Magnetite Nanoparticle Assemblies and Their Biological Applications: A Review
Abstract
1. Introduction
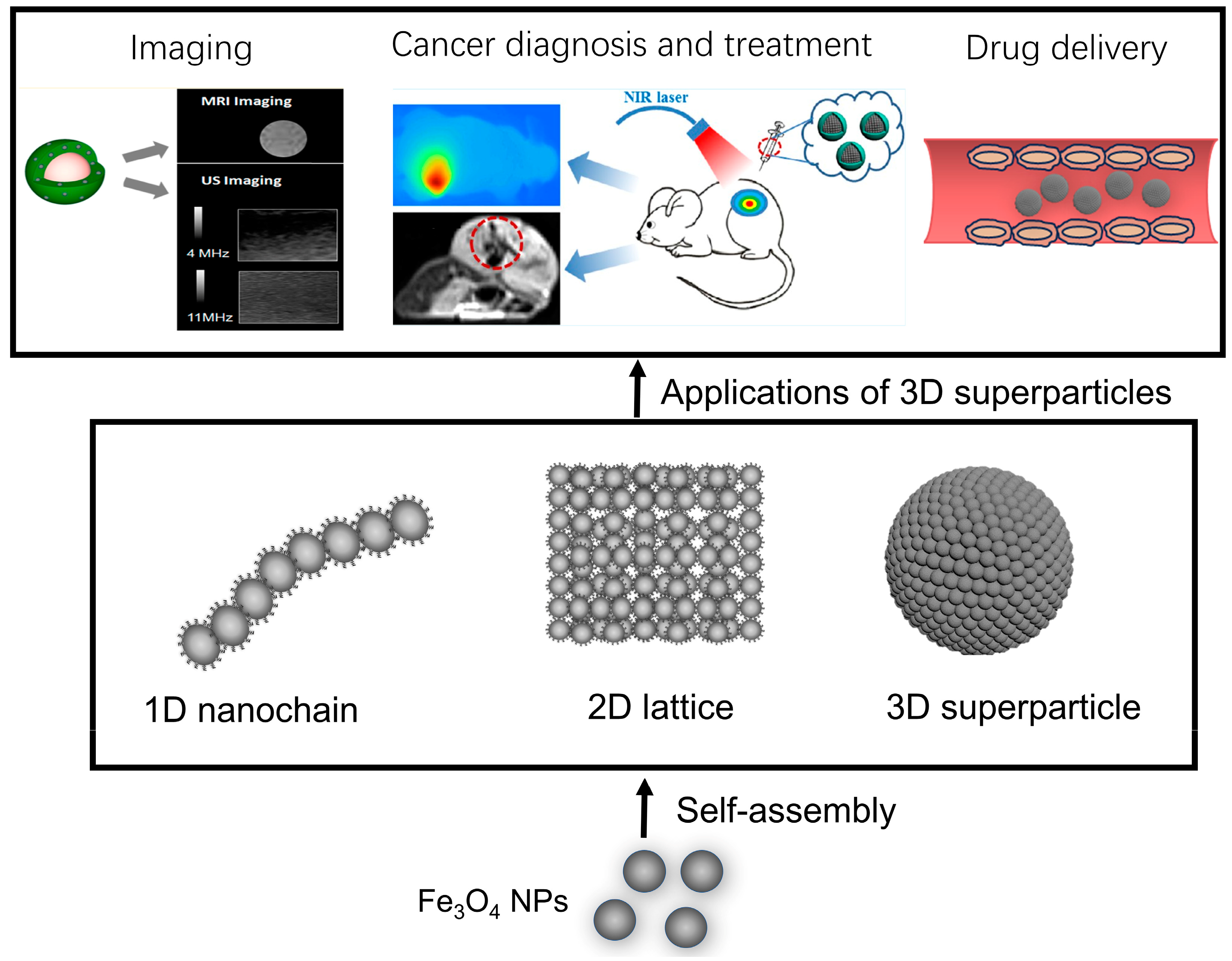
2. Self-Assembly of Fe3O4 NPs
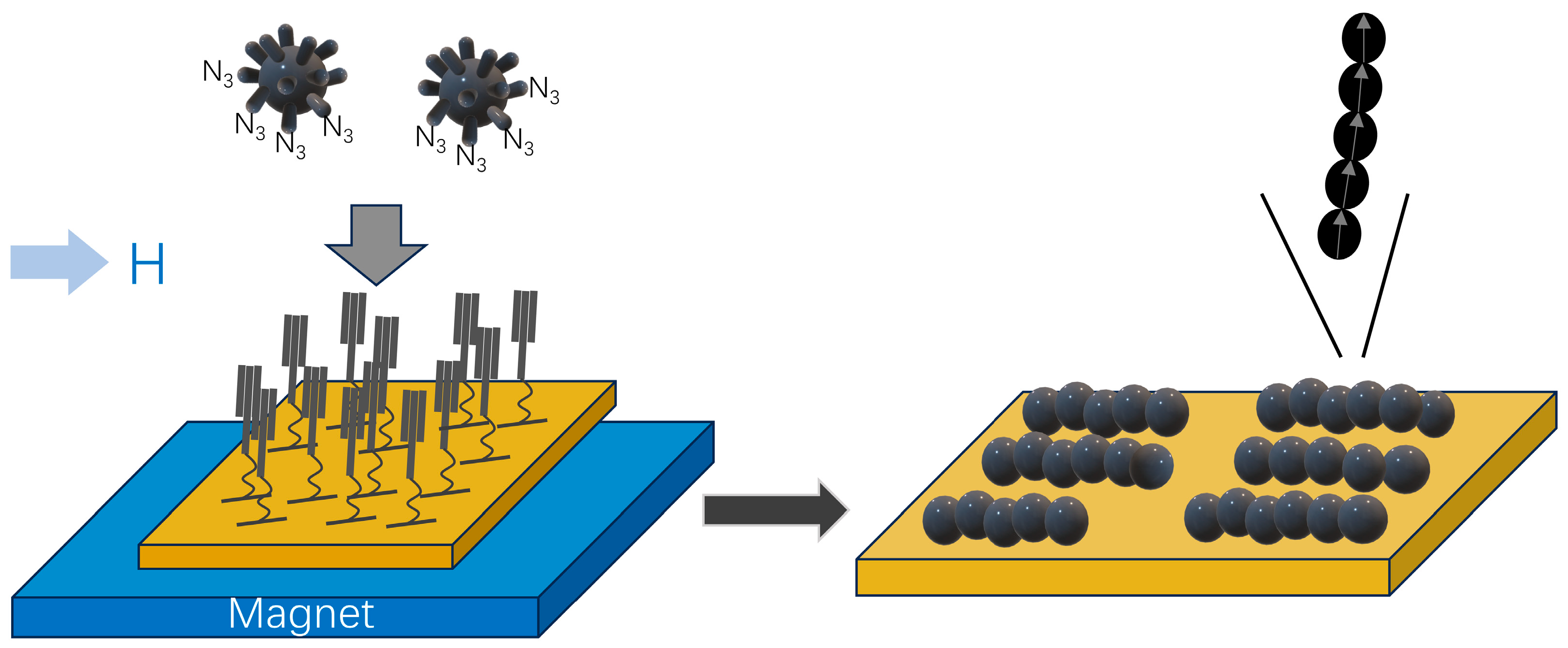
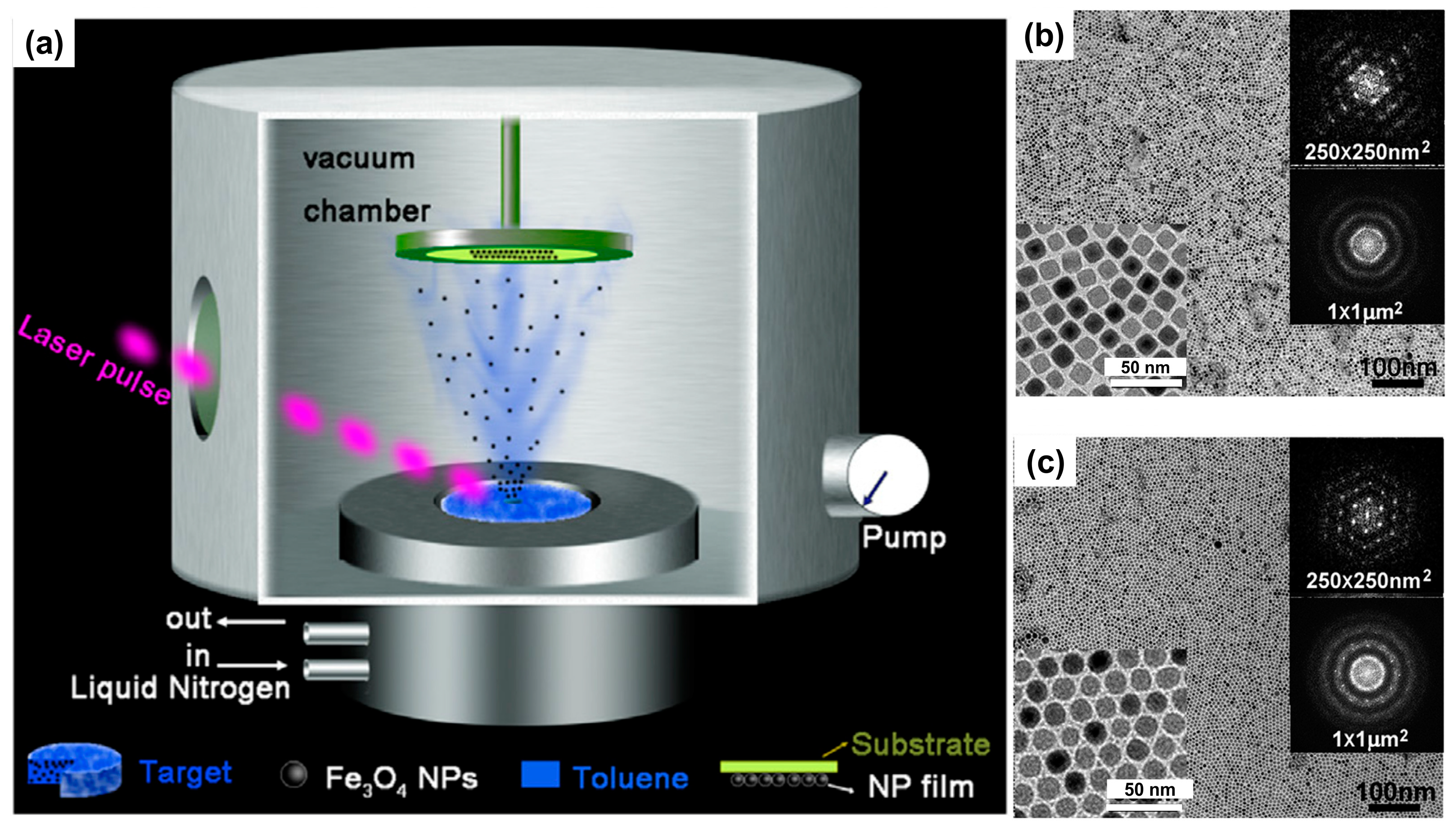
2.1. 1D Fe3O4 NP Nanoarrays
2.2. 2D Fe3O4 NP Nanoarrays
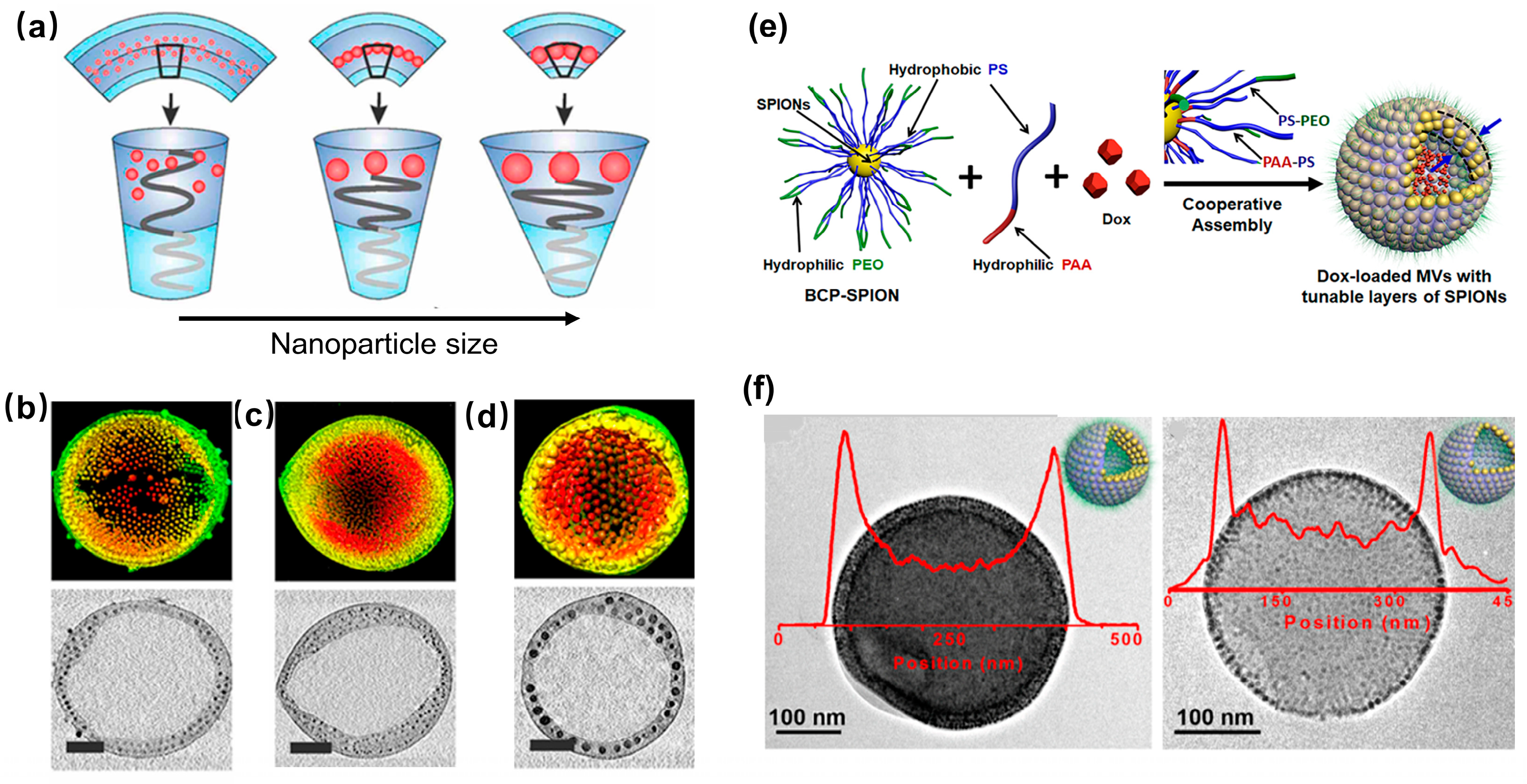
2.3. 3D Fe3O4 NP Superstructure
3. Application of Fe3O4 NP Assemblies
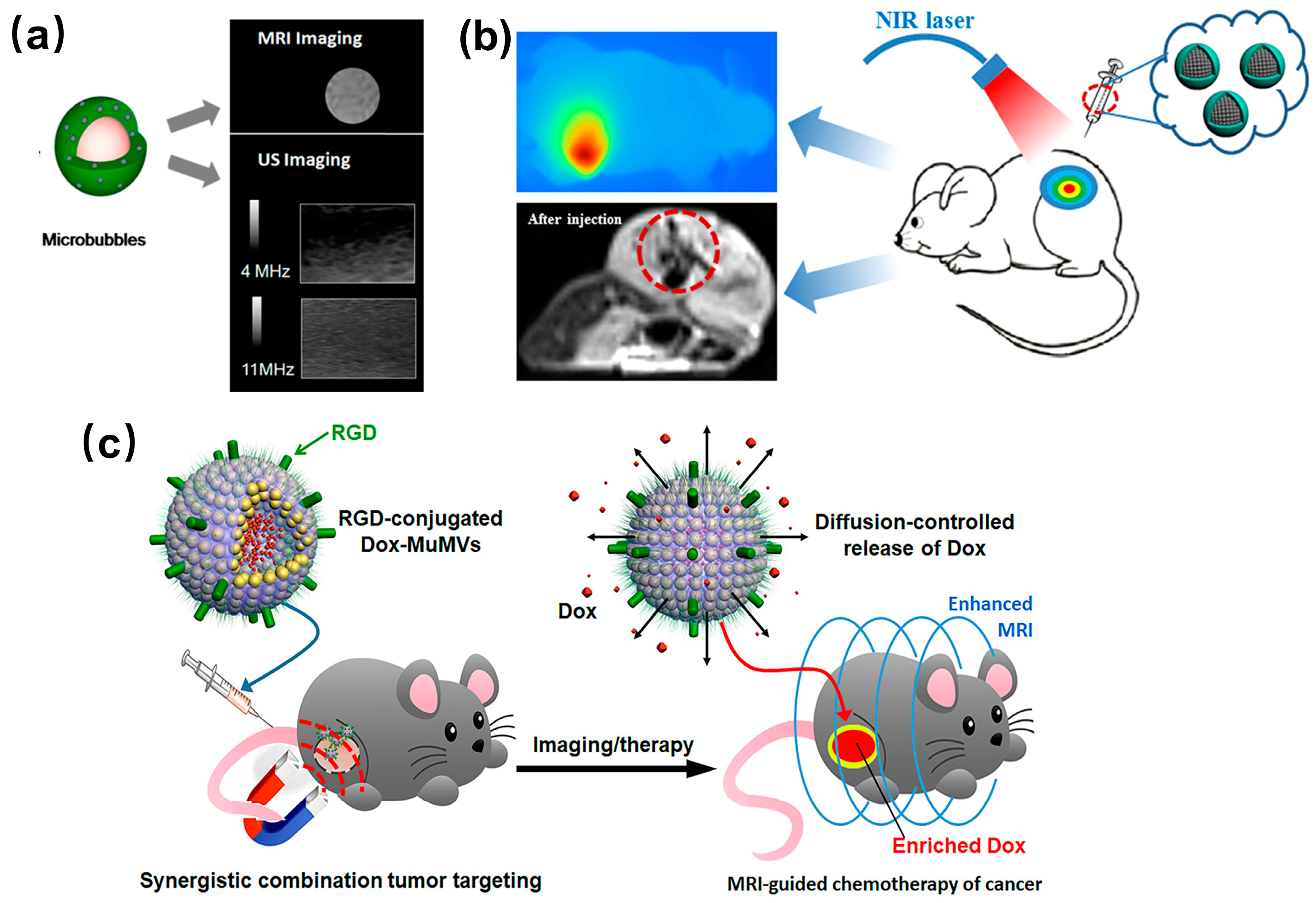
3.1. Imaging
3.2. Cancer Diagnosis and Therapy
3.3. Drug Delivery
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, S.; Wang, C.; Xie, J.; Sun, S. Synthesis and Stabilization of Monodisperse Fe Nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef] [PubMed]
- Dumestre, F.; Chaudret, B.; Amiens, C.; Renaud, P.; Fejes, P. Superlattices of Iron Nanocubes Synthesized from Fe[N(SiMe3)2]2. Science 2004, 303, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Puntes, V.F.; Krishnan, K.M.; Alivisatos, A.P. Colloidal Nanocrystal Shape and Size Control: The Case of Cobalt. Science 2001, 291, 2115–2117. [Google Scholar] [CrossRef]
- Park, J.; Kang, E.; Son, S.U.; Park, H.M.; Lee, M.K.; Kim, J.; Kim, K.W.; Noh, H.J.; Park, J.H.; Bae, C.J.; et al. Monodisperse Nanoparticles of Ni and NiO: Synthesis, Characterization, Self-Assembled Superlattices, and Catalytic Applications in the Suzuki Coupling Reaction. Adv. Mater. 2005, 17, 429–434. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef]
- Park, J.; Lee, E.; Hwang, N.; Kang, M.; Kim, S.; Hwang, Y.; Park, J.; Noh, H.; Kini, J.; Park, J.; et al. One-Nanometer-Scale Size-Controlled Synthesis of Monodisperse Magnetic Iron Oxide Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 2872–2877. [Google Scholar] [CrossRef]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Chavan, N.; Dharmaraj, D.; Sarap, S.; Surve, C. Magnetic Nanoparticles—A New Era in Nanotechnology. J. Drug Deliv. Sci. Technol. 2022, 77, 103899. [Google Scholar] [CrossRef]
- Jun, Y.W.; Lee, J.H.; Cheon, J. Chemical Design of Nanoparticle Probes for High-Performance Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2008, 47, 5122–5135. [Google Scholar] [CrossRef]
- Thiesen, B.; Jordan, A. Clinical Applications of Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef]
- Aquino, V.R.R.; Aquino, J.C.R.; Coaquira, J.A.H.; Bakuzis, A.F.; Sousa, M.H.; Morais, P.C. New Synthesis Route for High Quality Iron Oxide-Based Nanorings: Structural and Magnetothermal Evaluations. Mater. Des. 2023, 232, 112082. [Google Scholar] [CrossRef]
