Quantum Chemical Stability Analysis of Phthalocyanine Metal One-Dimensional Polymers with Bidentate Ligands
Abstract
1. Introduction
2. Results and Discussion
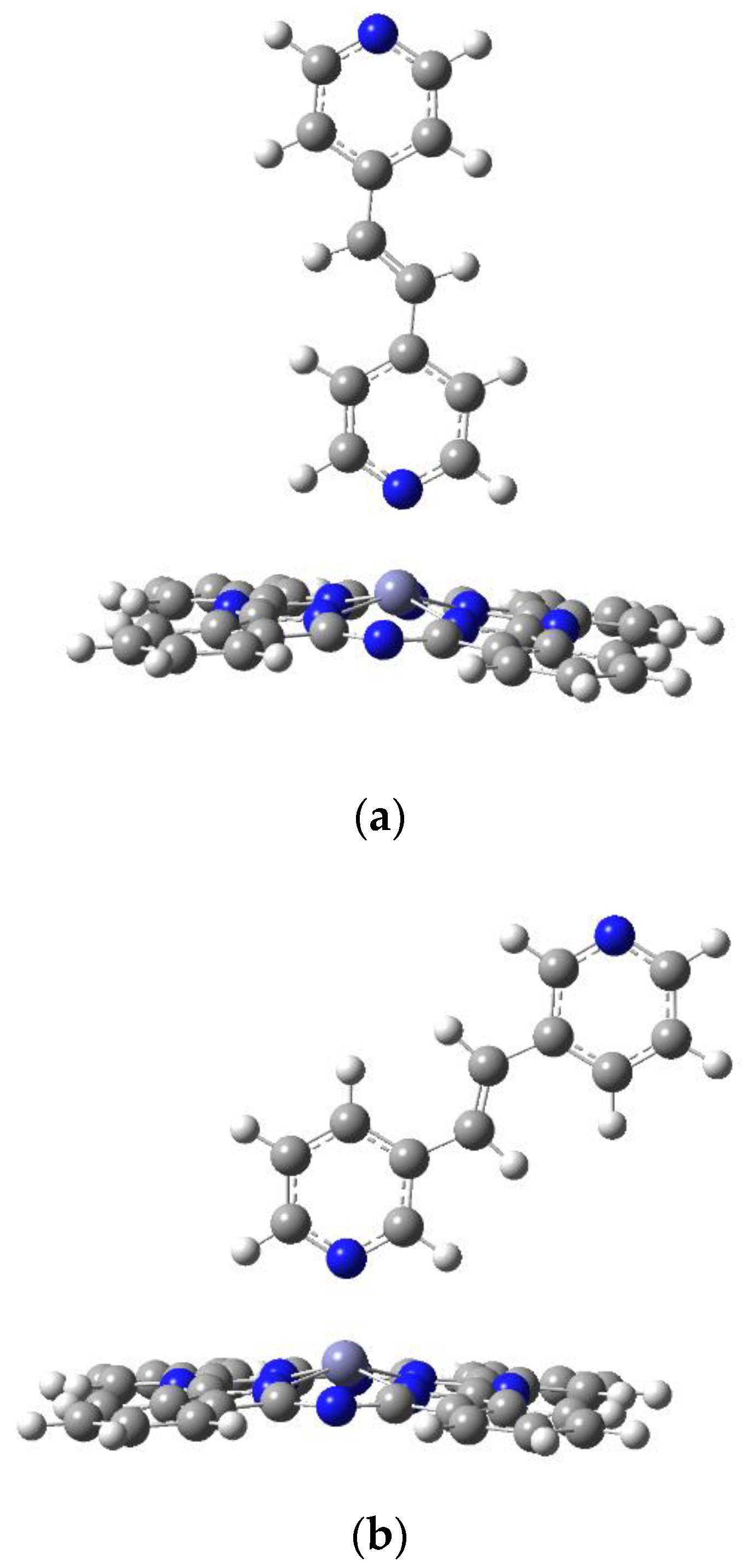
2.1. Geometry Optimization of PcZn Complex with Ligands (I–VI)
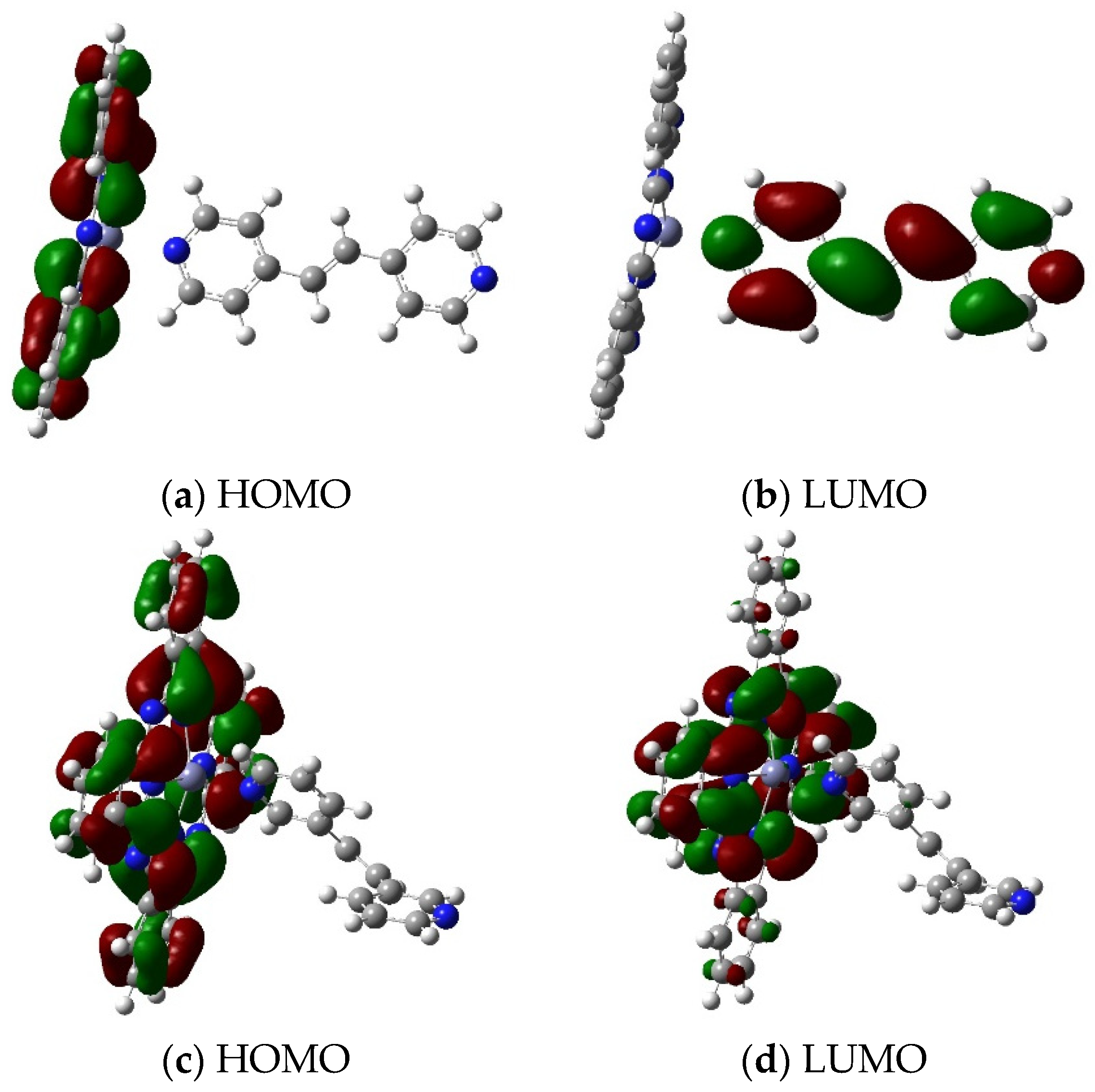
2.2. HOMO LUMO of PcZn Complex with Ligands (I–VI)
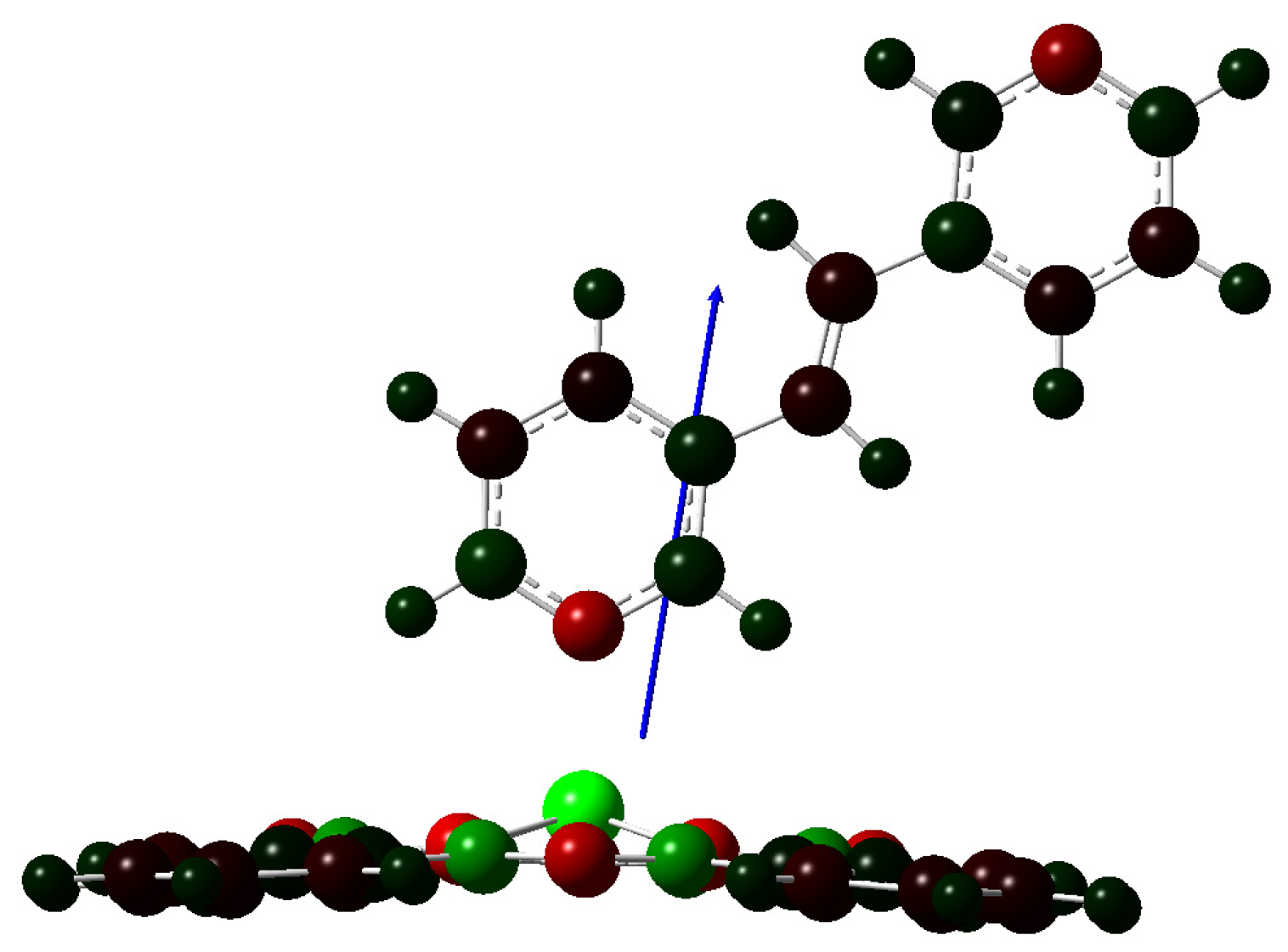
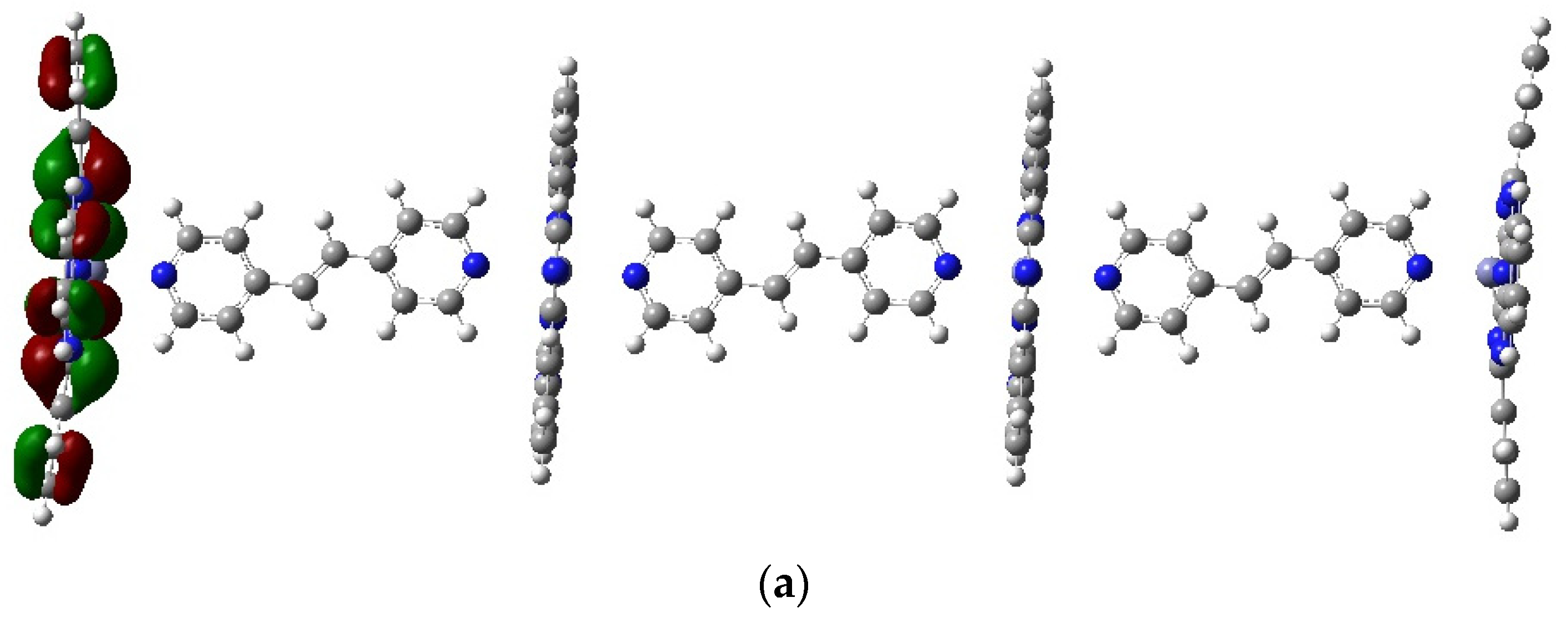
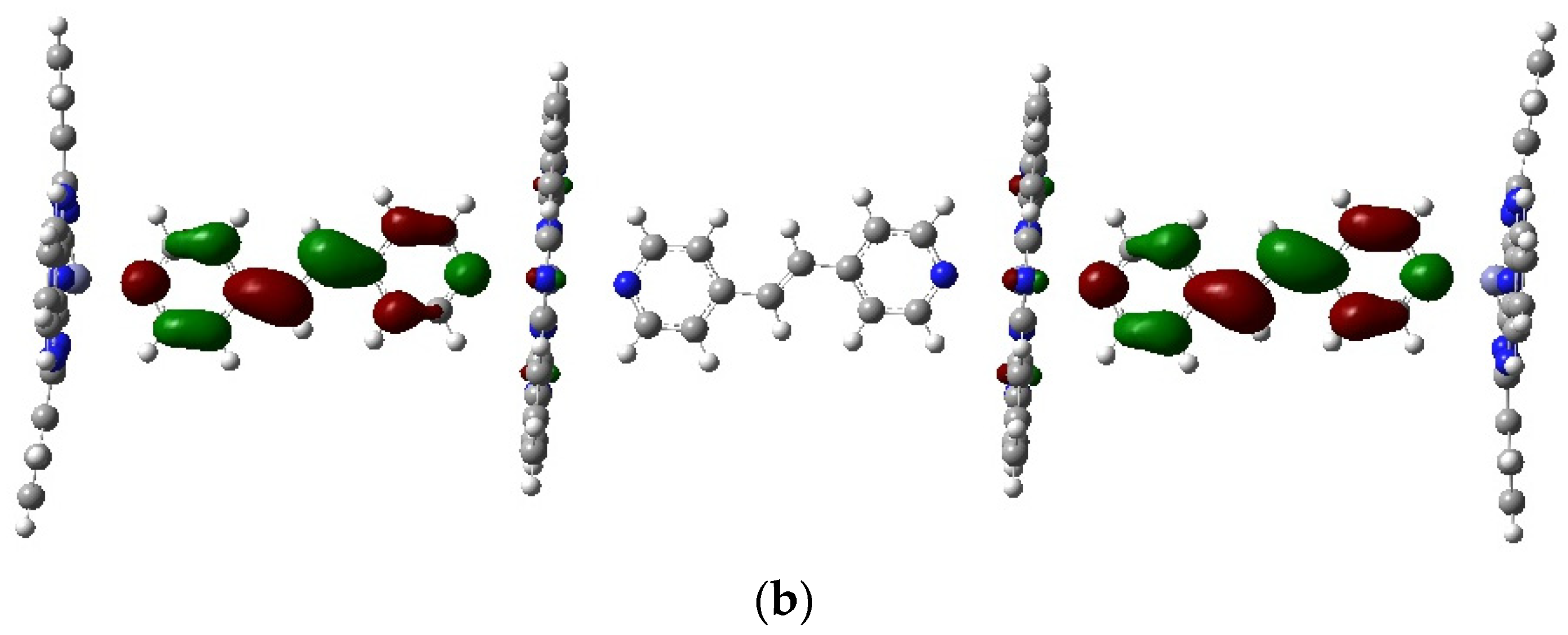
2.3. Geometry Optimization of Polymers Pc(PcZnL)3 (L = I and II)
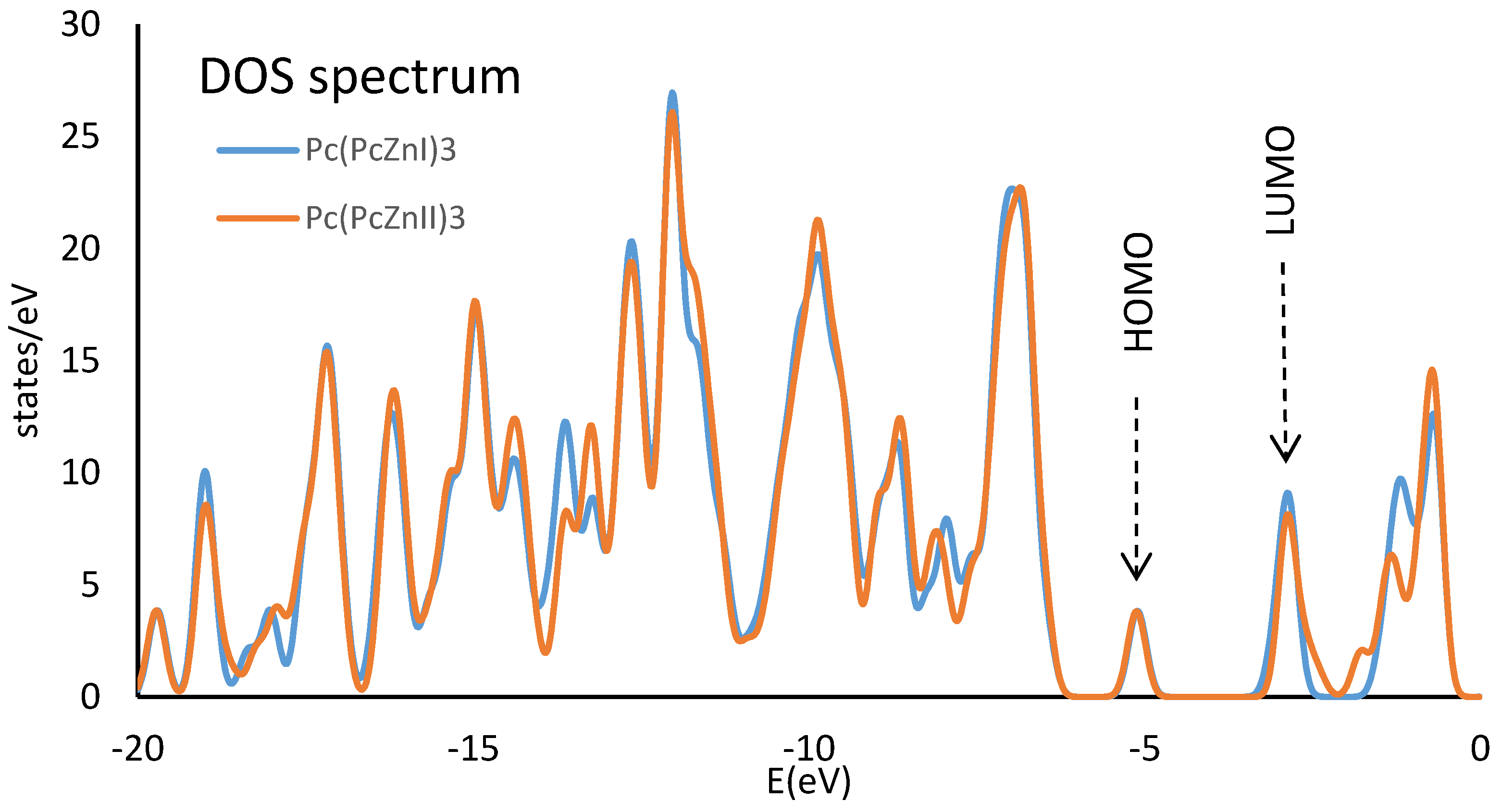
2.4. HOMO LUMO of Polymers Pc(PcZnL)3 (L = I and II)
3. Methods
Quantum Chemical Calculations Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, L.Y.; Yang, J.; Fu, Q.; Zhao, B.Z.; Tian, L.; Yu, H.L. Synthesis, crystal structure and characterization of a new zinc phthalocyanine complex. J. Mol. Struct. 2007, 827, 149–154. [Google Scholar] [CrossRef]
- Ferraudi, G.; Lappin, A.G. Review: Properties and chemical reactivity of metallo phthalocyanine and tetramethylbenzoannulene complexes grafted into a polymer backbone. J. Coord. Chem. 2014, 67, 3822–3839. [Google Scholar] [CrossRef]
