shang, E.-X.; Xiao, Q.-L.; Guo, S.; Duan, J.-A. Optimal compatibility proportional screening of Trichosanthis Pericarpium- Trichosanthis Radix and its anti- Inflammatory components effect on experimental zebrafish and coughing mice. J. Ethnopharmacol. 2024, 319, 117096. [Google Scholar] [CrossRef]
- Aljohani, O.S. Phytochemical evaluation of Cucumis prophetarum: Protective effects against carrageenan-induced prostatitis in rats. Drug Chem. Toxicol. 2022, 45, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.-B.; Jiang, B.; Shi, T.-S.; Li, W.-Y.; Chen, W.-J.; Zhu, B.-L.; Qin, Z.-H. Cucurbitacin B Exerts Significant Antidepressant-Like Effects in a Chronic Unpredictable Mild Stress Model of Depression: Involvement of the Hippocampal BDNF-TrkB System. Int. J. Neuropsychopharmacol. 2023, 26, 680–691. [Google Scholar] [CrossRef]
- Li, J.; Sun, K.; Muroi, M.; Gao, L.; Chang, Y.-T.; Osada, H.; Xiang, L.; Qi, J. Cucurbitacin B induces neurogenesis in PC12 cells and protects memory in APP/PS1 mice. J. Cell. Mol. Med. 2019, 23, 6283–6294. [Google Scholar] [CrossRef]
- Liu, Z.; Kumar, M.; Kabra, A. Cucurbitacin B exerts neuroprotection in a murine Alzheimer’s disease model by modulating oxidative stress, inflammation, and neurotransmitter levels. Front. Biosci. -Landmark 2022, 27, 71. [Google Scholar] [CrossRef]
- Arques, S. Serum albumin and cardiovascular disease: State-of-the-art review. Ann. De Cardiol. Et D’angeiologie 2020, 69, 192–200. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Z.; Wu, Q.-Q.; Jiang, X.-H.; Yuan, Y.; Chang, W.; Bian, Z.Y.; Zhu, J.X.; Tang, Q.-Z. Cucurbitacin B Protects Against Pressure Overload Induced Cardiac Hypertrophy. J. Cell. Biochem. 2017, 118, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Xu, M.; Chen, L.; Sun, Z.; Gong, J.; Li, Y.; Jiang, T. Cucurbitacin B protects against myocardial ischemia-reperfusion injury through activating JAK2/STAT3 signaling pathway. Cell. Mol. Biol. 2023, 69, 155–161. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Y.; Luo, Y.; Gu, F.; Zhu, Y.; Liu, X.; Yan, C.; Hu, W.; Wang, S.; Chao, X.; et al. Natural compounds modulating mitophagy: Implications for cancer therapy. Cancer Lett. 2024, 582, 216590. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Sflakidou, E.; Leonidis, G.; Foroglou, E.; Siokatas, C.; Sarli, V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules 2022, 27, 6632. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, X.-D.; Tang, P.Y.-Z.; Li, H.-M.; Liu, X.; Zhong, J.-J.; Tang, Y.-J. Advances of antitumor drug discovery in traditional Chinese medicine and natural active products by using multi-active components combination. Med. Res. Rev. 2023, 43, 1778–1808. [Google Scholar] [CrossRef]
- Luo, W.-W.; Zhao, W.-W.; Lu, J.-J.; Wang, Y.-T.; Chen, X.-P. Cucurbitacin B suppresses metastasis mediated by reactive oxygen species (ROS) via focal adhesion kinase (FAK) in breast cancer MDA-MB-231 cells. Chin. J. Nat. Med. 2018, 16, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, X.-L.; Yuan, J.-W.; Zhang, H.-R.; Liu, D.; Hao, J.; Ji, W.; Wu, X.-Z.; Chen, D. Cucurbitacin B inhibits the migration and invasion of breast cancer cells by altering the biomechanical properties of cells. Phytother. Res. 2019, 33, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Wakimoto, N.; Xing, H.; Lu, D.; Huynh, T.; Wang, X.; Black, K.L.; Koeffler, H.P. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int. J. Cancer 2008, 123, 1364–1375. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, Y.; Liu, W.; Ma, F.; Zhou, Y.; Chen, M.; Chang, J.; Wang, Y.; Yang, G.; He, G. Cucurbitacin B inhibits growth and induces apoptosis through the JAK2/STAT3 and MAPK pathways in SH-SY5Y human neuroblastoma cells. Mol. Med. Rep. 2014, 10, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-L.; Tao, W.-H.; Yang, T.-X.; Qiao, J.-G. Anticancer effect of cucurbitacin B on MKN-45 cells via inhibition of the JAK2/STAT3 signaling pathway. Exp. Ther. Med. 2016, 12, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Yang, R.; Zhou, T.; Ke, W.; Si, Y.; Yang, S.; Zhang, T.; Liu, X.; Zhang, L.; et al. Cucurbitacin B inhibits gastric cancer progression by suppressing STAT3 activity. Arch. Biochem. Biophys. 2020, 684, 108314. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Gao, M.X.; Yang, K. Cucurbitacin B inhibits cell proliferation and induces apoptosis in human osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK pathways. Exp. Ther. Med. 2017, 14, 805–812. [Google Scholar] [CrossRef]
- Wu, H.; Ma, T.; He, M.; Xie, W.; Wang, X.; Lu, L.; Wang, H.; Cui, Y. Cucurbitacin B modulates M2 macrophage differentiation and attenuates osteosarcoma progression via PI3K/AKT pathway. Phytother. Res. 2024, 38, 2215–2233. [Google Scholar] [CrossRef]
- Yu, Z.; Liang, S.; Ji, L.; Cheng, Y.; Yan, W.; Gao, R.; Zhang, F. Network pharmacological analysis and experimental study of cucurbitacin B in oral squamous cell carcinoma. Mol. Divers. 2023. [Google Scholar] [CrossRef]
- Tao, B.; Wang, D.; Yang, S.; Liu, Y.; Wu, H.; Li, Z.; Chang, L.; Yang, Z.; Liu, W. Cucurbitacin B Inhibits Cell Proliferation by Regulating X-Inactive Specific Transcript Expression in Tongue Cancer. Front. Oncol. 2021, 11, 651648. [Google Scholar] [CrossRef]
- Alafnan, A.; Khalifa, N.E.; Hussain, T.; Osman, M.E. Cucurbitacin-B instigates intrinsic apoptosis and modulates Notch signaling in androgen-dependent prostate cancer LNCaP cells (vol 14, 1206981, 2023). Front. Pharmacol. 2023, 14, 1206981. [Google Scholar]
- Alafnan, A.; Alamri, A.; Hussain, T.; Rizvi, S.M.D. Cucurbitacin-B Exerts Anticancer Effects through Instigation of Apoptosis and Cell Cycle Arrest within Human Prostate Cancer PC3 Cells via Downregulating JAK/STAT Signaling Cascade. Pharmaceuticals 2022, 15, 1229. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, M.; Chen, Y.; Xu, S.; Guo, Y.; Zhao, L. Cucurbitacin B suppresses proliferation of pancreatic cancer cells by ceRNA: Effect of miR-146b-5p and lncRNA-AFAP1-AS1. J. Cell. Physiol. 2019, 234, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Z.; Chen, Y.-Y.; Liu, Q.-P.; Feng, Z.-H.; Zhang, L.; Zhang, H. Cucurbitacin B suppresses hepatocellular carcinoma progression through inducing DNA damage-dependent cell cycle arrest. Phytomedicine 2024, 126, 155177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, Y.; Yan, X.; Li, J.; Lin, B.; Dai, L.; Xu, C.; Li, H.; Li, D.; Yang, T.; et al. Cucurbitacin B exhibits antitumor effects on CD133+HepG2 liver cancer stem cells by inhibiting JAK2/STAT3 signaling pathway. Anti-Cancer Drugs 2021, 32, 548–557. [Google Scholar] [CrossRef]