- Siqueira, E.R.L.; Pinheir, W.O.; Aquino, V.R.R.; Coelho, B.C.P.; Bakuzis, A.F.; Azevedo, R.B.; Sousa, M.H.; Paulo Cesar Morais, P.C. Engineering Gold Shelled Nanomagnets for Pre-Setting the Operating Temperature for Magnetic Hyperthermia. Nanomaterials 2022, 12, 2760. [Google Scholar] [CrossRef] [PubMed]
- Babincova, M.; Babinec, P. Magnetic Drug Delivery and Targeting: Principles and Applications. Biomed. Pap. 2009, 153, 243–250. [Google Scholar] [CrossRef]
- Polyak, B.; Friedman, G. Magnetic Targeting for Site-Specific Drug Delivery: Applications and Clinical Potential. Expert Opin. Drug Deliv. 2009, 6, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef]
- Blakemore, R. Magnetotactic Bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- Bazylinski, D.; Frankel, R. Magnetosome Formation in Prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230. [Google Scholar] [CrossRef]
- Acosta-Avalos, D.; Wajnberg, E.; Oliveira, P.S.; Leal, I.; Farina, M.; Esquivel, D.M.S. Isolation of Magnetic Nanoparticles from Pachycondyla Marginata Ants. J. Exp. Biol. 1999, 202, 2687–2692. [Google Scholar] [CrossRef]
- Lcal, I.R.; Oliveira, P.S. Behavioral Ecology of the Neotropical Termite-Hunting Ant Pachycondyla Marginata: Colony Founding, Group-Raiding and Migratory Patterns. Behav. Ecol. Sociobiol. 1995, 37, 373–383. [Google Scholar]
- Diebel, C.; Proksch, R.; Green, C.; Neilson, P.; Walker, M.M. Magnetite Defines a Vertebrate Magnetoreceptor. Nature 2000, 406, 299–302. [Google Scholar] [CrossRef]
- Falkenberg, G.; Fleissner, G.; Schuchardt, K.; Kuehbacher, M.; Thalau, P.; Mouritsen, H.; Heyers, D.; Wellenreuther, G.; Fleissner, G. Avian Magnetoreception: Elaborate Iron Mineral Containing Dendrites in the Upper Beak Seem to Be a Common Feature of Birds. PLoS ONE 2010, 5, e9231. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.; Noh, H.; Kim, J.; Park, J.; Hwang, N.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Chen, F.; Gao, Q.; Hong, G.; Ni, J. Synthesis and Characterization of Magnetite Dodecahedron Nanostructure by Hydrothermal Method. J. Magn. Magn. Mater. 2008, 320, 1775–1780. [Google Scholar] [CrossRef]
- Mikhaylova, M.; Kim, D.K.; Berry, C.C.; Zagorodni, A.; Toprak, M.; Curtis, A.S.G.; Muhammed, M. BSA Immobilization on Amine-Functionalized Superparamagnetic Iron Oxide Nanoparticles. Chem. Mater. 2004, 16, 2344–2354. [Google Scholar] [CrossRef]
- Itoh, H.; Sugimoto, T. Systematic Control of Size, Shape, Structure, and Magnetic Properties of uniform Magnetite and Maghemite Particles. J. Colloid Interface Sci. 2003, 265, 283–295. [Google Scholar] [CrossRef]