- Janczak, J. Water-Involved Hydrogen Bonds in Dimeric Supramolecular Structures of Magnesium and Zinc Phthalocyaninato Complexes. ACS Omega 2019, 4, 3673–3683. [Google Scholar] [CrossRef]
- De La Torre, G.; Claessens, C.G.; Torres, T. Phthalocyanines: Old dyes, new materials. Putting color in nanotechnology. Chem. Commun. 2007, 20, 2000–2015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Leznoff, D.B. Phthalocyanine as a redox-active platform for organometallic chemistry. Chem. Commun. 2018, 54, 1829–1832. [Google Scholar] [CrossRef]
- Han, B.; Liang, B.; Zhang, E.; Li, J.; Li, Y.; Zhang, Q.; Xie, Z.; Wang, H.; Jiang, J. Phthalocyanine Covalent Organic Frameworks: Dimensionality Effect on Third-Order Nonlinear Optical Properties. Adv. Funct. Mater. 2024, 2404289, 1–7. [Google Scholar] [CrossRef]
- Kareem, R.O.; Hamad, O.A.; Omer, P.K. Recent Development in Synthesize, Properties, Characterization, and Application of Phthalocyanine and Metal Phthalocyanine. J. Chem. Rev. 2024, 6, 39–75. [Google Scholar]