- Liu, J.-H.; Li, C.; Cao, L.; Zhang, C.-H.; Zhang, Z.-H. Cucurbitacin B regulates lung cancer cell proliferation and apoptosis via inhibiting the IL-6/STAT3 pathway through the lncRNA XIST/miR-let-7c axis. Pharm. Biol. 2022, 60, 154–162. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, Q.; Liang, X.; Han, S.; He, J.; Qin-Qin, W.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits TGF-β1-induced epithelial-mesenchymal transition (EMT) in NSCLC through regulating ROS and PI3K/Akt/mTOR pathways. Chin. Med. 2022, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, L.; Tang, H.; Wang, W.; Lin, Y. Cucurbitacin B enhances apoptosis in gefitinib resistant non-small cell lung cancer by modulating the miR-17-5p/STAT3 axis. Mol. Med. Rep. 2021, 24, 710. [Google Scholar] [CrossRef]
- Yu, X.; Chen, W.; Zhang, J.; Gao, X.; Cui, Q.; Song, Z.; Du, J.; Lv, W. Antitumor activity and mechanism of cucurbitacin B in A549/DDP cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2023, 396, 1095–1103. [Google Scholar] [CrossRef]
- Mao, D.; Liu, A.H.; Wang, Z.P.; Zhang, X.W.; Lu, H. Cucurbitacin B inhibits cell proliferation and induces cell apoptosis in colorectal cancer by modulating methylation status of BTG3. Neoplasma 2019, 66, 593–602. [Google Scholar] [CrossRef]
- Huang, J.-L.; Liang, L.; Xie, P.-E.; Sun, W.-L.; Wang, L.; Cai, Z.-W. Cucurbitacin B induces apoptosis in colorectal cells through reactive oxygen species generation and endoplasmic reticulum stress pathways. Exp. Ther. Med. 2023, 26, 484. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, B.; Wei, H.; Zeng, H.; Sheng, D.; Zhang, Y. Cucurbitacin B controls M2 macrophage polarization to suppresses metastasis via targeting JAK-2/STAT3 signalling pathway in colorectal cancer. J. Ethnopharmacol. 2022, 287, 114915. [Google Scholar] [CrossRef]
- Dandawate, P.; Subramaniam, D.; Panovich, P.; Standing, D.; Krishnamachary, B.; Kaushik, G.; Thomas, S.M.; Dhar, A.; Weir, S.J.; Jensen, R.A.; et al. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci. Rep. 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Kaewmeesri, P.; Kukongviriyapan, V.; Prawan, A.; Kongpetch, S.; Senggunprai, L. Cucurbitacin B Diminishes Metastatic Behavior of Cholangiocarcinoma Cells by Suppressing Focal Adhesion Kinase. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kurman, Y.; Kiliccioglu, I.; Dikmen, A.U.; Esendagli, G.; Bilen, C.Y.; Sozen, S.; Konac, E. Cucurbitacin B and cisplatin induce the cell death pathways in MB49 mouse bladder cancer model. Exp. Biol. Med. 2020, 245, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Kariya, R.; Sittithumcharee, G.; Okada, S. Cucurbitacin B induces apoptosis of primary effusion lymphoma via disruption of cytoskeletal organization. Phytomedicine 2021, 85, 153545. [Google Scholar] [CrossRef]
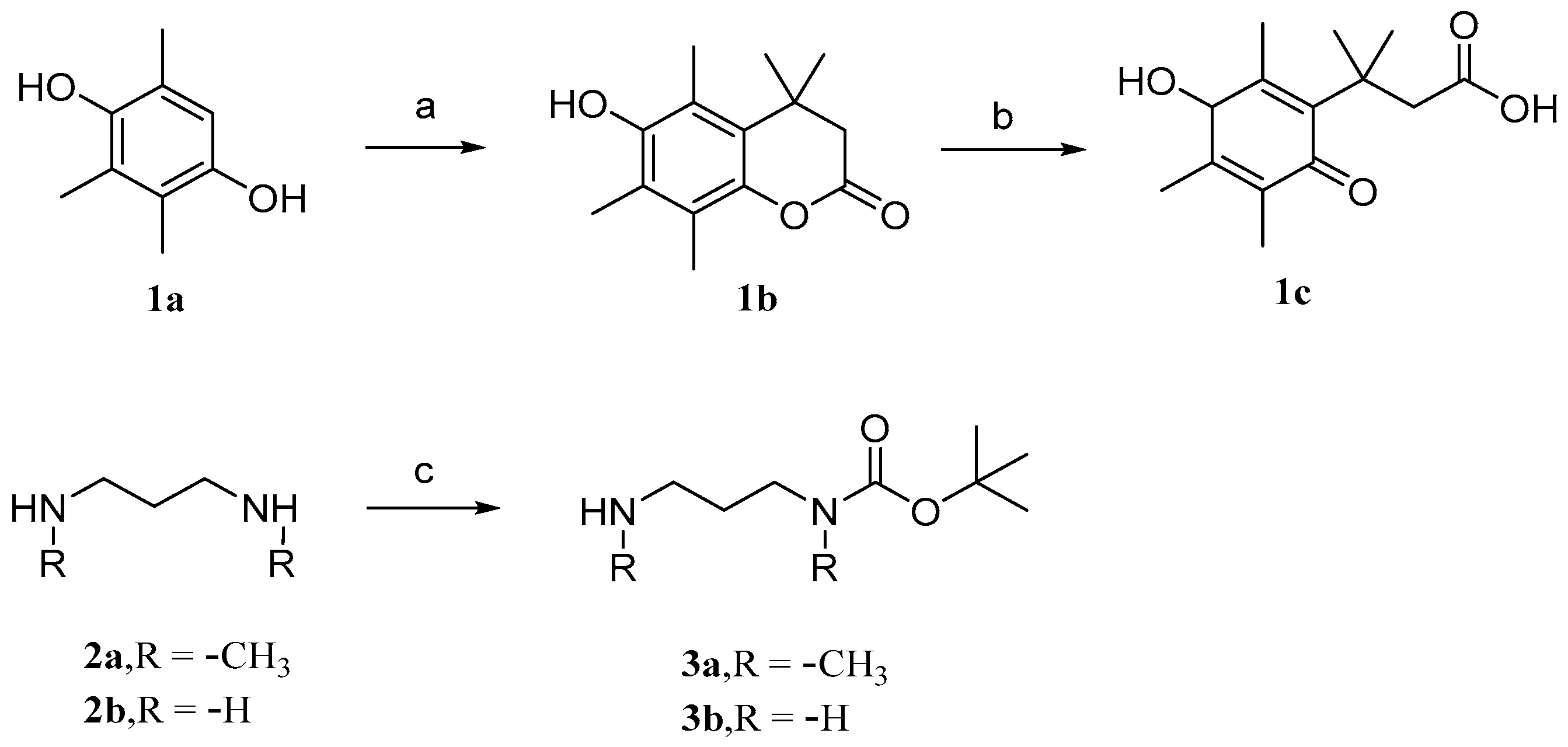
- Ding, Y.; Xue, X. Medicinal Chemistry Strategies for the Modification of Bioactive Natural Products. Molecules 2024, 29, 689. [Google Scholar] [CrossRef]
- Guo, M.; Jin, J.; Zhao, D.; Rong, Z.; Cao, L.-Q.; Li, A.-H.; Sun, X.-Y.; Jia, L.-Y.; Wang, Y.-D.; Huang, L.; et al. Research Advances on Anti-Cancer Natural Products. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef]
- Jung, M.E.; Lui, R.M. Studies toward the Total Syntheses of Cucurbitacins B and D. J. Org. Chem. 2010, 75, 7146–7158. [Google Scholar] [CrossRef]
- Suebsakwong, P.; Wang, J.; Ithetkam, P.; Weerapreeyaku, N.; Wu, J.; Du, Y.; Yao, Z.J.; Li, J.-X.; Suksamrarn, A. A Bioreductive Prodrug of Cucurbitacin B Significantly Inhibits Tumor Growth in the 4T1 Xenograft Mice Model. Acs Med. Chem. Lett. 2019, 10, 1400–1406. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Xing, Y.; Sun, Y.-Q.; Liu, C.; Xu, Q.; Shang, F.F.; Zhang, R.H.; Jin, X.-J.; Chen, F.; Lee, J.J.; et al. Ginsengenin derivatives synthesized from 20(R)-panaxotriol: Synthesis, characterization, and antitumor activity targeting HIF-1 pathway. J. Ginseng Res. 2022, 46, 738–749. [Google Scholar] [CrossRef]