- Jun, Y.; Huh, Y.-M.; Choi, J.; Lee, J.; Song, H.; Kim, S.; Yoon, S.; Kim, K.; Shin, J.; Suh, J.; et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Brooks, R.; Moiny, F.; Gillis, P. On T2-Shortening by Weakly Magnetized Particles: The Chemical Exchange Model. Magn. Reson. Med. 2001, 45, 1014–1020. [Google Scholar] [CrossRef]
- Gillis, P.; Moiny, F.; Brooks, R.A. On T2-Shortening by Strongly Magnetized Spheres: A Partial Refocusing Model. Magn. Reson. Med. 2002, 47, 257–263. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Q.; Yin, Y. Colloidal Self-Assembly Approaches to Smart Nanostructured Materials. Chem. Rev. 2022, 122, 4976–5067. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, W.; Wang, C. Magnetic Colloidal Supraparticles: Design, Fabrication and Biomedical Applications. Adv. Mater. 2013, 25, 5196–5214. [Google Scholar] [CrossRef]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte Adsorption, Interparticle Forces, and Colloidal Aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef] [PubMed]
- Ai, H. Layer-By-Layer Capsules for Magnetic Resonance Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 772–788. [Google Scholar] [CrossRef]
- Thomas, L.A.; Dekker, L.; Kallumadil, M.; Southern, P.; Wilson, M.; Nair, S.P.; Pankhurstc, Q.A.; Parkin, I.P. Carboxylic Acid-Stabilised Iron Oxide Nanoparticles for Use in Magnetic Hyperthermia. J. Mater. Chem. 2009, 19, 6529–6535. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Khaniabadi, P.M.; Mehrdel, B. Simple Rapid Stabilization Method through Citric Acid Modification for Magnetite Nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Hackett, M.J.; Hyeon, T. Surface Ligands in Synthesis, Modification, Assembly and Biomedical Applications of Nanoparticles. Nano Today 2014, 9, 457–477. [Google Scholar] [CrossRef]
- Amstad, E.; Gillich, T.; Bilecka, I.; Textor, M.; Reimhult, E. Ultrastable Iron Oxide Nanoparticle Colloidal Suspensions Using Dispersants with Catechol-Derived Anchor Groups. Nano Lett. 2009, 9, 4042–4048. [Google Scholar] [CrossRef]
- Zvarec, O.; Purushotham, S.; Masic, A.; Ramanujan, R.V.; Miserez, A. Catechol-Functionalized Chitosan/Iron Oxide Nanoparticle Composite Inspired by Mussel Thread Coating and Squid Beak Interfacial Chemistry. Langmuir 2013, 29, 10899–10906. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces Preface to the Third Edition. In Intermolecular and Surface Forces; University of California: Santa Barbara, CA, USA, 2011; p. xvii. [Google Scholar]