- Martynov, A.G.; Mack, J.; May, A.K.; Nyokong, T.; Gorbunova, Y.G.; Tsivadze, A.Y. Methodological Survey of Simplified TD-DFT Methods for Fast and Accurate Interpretation of UV-Vis-NIR Spectra of Phthalocyanines. ACS Omega 2019, 4, 7265–7284. [Google Scholar] [CrossRef]
- Gajda, Ł.; Kupka, T.; Broda, M.A. Solvent impact on the planarity and aromaticity of free and monohydrated zinc phthalocyanine: A theoretical study. Struct. Chem. 2018, 29, 667–679. [Google Scholar] [CrossRef]
- Ueno, L.T.; Machado, A.E.H.; Machado, F.B.C. Theoretical studies of zinc phthalocyanine monomer, dimer and trimer forms. J. Mol. Struct. THEOCHEM 2009, 899, 71–78. [Google Scholar] [CrossRef]
- Lee, A.; Kim, D.; Choi, S.H.; Park, J.W.; Jaung, J.Y.; Jung, D.H. Theoretical study on phthalocyanine, pyrazinoporphyrazine and their complexation with Mg2+ and Zn2+. Mol. Simul. 2010, 36, 192–198. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, D.; Wang, C.; Lu, J.; Ma, B.; Shu, C.; Ma, H.; Li, Y.; Bai, C. Bipyridine conformations control the solid-state supramolecular chemistry of zinc(II) phthalocyanine with bipyridines. CrystEngComm 2005, 7, 243–248. [Google Scholar] [CrossRef]
- Janczak, J.; Kubiak, R. Pyrazine control of the solid-state supramolecular chemistry of zinc phthalocyanine. Polyhedron 2009, 28, 2391–2396. [Google Scholar] [CrossRef]