- Liu, H.-J.; Huang, X.; Shen, Q.-K.; Deng, H.; Li, Z.; Quan, Z.-S. Design, Synthesis, and Anticancer Activity Evaluation of Hybrids of Azoles and Barbituric Acids. Iran. J. Pharm. Res. 2021, 20, 144–155. [Google Scholar]
- Ma, Q.; Bian, M.; Gong, G.; Bai, C.; Liu, C.; Wei, C.; Quan, Z.-S.; Du, H.-H. Synthesis and Evaluation of Bakuchiol Derivatives as Potent Anti-inflammatory Agents in Vitro and in Vivo. J. Nat. Prod. 2022, 85, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Liu, C.-Y.; Gong, G.-H.; Quan, Z.-S. Synthesis, in vitro and in vivo biological evaluation of novel lappaconitine derivatives as potential anti-inflammatory agents. Acta Pharm. Sin. B 2020, 10, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.-F.; Lu, Q.; Lin, T.; Pu, M.; Xiao, R.; Liu, W.; Deng, H.; Guo, H.; Quan, Z.-S.; Ding, C.; et al. Discovery of Triazolyl Derivatives of Cucurbitacin B Targeting IGF2BP1 against Non-Small Cell Lung Cancer. J. Med. Chem. 2023, 66, 12931–12949. [Google Scholar] [CrossRef]
- Lang, K.L.; Silva, I.T.; Zimmermann, L.A.; Machado, V.R.; Teixeira, M.R.; Ivana Lapuh, M.; Alejandra Galetti, M.; Alejandro Palermo, J.; Myriam Cabrera, G.; Campos Bernardes, L.S.; et al. Synthesis and cytotoxic activity evaluation of dihydrocucurbitacin B and cucurbitacin B derivatives. Bioorganic Med. Chem. 2012, 20, 3016–3030. [Google Scholar] [CrossRef]
- Ge, W.; Chen, X.; Han, F.; Liu, Z.; Wang, T.; Wang, M.; Chen, Y.; Ding, Y.; Zhang, Q. Synthesis of Cucurbitacin B Derivatives as Potential Anti-Hepatocellular Carcinoma Agents. Molecules 2018, 23, 3345. [Google Scholar] [CrossRef]
- Ai, Y.; Kang, F.; Huang, Z.; Xue, X.; Lai, Y.; Peng, S.; Tian, J.; Zhang, Y. Synthesis of CDDO-Amino Acid-Nitric Oxide Donor Trihybrids as Potential Antitumor Agents against Both Drug-Sensitive and Drug-Resistant Colon Cancer. J. Med. Chem. 2015, 58, 2452–2464. [Google Scholar] [CrossRef]
- Duan, W.; Li, J.; Inks, E.S.; Chou, C.J.; Jia, Y.; Chu, X.; Li, X.; Xu, W.; Zhang, Y. Design, Synthesis, and Antitumor Evaluation of Novel Histone Deacetylase Inhibitors Equipped with a Phenylsulfonylfuroxan Module as a Nitric Oxide Donor. J. Med. Chem. 2015, 58, 4325–4338. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Liu, M.-M.; Chen, X.-Y.; Huang, Y.-Q.; Feng, P.; Guo, Y.-L.; Yang, G.; Chen, Y. Hybrids of Phenylsulfonylfuroxan and Coumarin as Potent Antitumor Agents. J. Med. Chem. 2014, 57, 9343–9356. [Google Scholar]
- Tang, W.; Xie, J.; Xu, S.; Lv, H.; Lin, M.; Yuan, S.; Bai, J.; Hou, Q.; Yu, S. Novel Nitric Oxide-Releasing Derivatives of Brusatol as Anti-Inflammatory Agents: Design, Synthesis, Biological Evaluation, and Nitric Oxide Release Studies. J. Med. Chem. 2014, 57, 7600–7612. [Google Scholar] [CrossRef]
- Zhuo, N.; Ma, J.; Cao, L.; Chen, L.; Nan, F. Protecting-Group-Free One-Step Palladium-Catalyzed Coupling on C25 of Cucurbitacin B Expands Chemical Diversity with Improved Cytotoxicity against A549 Cells. Chin. J. Chem. 2022, 40, 1662–1666. [Google Scholar] [CrossRef]