- Min, Y.J.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The Role of Interparticle and External Forces in Nanoparticle Assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Huang, X.; Li, F.; Davis, T.P.; Qiao, R.; Ling, D. Polymer-Assisted Magnetic Nanoparticle Assemblies for Biomedical Applications. ACS Appl. Bio Mater. 2020, 3, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, A.; Yatvin, J.; Trotsenko, O.; Locklin, J.; Minko, S. Nanostructured Soft Matter with Magnetic Nanoparticles. Adv. Funct. Mater. 2016, 26, 3761–3782. [Google Scholar] [CrossRef]
- Song, S.; Guo, H.; Jiang, Z.; Jin, Y.; Zhang, Z.; Sun, K.; Dou, H. Self-Assembled Fe3O4/Polymer Hybrid Microbubble with MRI/ Ultrasound Dual-Imaging Enhancement. Langmuir 2014, 30, 10557–10561. [Google Scholar] [CrossRef]
- Ge, R.; Li, X.; Lin, M.; Wang, D.; Li, S.; Liu, S.; Tang, Q.; Liu, Y.; Jiang, J.; Liu, L.; et al. Fe3O4@polydopamine Composite Theranostic Superparticles Employing Preassembled Fe3O4 Nanoparticles as the Core. ACS Appl. Mater. Interfaces 2016, 8, 22942–22952. [Google Scholar] [CrossRef]
- Dreyer1, A.; Feld, A.; Kornowski, A.; Yilmaz, E.D.; Noei, H.; Meyer, A.; Krekeler, T.; Jiao, C.; Stierle, A.; Abetz, V.; et al. Organically Linked Iron Oxide Nanoparticle Supercrystals with Exceptional Isotropic Mechanical Properties. Nat. Mater. 2016, 15, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Dhak, P.; Kim, M.-K.; Lee, J.H.; Kim, M.; Kim, S.-K. Linear-Chain Assemblies of Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2017, 433, 47–52. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.-C. Fabrication of One-Dimensional Iron Oxide/Silica Nanostructures with High Magnetic Sensitivity by Dipole-Directed Self-Assembly. J. Phys. Chem. C 2008, 112, 15151–15156. [Google Scholar] [CrossRef]
- Toulemon, D.; Rastei, M.V.; Schmool, D.; Garitaonandia, J.S.; Lezama, L.; Cattoën, X.; Bégin-Colin, S.; Pichon, B.P. Enhanced Collective Magnetic Properties Induced by the Controlled Assembly of Iron Oxide Nanoparticles in Chains. Adv. Funct. Mater. 2016, 26, 2454–2462. [Google Scholar] [CrossRef]
- Park, M.; Kang, S.; Nam, C.; Narasimha, K.; Lee, W.B.; Park, S.-J. Magnetic Field-Induced Self-Assembly of Conjugated Block Copolymers and Nanoparticles at the Air–Water Interface. ACS Appl. Mater. Interfaces 2022, 14, 8266–8273. [Google Scholar] [CrossRef]