- Janczak, J. Peripherally octamethyl zinc(II) phthalocyanines with various axial substituents. Polyhedron 2021, 197, 115024. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Chen, Y.; Fan, X.; Zeng, Q. Solid-state supramolecular chemistry of zinc tetraphenylporphyrin and zinc phthalocyanine with bis(pyridyl) ligands. J. Mol. Struct. 2011, 1002, 145–150. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, K.Q.; Zheng, W.X.; Huang, C.C.; Zheng, B.Y.; Ke, M.R.; Huang, J.D. Solid-state supramolecular structures and excellent photothermal activities of dimeric zinc(II) phthalocyanines axially bridged with bipyridine derivatives. Dye. Pigment. 2022, 199, 110037. [Google Scholar] [CrossRef]
- Cordeiro, M.R.; Moreira, W.C. Thermal behavior of tetrapyrrole derivatives and their mixed complexes. Thermochim. Acta 2009, 486, 52–56. [Google Scholar] [CrossRef]
- Shukla, A.D.; Dave, P.C.; Suresh, E.; Das, A.; Dastidar, P. Multicomponent Zn-tetraphenylporphyrins: Syntheses, characterization and their self assembly in the solid state. J. Chem. Soc. Dalton Trans. 2000, 4459–4463. [Google Scholar] [CrossRef]