- Ahmadi, M.; Siavashy, S.; Ayyoubzadeh, S.M.; Kecili, R.; Ghorbani-Bidkorbeh, F. Controllable Synthesis of Polymeric Micelles by Microfluidic Platforms for Biomedical Applications: A Systematic Review. Iran. J. Pharm. Res. 2021, 20, e124514. [Google Scholar]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnuerch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, J.; Zhang, Q.; Fang, L.; Liu, C.; Xu, H. Development and Evaluation of Cucurbitacin B Microemulsion: The Effect of Oil Phase and Aqueous Phase on Drug Percutaneous Absorption Based on ATR-FTIR Spectroscopy and Molecular Modeling. Aaps Pharmscitech 2020, 21, 258. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, Z.; Xiao, Y.; Chang, J.; Liu, P.; Liu, C.; Liu, X. Preparation, characterization and pharmacokinetics of Cucurbitacin B solid dispersion. OpenNano 2022, 8, 100088. [Google Scholar] [CrossRef]
- Lv, Q.; Shen, C.; Li, X.; Shen, B.; Yu, C.; Xu, P.; Xu, H.; Han, J.; Yuan, H. Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for Cucurbitacin B delivery. Drug Deliv. 2015, 22, 351–358. [Google Scholar] [CrossRef]
- Hu, H.; Liu, D.; Zhao, X.; Qiao, M.; Chen, D. Preparation, characterization, cellular uptake and evaluation in vivo of solid lipid nanoparticles loaded with cucurbitacin B. Drug Dev. Ind. Pharm. 2013, 39, 770–779. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, M.; Lu, X.; Li, Y.; Lu, C. Diselenium-linked dimeric prodrug nanomedicine breaking the intracellular redox balance for triple-negative breast cancer targeted therapy. Eur. J. Pharm. Biopharm. 2023, 193, 16–27. [Google Scholar] [CrossRef]
- Tang, L.; Fu, L.; Zhu, Z.; Yang, Y.; Sun, B.; Shan, W.; Zhang, Z. Modified mixed nanomicelles with collagen peptides enhanced oral absorption of Cucurbitacin B: Preparation and evaluation. Drug Deliv. 2018, 25, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Allen-Perkins, A.; Estrada, E. Mathematical modelling for sustainable aphid control in agriculture via intercropping. Proc. R. Soc. A-Math. Phys. Eng. Sci. 2019, 475, 20190136. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. Biomed Res. Int. 2018, 2018, 4308054. [Google Scholar] [CrossRef] [PubMed]
- Zust, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.R.; Hoffman, C.A. Chemical Feeding Deterrent Mobilized in Response to Insect Herbivory and Counteradaptation by Epilachna tredecimnotata. Science 1980, 209, 414–416. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar]
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (Review). Int. J. Oncol. 2018, 52, 19–37. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Metcalf, R.A.; Rhodes, A.M. Cucurbitacins as kairomones for diabroticite beetles. Proc. Natl. Acad. Sci. USA 1980, 77, 3769–3772. [Google Scholar] [CrossRef]
- Yousaf, H.K.; Shan, T.; Chen, X.; Ma, K.; Shi, X.; Desneux, N.; Biondi, A.; Gao, X. Impact of the secondary plant metabolite Cucurbitacin B on the demographical traits of the melon aphid, Aphis gossypii. Sci. Rep. 2018, 8, 16473. [Google Scholar] [CrossRef]
- Clyne, P.J.; Warr, C.G.; Carlson, J.R. Candidate taste receptors in Drosophila. Science 2000, 287, 1830–1834. [Google Scholar] [CrossRef]
- Rimal, S.; Sang, J.; Dhakal, S.; Lee, Y. Cucurbitacin B Activates Bitter-Sensing Gustatory Receptor Neurons via Gustatory Receptor 33a in Drosophila melanogaster. Mol. Cells 2020, 43, 530–538. [Google Scholar] [PubMed]
- Cooke, J.; Sang, J.H. Utilization of sterols by larvae of Drosophila melanogaster. J. Insect Physiol. 1970, 16, 801–812. [Google Scholar] [CrossRef]
- Niwa, R.; Niwa, Y.S. The Fruit Fly Drosophila melanogaster as a Model System to Study Cholesterol Metabolism and Homeostasis. Cholesterol 2011, 2011, 176802. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Connacher, R.P.; O’Connor, M.B. Control of the insect metamorphic transition by ecdysteroid production and secretion. Curr. Opin. Insect Sci. 2021, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Fujinaga, D.; Inaba, K.; Funahashi, T.; Fujikawa, Y.; Inoue, H.; Kataoka, H.; Niwa, R.; Ono, H. The plant-derived triterpenoid, cucurbitacin B, but not cucurbitacin E, inhibits the developmental transition associated with ecdysone biosynthesis in Drosophila melanogaster. J. Insect Physiol. 2021, 134, 104294. [Google Scholar] [CrossRef]