- Liao, X.; Ulusoy, S.; Huang, R.; Wetterskog, E.; Gunnarsson, K.; Wang, Y.; Liang, H.; Zeng, Y.-J.; Salazar-Alvarez, G.; Svedlindh, P. Low-Field-Induced Spin-Glass Behavior and Controllable Anisotropy in Nanoparticle Assemblies at a Liquid-Air Interface. Sci. China Mater. 2022, 65, 193–200. [Google Scholar] [CrossRef]
- Ekeroth, S.; Münger, E.P.; Boyd, R.; Ekspong, J.; Wågberg, T.; Edman, L.; Brenning, N.; Helmersson, U. Catalytic Nanotruss Structures Realized by Magnetic Self-Assembly in Pulsed Plasma. Nano Lett. 2018, 18, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Bennet, M.; Bertinetti, L.; Neely, R.K.; Schertel, A.; Körnig, A.; Flors, C.; Müller, F.D.; Schüler, D.; Klumpp, S.; Faivre, D. Biologically Controlled Synthesis and Assembly of Magnetite Nanoparticles. Faraday Discuss. 2015, 181, 71–83. [Google Scholar] [CrossRef]
- Alphandéry, E.; Ding, Y.; Ngo, A.T.; Wang, Z.L.; Wu, L.F.; Pileni, M.P. Assemblies of Aligned Magnetotactic Bacteria and Extracted Magnetosomes: What Is the Main Factor Responsible for the Magnetic Anisotropy? ACS Nano 2009, 3, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Eloi, M.T.A.; Santos, J.L., Jr.; Morais, P.C.; Bakuzis, A.F. Field-Induced Columnar Transition of Biocompatible Magnetic Colloids: An Aging Study by Magnetotransmissivity. Phy. Rev. E 2010, 82, 021407. [Google Scholar] [CrossRef]
- Hu, J.; Pan, B.; Makihara, T.; Garcia, R.D.J.; Herman, I.P. Brewster Angle Optical Reflection Observation of Self-limiting Nanoparticle Monolayer Self-assembly at a Liquid/Liquid Interface. AIP Adv. 2019, 9, 065022. [Google Scholar] [CrossRef]
- Sachan, M.; Bonnoit, C.; Hogg, C.; Evarts, E.; Bain, J.A.; Majetich, S.A.; Park, J.-H.; Zhu, J.-G. Self-Assembled Nanoparticle Arrays as Nanomasks for Pattern Transfer. J. Phys. D Appl. Phys. 2008, 41, 134001. [Google Scholar] [CrossRef]
- Vaknin, D.; Wang, W.; Islam, F.; Zhang, H. Polyethylene-Glycol-Mediated Self-Assembly of Magnetite Nanoparticles at the Liquid/Vapor Interface. Adv. Mater. Interfaces 2018, 5, 1701149. [Google Scholar] [CrossRef]
- Fleutot, S.; Nealon, G.L.; Pauly, M.; Pichon, P.; Leuvrey, C.; Drillon, M.; Gallani, J.-L.; Guillon, D.; Donnio, B.; Begin-Colin, S. Spacing-Dependent Dipolar Interactions in Dendronized Magnetic Iron Oxide Nanoparticle 2D Arrays and Powders. Nanoscale 2013, 5, 1507–1516. [Google Scholar] [CrossRef]
- Disch, S.; Wetterskog, E.; Hermann, R.P.; Salazar-Alvarez, G.; Busch, P.; Brückel, T.; Bergström, L.; Kamali, S. Shape Induced Symmetry in Self-Assembled Mesocrystals of Iron Oxide Nanocubes. Nano Lett. 2011, 11, 1651–1656. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, F.; Zhang, J.; Bian, B.; Hu, Y.; Xue, F.; Du, J. Large-Scale Area of Magnetically Anisotropic Nanoparticle Monolayer Films Deposited by MAPLE. J. Mater. Sci. Technol. 2022, 106, 28–32. [Google Scholar] [CrossRef]