- Schneider, O.; Hanack, M. Phthalocyaninatoiron with Pyrazine as a Bidentate Bridging Ligand. Angew. Chem. Int. Ed. Engl. 1980, 19, 392–393. [Google Scholar] [CrossRef]
- Yuan, N. Coordination Polymers and Clusters Based on the Versatile Mercaptonicotinate Ligands. Eur. J. Inorg. Chem. 2019, 2019, 4607–4620. [Google Scholar] [CrossRef]
- De, S.; Devic, T.; Fateeva, A. Porphyrin and phthalocyanine-based metal organic frameworks beyond metal-carboxylates. Dalton Trans. 2021, 50, 1166–1188. [Google Scholar] [CrossRef] [PubMed]
- Al-Anber, M.; Vatsadze, S.; Holze, R.; Lang, H.; Thiel, W.R. π-Conjugated N-heterocyclic compounds: Correlation of computational and electrochemical data. Dalton Trans. 2005, 22, 3632–3637. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.J.; Fang, X.; Yu, H.Y.; Wang, J.D. (4-amino-pyridine-κN 1)(phthalo-cyan-inato- κ4 N)zinc(II) tetra-hydro-furan disolvate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2008, 64, 375–377. [Google Scholar] [CrossRef]
- Przybył, B.; Janczak, J. 1,8-Diazabicyclo[5.4.0]Undec-7-En-8-Ium Bromido(Phthalocyaninato)Zincate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m231–m232. [Google Scholar] [CrossRef]