- Huang, A.C.; Jiang, T.; Liu, Y.-X.; Bai, Y.-C.; Reed, J.; Qu, B.; Goossens, A.; Nutzmann, H.-W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef]
- Koprivova, A.; Schuck, S.; Jacoby, R.P.; Klinkhammer, I.; Welter, B.; Leson, L.; Martyn, A.; Nauen, J.; Grabenhorst, N.; Mandelkow, J.F.; et al. Root-specific camalexin biosynthesis controls the plant growth-promoting effects of multiple bacterial strains. Proc. Natl. Acad. Sci. USA 2019, 116, 15735–15744. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xun, W.; Wang, X.; Tian, S.; Zhang, Y.; Li, D.; Zhou, Y.; Qin, Y.; Zhang, B.; Zhao, G.; et al. Root-secreted bitter triterpene modulates the rhizosphere microbiota to improve plant fitness. Nat. Plants 2022, 8, 887–896. [Google Scholar] [CrossRef]
- Cheke, R.A. New pests for old as GMOs bring on substitute pests. Proc. Natl. Acad. Sci. USA 2018, 115, 8239–8240. [Google Scholar] [CrossRef]
- Constantine, K.L.; Kansiime, M.K.; Mugambi, I.; Nunda, W.; Chacha, D.; Rware, H.; Makale, F.; Mulema, J.; Lamontagne-Godwin, J.; Williams, F.; et al. Why don’t smallholder farmers in Kenya use more biopesticides? Pest Manag. Sci. 2020, 76, 3615–3625. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, C.; Luo, J.; Niu, L.; Hua, H.; Zhang, S.; Cui, J. Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii. Insects 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Whiting, P.; Girault, J.P.; Lafont, R.; Dhadialla, T.S.; Cress, D.E.; Mugat, B.; Antoniewski, C.; Lepesant, J.A. Cucurbitacins are insect steroid hormone antagonists acting at the ecdysteroid receptor. Biochem. J. 1997, 327 Pt 3, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M.; Cherbas, P.; Truman, J.W. Ecdysone receptors and their biological actions. Vitam. Horm. 2000, 60, 1–73. [Google Scholar]
- Zou, C.; Liu, G.; Liu, S.; Liu, S.; Song, Q.; Wang, J.; Feng, Q.; Su, Y.; Li, S. Cucurbitacin B acts a potential insect growth regulator by antagonizing 20-hydroxyecdysone activity. Pest Manag. Sci. 2018, 74, 1394–1403. [Google Scholar] [CrossRef]
- Pofu, K.M.; Mashela, P.W. Interactive Effects of Filamentous Fungi and Cucurbitacin Phytonematicide on Growth of Cowpea and Suppression of Meloidogyne enterolobii. Front. Microbiol. 2022, 13, 765051. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based Approaches in Pharmacology. Mol. Inform. 2017, 36, 1700048. [Google Scholar] [CrossRef]
- Dai, S.; Wu, R.; Fu, K.; Li, Y.; Yao, C.; Liu, Y.; Zhang, F.; Zhang, S.; Guo, Y.; Yao, Y.; et al. Exploring the effect and mechanism of cucurbitacin B on cholestatic liver injury based on network pharmacology and experimental verification. J. Ethnopharmacol. 2024, 322, 117584. [Google Scholar] [CrossRef]
- Aizawa, H.; Kimura, S.; Abe, S.; Sano, M. Gel network amplifies Nano-Scale adsorption at Solid/Liquid interface to Sub-Millimeter-Scale. J. Colloid Interface Sci. 2022, 626, 276–282. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)—challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef]
- Eilers, A.; Witt, S.; Walter, J. Aptamer-Modified Nanoparticles in Medical Applications. Aptamers Biotechnol. 2020, 174, 161–193. [Google Scholar]