- Oberdick, D.; Majetich, S.A. Electrophoretic Deposition of Iron Oxide Nanoparticles on Templates. J. Phys. Chem. C 2013, 117, 18709–18718. [Google Scholar] [CrossRef]
- Xiang, Q.; Teixeira, C.B.; Sun, L.; Morais, P.C. Magnetic Nanoparticle Film Reconstruction Modulated by Immersion within DMSA Aqueous Solution. Sci. Rep. 2016, 6, 18202. [Google Scholar] [CrossRef] [PubMed]
- Benitez, M.J.; Mishra, D.; Szary, P.; Badini Confalonieri, G.A.; Feyen, M.; Lu, A.H.; Agudo, L.; Eggeler, G.; Petracic, O.; Zabel, H. Structural and Magnetic Characterization of Self-Assembled Iron Oxide Nanoparticle Arrays. J. Phys. Condens. Matter 2011, 23, 126003. [Google Scholar] [CrossRef][Green Version]
- Szyndler, M.W.; Corn, R.M. Self-Assembly of Flux-Closure Polygons from Magnetite Nanocubes. J. Phys. Chem. Lett. 2012, 3, 2320–2325. [Google Scholar] [CrossRef]
- Schmidtke, C.; Eggers, R.; Zierold, R.; Feld, A.; Kloust, H.; Wolter, C.; Ostermann, J.; Merkl, J.-P.; Schotten, T.; Nielsch, K.; et al. Polymer-Assisted Self-Assembly of Superparamagnetic Iron Oxide Nanoparticles into Well-Defined Clusters: Controlling the Collective Magnetic Properties. Langmuir 2014, 30, 11190–11196. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic Magnetite Colloidal Nanocrystal Clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.; Gossuin, Y.; Muller, R.N.; Gillis, P. Superparamagnetic Colloid Suspensions: Water Magnetic Relaxation and Clustering. J. Magn. Magn. Mater. 2005, 293, 532–539. [Google Scholar] [CrossRef]
- Pöselt, E.; Kloust, H.; Tromsdorf, U.; Janschel, M.; Hahn, C.; Maßlo, C.; Weller, H. Relaxivity Optimization of a Pegylated Iron-Oxide-Based Negative Magnetic Resonance Contrast Agent for T2-Weighted Spin-Echo Imaging. ACS Nano 2012, 6, 1619–1624. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Lee, H.; Shao, H.; Hilderbrand, S.A.; Weissleder, R. Multicore Assemblies Potentiate Magnetic Properties of Biomagnetic Nanoparticles. Adv. Mater. 2011, 23, 4793–4797. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Highly Water-Dispersible Biocompatible Magnetite Particles with Low Cytotoxicity Stabilized by Citrate Groups. Angew. Chem. Int. Ed. 2009, 48, 5875–5879. [Google Scholar] [CrossRef]
- Stolarczyk, J.K.; Deak, A.; Brougham, D.F. Nanoparticle Clusters: Assembly and Control Over Internal Order, Current Capabilities, and Future Potential. Adv. Mater. 2016, 28, 5400–5424. [Google Scholar] [CrossRef]