- Mendizabal, F. Phthalocyanoruthenium complexes and polymers with bridged ligands. J. Mol. Struct. THEOCHEM 2002, 579, 169–179. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. G16_C01 2016, Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. A Library for Package-Independent Computational Chemistry Algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]


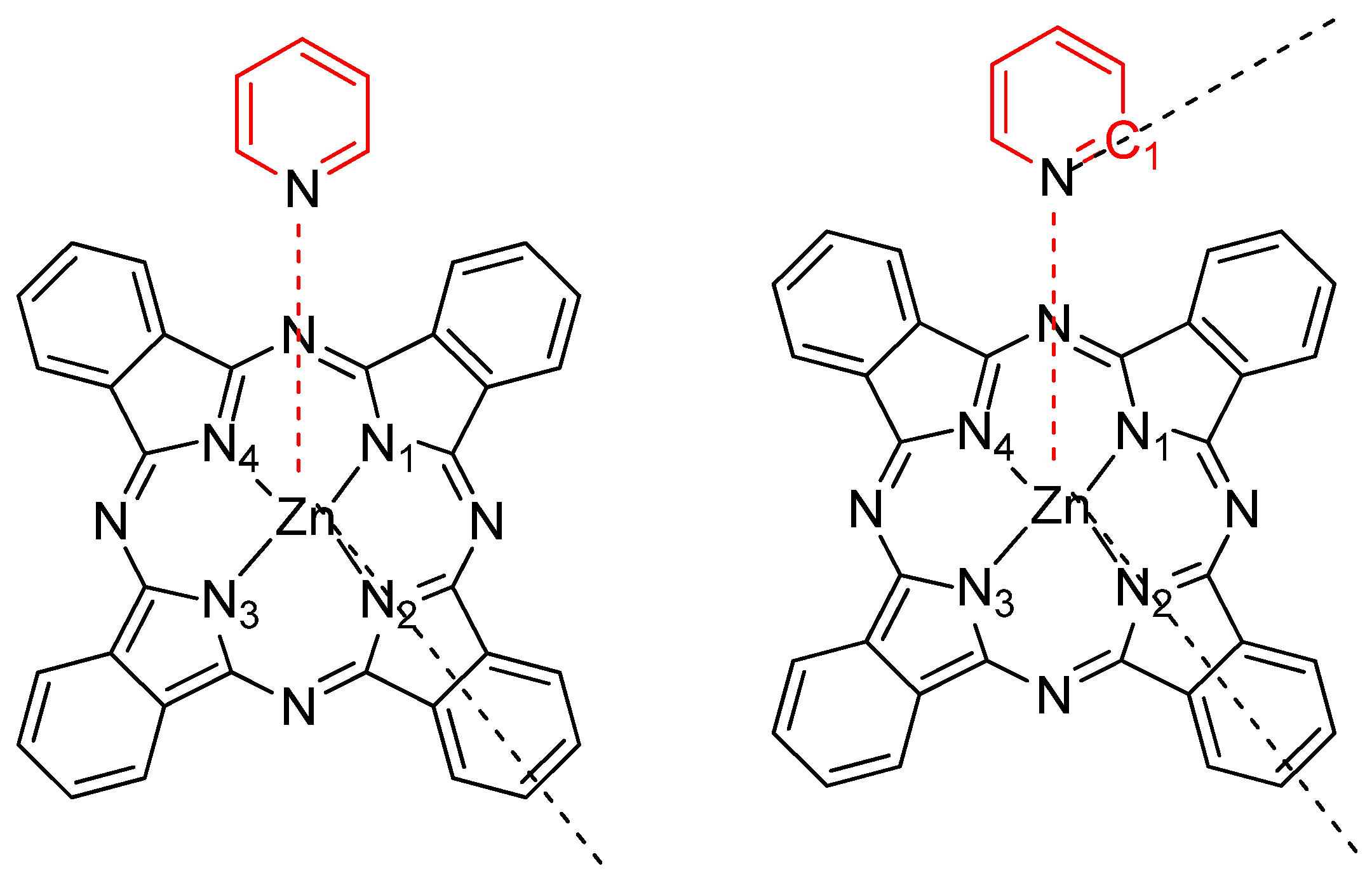


| PcZnI | PcZnII | PcZnIII | PcZnIV | PcZnV | PcZnVI | |
|---|---|---|---|---|---|---|
| Bond length [Å] | ||||||
| Zn-N1 | 2.0446 | 2.0431 | 2.0439 | 2.0434 | 2.0430 | 2.0429 |
| Zn-N2 | 2.0445 | 2.0447 | 2.0444 | 2.0437 | 2.0440 | 2.0447 |
| Zn-N3 | 2.0446 | 2.0447 | 2.0448 | 2.0436 | 2.0440 | 2.0447 |
| Zn-N4 | 2.0446 | 2.0431 | 2.0444 | 2.0443 | 2.0431 | 2.0430 |
| Zn-NPy | 2.1507 | 2.1561 | 2.1520 | 2.1566 | 2.1665 | 2.1545 |
| Bond angle [°] | ||||||
| N1-Zn-NPy | 103.3821 | 103.5133 | 103.5577 | 103.4910 | 103.4002 | 103.5540 |
| N1-Zn-NPy-C1Py | −44.9626 | −44.9945 | 43.7557 | 46.0764 | 44.4751 | −45.0138 |
| PcZnI | PcZnII | PcZnIII | PcZnIV | PcZnV | PcZnVI | |