- Kovalchuk, N.M.; Johnson, D.; Sobolev, V.; Hilal, N.; Starov, V. Interactions between nanoparticles in nanosuspension. Adv. Colloid Interface Sci. 2019, 272, 102020. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Jiang, Y.; McClements, D.J.; Liu, F.; Liu, X. Self-assembled nano-micelles of lactoferrin peptides: Structure, physicochemical properties, and application for encapsulating and delivering curcumin. Food Chem. 2022, 387, 132790. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Sporn, M.B. Synthetic Oleanane Triterpenoids: Multifunctional Drugs with a Broad Range of Applications for Prevention and Treatment of Chronic Disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef]
- Dai, S.; Wang, C.; Zhao, X.; Ma, C.; Fu, K.; Liu, Y.; Peng, C.; Li, Y. Cucurbitacin B: A review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol. Res. 2023, 187, 106587. [Google Scholar] [CrossRef]
- Guedes Silvestre, G.F.; de Lucena, R.P.; Alves, H.d.S. Cucurbitacins and the Immune System: Update in Research on Anti-inflammatory, Antioxidant, and Immunomodulatory Mechanisms. Curr. Med. Chem. 2022, 29, 3774–3789. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Ras, R.; Widmer, G. Effect of Ginsenoside-Rh2 and Curcurbitacin-B on Cryptosporidium parvum in vitro. Exp. Parasitol. 2020, 212, 107873. [Google Scholar] [CrossRef]
- Chen, J.-C.; Zhang, G.-H.; Zhang, Z.-Q.; Qiu, M.-H.; Zheng, Y.-T.; Yang, L.-M.; Yu, K.-B. Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod. 2008, 71, 153–155. [Google Scholar] [CrossRef]




| Compounds | IC50 (μM) | |
|---|---|---|
| MCF-7 | Vero | |
| Cu B | 12.0 ± 0.4 | 0.04 ± 0.02 |
| 1 | 18.1 ± 0.4 | 12.4 ± 0.5 |
| 2 | 15.4 ± 0.2 | 1.87 ± 0.24 |
| 3 | 16.6 ± 0.9 | 0.07 ± 0.02 |
| TAM | 22.6 ± 0.3 | - |
| Compounds | IC50 (μM) | SI | |
|---|---|---|---|
| HCT116 | L02 | ||
| 15 | 0.031 ± 0.003 | 0.080 ± 0.010 | 2.58 |
| 20 | 0.051 ± 0.003 | 0.080 ± 0.008 | 1.57 |
| 21 | 0.034 ± 0.005 | 0.100 ± 0.005 | 2.94 |
| 22 | 0.063 ± 0.074 | 0.031 ± 0.005 | 0.49 |
| 23 | 0.076 ± 0.039 | 0.031 ± 0.004 | 0.41 |
| 24 | 0.121 ± 0.009 | 0.040 ± 0.000 | 0.33 |
| 25 | 0.044 ± 0.006 | 0.033 ± 0.011 | 0.75 |
| 26 | 0.037 ± 0.014 | 0.034 ± 0.004 | 0.43 |
| 27 | 0.079 ± 0.014 | 0.034 ± 0.004 | 0.43 |
| Cu B | 0.019 ± 0.022 | 0.011 ± 0.003 | 0.57 |
| Cell Lines | IC50 (μM) | SI | ||
|---|---|---|---|---|
| Cu B | 21 | Cu B | 21 | |
| A549 | 0.088 ± 0.005 | 0.009 ± 0.002 | 0.13 | 11.11 |
| HCT-116 | 0.019 ± 0.003 | 0.080 ± 0.010 | 0.58 | 1.25 |
| MDA-MB-231 | 0.037 ± 0.002 | 0.049 ± 0.005 | 0.30 | 2.04 |
| MCF-7 | 0.040 ± 0.002 | 0.056 ± 0.003 | 0.23 | 1.79 |
| SK-OV-3 | 0.047 ± 0.004 | 0.054 ± 0.006 | 0.23 | 1.85 |
| HeLa | 0.024 ± 0.004 | 0.015 ± 0.004 | 0.46 | 6.67 |
| HepG-2 | 0.051 ± 0.003 | 0.080 ± 0.008 | 0.22 | 1.25 |
| BXPC-3 | 0.022 ± 0.001 | 0.018 ± 0.003 | 0.50 | 5.56 |
| PANC-1 | 0.067 ± 0.008 | 0.088 ± 0.012 | 0.16 | 1.14 |
| CFPAC-1 | 0.023 ± 0.002 | 0.037 ± 0.005 | 0.49 | 2.70 |
| L02 | 0.011 ± 0.030 | 0.011 ± 0.005 | ||
| Compounds | IC50 (μM) | TI | |
|---|---|---|---|
| HepG2 | L02 | ||
| Cu B | 0.060 ± 0.02 | 0.019 ± 0.003 | 0.32 |
| 31 | 1.01 ± 0.04 | 1.02 ± 0.58 | 1 |
| 32 | 0.54 ± 0.04 | 0.52 ± 0.08 | 0.96 |
| 33 | 2.45 ± 1.75 | 2.37 ± 0.09 | 0.97 |
| 34 | 4.89 ± 0.92 | 8.01 ± 0.47 | 1.64 |
| 35 | 9.28 ± 0.37 | 5.50 ± 2.48 | 0.59 |
| 36 | 0.93 ± 0.06 | 0.41 ± 0.11 | 0.44 |
| 37 | 0.22 ± 0.11 | 0.042 ± 0.001 | 0.19 |
| 38 | 9.62 ± 1.34 | 6.90 ± 1.37 | 0.72 |
| 39 | 7.42 ± 0.40 | 4.42 ± 0.97 | 0.60 |