- Lee, S.H.; Yu, S.-H.; Lee, J.E.; Jin, A.; Lee, D.J.; Lee, N.; Jo, H.; Shin, K.; Ahn, T.-Y.; Kim, Y.-W.; et al. Self-Assembled Fe3O4 Nanoparticle Clusters as High-Performance Anodes for Lithium Ion Batteries via Geometric Confinement. Nano Lett. 2013, 13, 4249–4256. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C. Controllable Assembly of Hydrophobic Superparamagnetic Iron Oxide Nanoparticle withmPEG-PLA Copolymer and Its Effect on MR Transverse Relaxation Rate. J. Nanomater. 2010, 2011, 1–7. [Google Scholar] [CrossRef]
- Qiu, P.; Jensen, C.; Charity, N.; Towner, R.; Mao, C. Oil Phase Evaporation-Induced Self-Assembly of Hydrophobic Nanoparticles into Spherical Clusters with Controlled Surface Chemistry in an Oil-in-Water Dispersion and Comparison of Behaviors of Individual and Clustered Iron Oxide Nanoparticles. J. Am. Chem. Soc. 2010, 132, 17724–17732. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Sobal, N.S.; Giersig, M.; Kurth, D.G.; Möhwald, H. Magnetic Colloidosomes Derived from Nanoparticle Interfacial Self-Assembly. Nano Lett. 2005, 5, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Haynes, A.S.; Kikkawa, J.M.; Park, S.-J. Controlling the Self-Assembly Structure of Magnetic Nanoparticles and Amphiphilic Block-Copolymers: From Micelles to Vesicles. J. Am. Chem. Soc. 2011, 133, 1517–1525. [Google Scholar] [CrossRef]
- Hickey, R.J.; Koski, J.; Meng, X.; Riggleman, R.A.; Zhang, P.; Park, S.-J. Size-Controlled Self-Assembly of Superparamagnetic Polymersomes. ACS Nano 2014, 8, 495–502. [Google Scholar] [CrossRef]
- Hickey, R.J.; Meng, X.; Zhang, P.; Park, S.-J. Low-Dimensional Nanoparticle Clustering in Polymer Micelles and Their Transverse Relaxivity Rates. ACS Nano 2013, 7, 5824–5833. [Google Scholar] [CrossRef]
- Yang, K.; Liu, Y.; Liu, Y.; Zhang, Q.; Kong, C.; Yi, C.; Zhou, Z.; Wang, Z.; Zhang, G.; Zhang, Y.; et al. Cooperative Assembly of Magneto-Nanovesicles with Tunable Wall Thickness and Permeability for MRI-Guided Drug Delivery. J. Am. Chem. Soc. 2018, 140, 4666–4677. [Google Scholar] [CrossRef]
- Singamaneni, S.; Bliznyuk, V.N.; Binekc, C.; Tsymbalc, E.Y. Magnetic Nanoparticles: Recent Advances in Synthesis, Self-Assembly and Applications. J. Mater. Chem. 2011, 21, 16819–16845. [Google Scholar] [CrossRef]
- Majetich, S.A.; Wen, T.; Booth, R.A. Functional Magnetic Nanoparticle Assemblies: Formation, Collective Behavior, and Future Directions. ACS Nano. 2011, 5, 6081–6084. [Google Scholar] [CrossRef]