|---|---|---|---|---|---|---|
| E | −0.7681 | −0.7584 | −0.7668 | −0.7568 | −0.7444 | −0.7518 |
| μ | 5.1699 | 4.5939 | 7.1704 | 4.6423 | 4.8229 | 6.1184 |
| PcZnI | PcZnII | PcZnIII | PcZnIV | PcZnV | PcZnVI | |
|---|---|---|---|---|---|---|
| LUMO | −4.6615 | −4.6800 | −4.6498 | −4.6860 | −4.6849 | −4.6699 |
| Eg | 1.8732 | 2.1975 | 2.0958 | 2.2000 | 2.1796 | 1.9624 |
| HOMO | −2.7883 | −2.4824 | −2.5540 | −2.4860 | −2.5053 | −1.8095 |
| Bond Length [Å] | Zn-N1 | Zn-N2 | Zn-N3 | Zn-N4 | Zn-NPy | Bond Angle [°] | N1-Zn-NPy | N1-Zn-NPy-C1Py |
|---|---|---|---|---|---|---|---|---|
| Pc(PcZnI)3 | ||||||||
| 2.0689 | 2.0670 | 2.0689 | 2.0670 | 2.2074 | 101.7536 | −0.0422 | ||
| 2.0454 | 2.0454 | 2.0456 | 2.0456 | 2.4225 | 90.8825 | −44.7312 | ||
| 2.0454 | 2.0454 | 2.0456 | 2.0456 | 2.4221 | 90.8705 | 44.7480 | ||
| (X-ray) a | 2.0009 | 2.0044 | 2.0100 | 2.0080 | 2.1558 | 104.3568 | 107.2625 | |
| Pc(PcZnII)3 | ||||||||
| 2.0676 | 2.0686 | 2.0658 | 2.0686 | 2.2115 | 100.9246 | −1.1096 | ||
| 2.0452 | 2.0453 | 2.0457 | 2.0456 | 2.4315 | 90.7744 | −43.6966 | ||
| 2.0452 | 2.0454 | 2.0457 | 2.0456 | 2.4866 | 90.7829 | −43.7065 | ||