| 40 | 4.67 ± 0.28 | 3.57 ± 2.58 | 0.76 |
| 41 | 2.99 ± 2.06 | 1.93 ± 0.32 | 0.65 |
| 42 | 3.71 ± 1.69 | 2.47 ± 0.47 | 0.67 |
| 43 | 0.92 ± 0.44 | 0.74 ± 0.15 | 0.8 |
| 44 | 4.05 ± 1.39 | 2.48 ± 0.52 | 0.61 |
| 45 | 3.31 ± 0.24 | 9.59 ± 2.38 | 2.90 |
| 46 | 2.34 ± 0.91 | 1.47 ± 0.36 | 0.63 |
| 47 | 3.85 ± 0.63 | 5.25 ± 1.63 | 1.36 |
| Compounds | IC50 (μM) | TI | |
|---|---|---|---|
| HepG2 | L02 | ||
| Cu B | 0.060 ± 0.02 | 0.019 ± 0.003 | 0.32 |
| 48 | 0.82 ± 0.33 | 2.04 ± 0.67 | 2.49 |
| 49 | 0.63 ± 0.29 | 2.97 ± 0.23 | 4.71 |
| 50 | 0.52 ± 0.09 | 2.10 ± 1.41 | 4.03 |
| 51 | 0.64 ± 0.26 | 2.62 ± 0.52 | 4.1 |
| 52 | 1.15 ± 0.24 | 3.21 ± 0.09 | 2.79 |
| 53 | 0.83 ± 0.38 | 3.25 ± 0.40 | 3.92 |
| 54 | 1.19 ± 0.55 | 4.29 ± 0.66 | 3.61 |
| 55 | 1.15 ± 0.24 | 1.17 ± 0.08 | 1.02 |
| 56 | 6.92 ± 0.75 | 3.27 ± 0.43 | 0.47 |
| 57 | 10.28 ± 1.11 | 6.69 ± 3.95 | 0.65 |
| 58 | - | - | - |
| 59 | 1.01 ± 0.40 | 2.72 ± 0.38 | 2.69 |
| 60 | 1.35 ± 0.10 | 2.94 ± 0.09 | 2.18 |
| 61 | 1.11 ± 0.22 | 3.31 ± 0.18 | 0.34 |
| 62 | 1.02 ± 0.28 | 3.00 ± 0.26 | 2.94 |
| 63 | 0.46 ± 0.14 | 0.69 ± 0.43 | 1.5 |
| 64 | 0.53 ± 0.01 | 0.80 ± 0.40 | 1.51 |
| 65 | 0.92 ± 0.16 | 2.61 ± 1.77 | 2.84 |
| Compounds | IC50 (nM) |
|---|---|
| A549 | |
| Cu B | 12.3 ± 2.3 |
| 67 | 61.0 ± 6.8 |
| 68 | 171.6 ± 37.3 |
| 69 | 34.5 ± 2.1 |
| 70 | 13.0 ± 1.3 |
| 71 | 44.4 ± 3.6 |
| 72 | 97.2 ± 21.6 |
| 73 | 30.6 ± 7.8 |
| 74 | 61.3 ± 11.4 |
| 75 | 23.3 ± 4.8 |
| 76 | 36.7 ± 9.8 |
| 77 | 13.2 ± 2.6 |
| 78 | 46.0 ± 8.4 |
| 79 | 35.3 ± 6.1 |
| 80 | 11.6 ± 1.9 |
| 81 | 10.7 ± 1.9 |
| 82 | 17.7 ± 2.1 |
| 83 | 8.5 ± 1.0 |
| 84 | 88.1 ± 19.5 |
| 85 | 81.4 ± 13.4 |
| 86 | 16.2 ± 2.7 |
| 87 | 6.8 ± 0.9 |
| 88 | 47.7 ± 10.3 |
| 89 | 160.1 ± 31.2 |
| 90 | 1460 ± 310 |
| 91 | 25.7 ± 6.7 |
| 92 | 14.4 ± 4.6 |
| 93 | 29.2 ± 6.4 |
| 94 | 49.7 ± 8.4 |
| 95 | 16.5 ± 4.6 |
| Compounds | IC50 (μM) | Increase Toxicity (CC48/CC72) | |
|---|---|---|---|
| 48 h | 72 h | ||
| Cu B | 19.91 | 12.09 | 1.6 |
| 100 | 54.42 | 26.49 | 2.1 |
| 101 | - | - | - |
| 102 | - | - | - |
| 103 | - | - | - |
| 104 | 14.65 | 2.64 | 5.5 |
| 105 | - | - | - |
| 106 | 28.80 | 11.52 | 2.5 |
| 107 | 1.33 | 0.12 | 1.2 |
| 108 | 0.42 | 0.12 | 11.1 |
| 109 | - | - | - |
| 110 | 14.65 | 6.90 | 2.1 |
| 111 | - | - | - |
| 112 | - | - | - |
| 113 | - | - | - |
| 114 | - | - | - |
| 115 | - | - | - |
| 116 | - | - | - |
| Doxorubicin | 3.69 | 1.18 | 3.1 |
| Paclitaxel | 1.16 | 0.19 | 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; Wang, Y.; Tian, X.; Liu, J.; Jin, Z.; Xu, J.; He, M.; Shen, Q.; Guo, H.; Luan, T. Cucurbitacin B and Its Derivatives: A Review of Progress in Biological Activities. Molecules 2024, 29, 4193. https://doi.org/10.3390/molecules29174193
Nie W, Wang Y, Tian X, Liu J, Jin Z, Xu J, He M, Shen Q, Guo H, Luan T. Cucurbitacin B and Its Derivatives: A Review of Progress in Biological Activities. Molecules. 2024; 29(17):4193. https://doi.org/10.3390/molecules29174193
Chicago/Turabian StyleNie, Wenzhe, Yalan Wang, Xinlu Tian, Jinying Liu, Zhanhui Jin, Junjie Xu, Miaohai He, Qingkun Shen, Hongyan Guo, and Tian Luan. 2024. "Cucurbitacin B and Its Derivatives: A Review of Progress in Biological Activities" Molecules 29, no. 17: 4193. https://doi.org/10.3390/molecules29174193
APA StyleNie, W., Wang, Y., Tian, X., Liu, J., Jin, Z., Xu, J., He, M., Shen, Q., Guo, H., & Luan, T. (2024). Cucurbitacin B and Its Derivatives: A Review of Progress in Biological Activities. Molecules, 29(17), 4193. https://doi.org/10.3390/molecules29174193