- Wan, Q.; Xie, L.; Gao, L.; Wang, Z.; Nan, X.; Lei, H.; Long, X.; Chen, Z.-Y.; He, C.-Y.; Liu, G.; et al. Self-Assembled Magnetic Theranostic Nanoparticles for Highly Sensitive MRI of Minicircle DNA Delivery. Nanoscale 2013, 5, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Glover, A.L.; Bennett, J.B.; Pritchett, J.S.; Nikles, S.M.; Nikles, D.E.; Nikles, J.A.; Brazel, C.S. Magnetic Heating of Iron Oxide Nanoparticles and Magnetic Micelles for Cancer Therapy. IEEE Trans. Magn. 2013, 49, 231–235. [Google Scholar] [CrossRef]
- Lin, J.-J.; Chen, J.-S.; Huang, S.-J.; Ko, J.-H.; Wang, Y.-M.; Chen, T.-L.; Wang, L.-F. Folic Acid–Pluronic F127 Magnetic Nanoparticle Clusters for Combined Targeting, Diagnosis, and Therapy Applications. Biomaterials 2009, 30, 5114–5124. [Google Scholar] [CrossRef]
- Ling, D.; Park, W.; Park, S.-J.; Lu, Y.; Kim, K.S.; Hackett, M.J.; Kim, B.H.; Yim, H.; Jeon, Y.S.; Na, K.; et al. Multifunctional Tumor pH-Sensitive Self-Assembled Nanoparticles for Bimodal Imaging and Treatment of Resistant Heterogeneous Tumors. J. Am. Chem. Soc. 2014, 136, 5647–5655. [Google Scholar] [CrossRef]
- Jeong, M.; Lee, S.; Song, D.Y.; Kang, S.; Shin, T.-H.; Choi, J.-S. Hyperthermia Effect of Nanoclusters Governed by Interparticle Crystalline Structures. ACS Omega 2021, 6, 31161–31167. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-L.; Zhou, J.; Zhou, H.; Wu, Y.; Liu, R.; Wang, L.-L.; Lin, W.-W.; Huang, G.; Yang, H.-H. Bioinspired “Active” Stealth Magneto-Nanomicelles for Theranostics Combining Efficient MRI and Enhanced Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 30502–30509. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Xu, H.; Sun, Y.; Liu, Y.; Yan, R.; Chen, Y.; Zhang, Z. Magnetite Nanoparticle Assemblies and Their Biological Applications: A Review. Molecules 2024, 29, 4160. https://doi.org/10.3390/molecules29174160
Wei J, Xu H, Sun Y, Liu Y, Yan R, Chen Y, Zhang Z. Magnetite Nanoparticle Assemblies and Their Biological Applications: A Review. Molecules. 2024; 29(17):4160. https://doi.org/10.3390/molecules29174160
Chicago/Turabian StyleWei, Jinjian, Hong Xu, Yating Sun, Yingchun Liu, Ran Yan, Yuqin Chen, and Zhide Zhang. 2024. "Magnetite Nanoparticle Assemblies and Their Biological Applications: A Review" Molecules 29, no. 17: 4160. https://doi.org/10.3390/molecules29174160
APA StyleWei, J., Xu, H., Sun, Y., Liu, Y., Yan, R., Chen, Y., & Zhang, Z. (2024). Magnetite Nanoparticle Assemblies and Their Biological Applications: A Review. Molecules, 29(17), 4160. https://doi.org/10.3390/molecules29174160