| Pc(PcZnI)3 | Pc(PcZnII)3 | |
|---|---|---|
| DFT | −3.3263 | −3.3281 |
| Pc(PcZnI)3 | Pc(PcZnII)3 | |
|---|---|---|
| LUMO | −5.0582 | −5.0702 |
| Eg | 2.0092 | 2.1766 |
| HOMO | −3.0490 | −2.8936 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwajca, A.; Pankiewicz, R. Quantum Chemical Stability Analysis of Phthalocyanine Metal One-Dimensional Polymers with Bidentate Ligands. Molecules 2024, 29, 4111. https://doi.org/10.3390/molecules29174111
Szwajca A, Pankiewicz R. Quantum Chemical Stability Analysis of Phthalocyanine Metal One-Dimensional Polymers with Bidentate Ligands. Molecules. 2024; 29(17):4111. https://doi.org/10.3390/molecules29174111
Chicago/Turabian StyleSzwajca, Anna, and Radosław Pankiewicz. 2024. "Quantum Chemical Stability Analysis of Phthalocyanine Metal One-Dimensional Polymers with Bidentate Ligands" Molecules 29, no. 17: 4111. https://doi.org/10.3390/molecules29174111
APA StyleSzwajca, A., & Pankiewicz, R. (2024). Quantum Chemical Stability Analysis of Phthalocyanine Metal One-Dimensional Polymers with Bidentate Ligands. Molecules, 29(17), 4111. https://doi.org/10.3390/molecules29174111







