Thevetia thevetioides Cardenolide and Related Cardiac Glycoside Profile in Mature and Immature Seeds by High-Resolution Thin-Layer Chromatography (HPTLC) and Quadrupole Time of Flight–Tandem Mass Spectrometry (Q-TOF MS/MS) Reveals Insights of the Cardenolide Biosynthetic Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. HPTLC
2.4. Q-TOF MS/MS
2.5. GC-MS Analysis of Aglycones
3. Results
3.1. Mature Seed Cardenolide Profile
3.2. Immature Seeds Cardenolide Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- El-Mallakh, R.S.; Brar, K.S.; Yeruva, R.R. Cardiac glycosides in human physiology and disease: Update for entomologists. Insects 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bandara, V.; Weinstein, S.A.; White, J.; Eddleston, M. A review of the natural history, toxinology, diagnosis and clinical management of Nerium oleander (common oleander) and Thevetia peruviana (yellow oleander) poisoning. Toxicon 2010, 56, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Anaeigoudari, A.; Azdaki, N.; Reza Khazdair, M. A comprehensive review of cardiotoxic effects of selected plants. Toxin Rev. 2020, 40, 535–544. [Google Scholar] [CrossRef]
- Demiryurek, A.T.; Demiryurek, S. Cardiotoxicity of digitalis glycosides: Roles of autonomic pathways, autacoids and ion channels. Auton. Autacoid Pharmacol. 2005, 25, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Kohls, S.; Scholz-Böttcher, B.M.; Teske, J.; Zark, P.; Rullkötter, J. Cardiac glycosides from yellow oleander (Thevetia peruviana) seeds. Phytochemistry 2012, 75, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Souza e Souza, K.F.C.; Moraes, B.P.T.; Paixao, I.C.N.D.P.; Burth, P.; Silva, A.R.; Goncalves-de-Albuquerque, C.F. Na+/K+-ATPase as a target of cardiac glycosides for the treatment of SARS-CoV-2 infection. Front. Pharmacol. 2021, 12, 624704. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, C.M.; Munsamy, A.; Naidoo, Y.; Dewir, Y.H. Chemistry, Biological Activities, and Uses of Latex from Selected Species of Apocynaceae. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Springer International Publishing: Cham, Switzerland, 2022; pp. 845–868. [Google Scholar]
- Rzedowski, J.; Calderón de Rzedowski, G. Familia Apocynaceae. Flora del Bajío y de Regiones Adyacentes, 2nd ed.; Instituto de Ecología, A.C.: Patzcuaro, Mexico, 2003; Fascículo 70; pp. 1–64. [Google Scholar]
- Standley, P.C. Trees and shrubs of Mexico Part II, 1st ed.; Smithsonian Press: Washinton, DC, USA, 1926; pp. 1151–1152. [Google Scholar]
- Martínez, M. Las Plantas Medicinales de México, 1st ed.; Editorial Botas: Mexico City, Mexico, 1933. [Google Scholar]
- Lozoya, X. Xiuhpatli, Herba officinalis, 1st ed.; Secretaría de Salud, Universidad Nacional Autónoma de México: Mexico City, Mexico, 1999. [Google Scholar]
- Nesy, E.A.; Mathew, L. Detection and Quantification of Cardiotonic Drug Peruvoside Using HPTLC from Thevetia neriifolia, Juss Seed Extracts. Int. J. Pharm. Sci. Invent. 2014, 3, 11–16. [Google Scholar]
- Helfenberger, H.; Reichstein, T. Thevetin. I. Glycoside and Aglykone. Helv. Chim. Acta 1948, 31, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Frèrejacque, M. La nériifoline, nouvel hétéroside digitalique de Thevetia neriifolia. C. R. Hebd. Seances Acad. Sci. 1945, 221, 645–646. [Google Scholar]
- Abe, F.; Yamauchi, T.; Yahara, S.; Nohara, T. Glycosides of 19-formylthevetiogenin and 5a-thevetiogenin from Thevetia neriifolia. Phytochemistry 1994, 37, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Balderas-López, J.L.; Barbonetti, S.; Pineda-Rosas, E.L.; Tavares-Carvalho, J.C.; Navarrete, A. Cardiac glycosides from Cascabela thevetioides by HPLC-MS analysis. Rev. Bras. Farmacogn. 2019, 29, 441–444. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis. A Thin Layer Chromatography Atlas (Cardenolides), 2nd ed.; Springer: Berlin, Germany, 1996; pp. 102, 104, 261. [Google Scholar]
- Bisset, N.G. Cardiac glycosides part V. Apocynaceae: A preliminary paper-chromatographic study of the glycosides from some species of Cerbera L. (including Tanghinia Thouars). Ann. Bogor. 1961, IV, 153–161. [Google Scholar]
- Adams, S.J.; Avula, B.; Katragunta, K.; Saroja, S.G.; Zhao, J.; Chittiboyina, A.G.; Khan, I.A. Microscopy, HPTLC, and LC-DAD-Q-ToF validation of nut-based weight-loss dietary supplements, Aleurites moluccanus (candlenut) and Bertholletia excelsa (Brazil nut). Food Addit. Contam. Part A 2024, 41, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Kytidou, K.; Artola, M.; Overkleeft, H.S.; Aerts, J.M. Plant glycosides and glycosidases: A treasure-trove for therapeutics. Front. Plant Sci. 2020, 11, 357. [Google Scholar] [CrossRef] [PubMed]
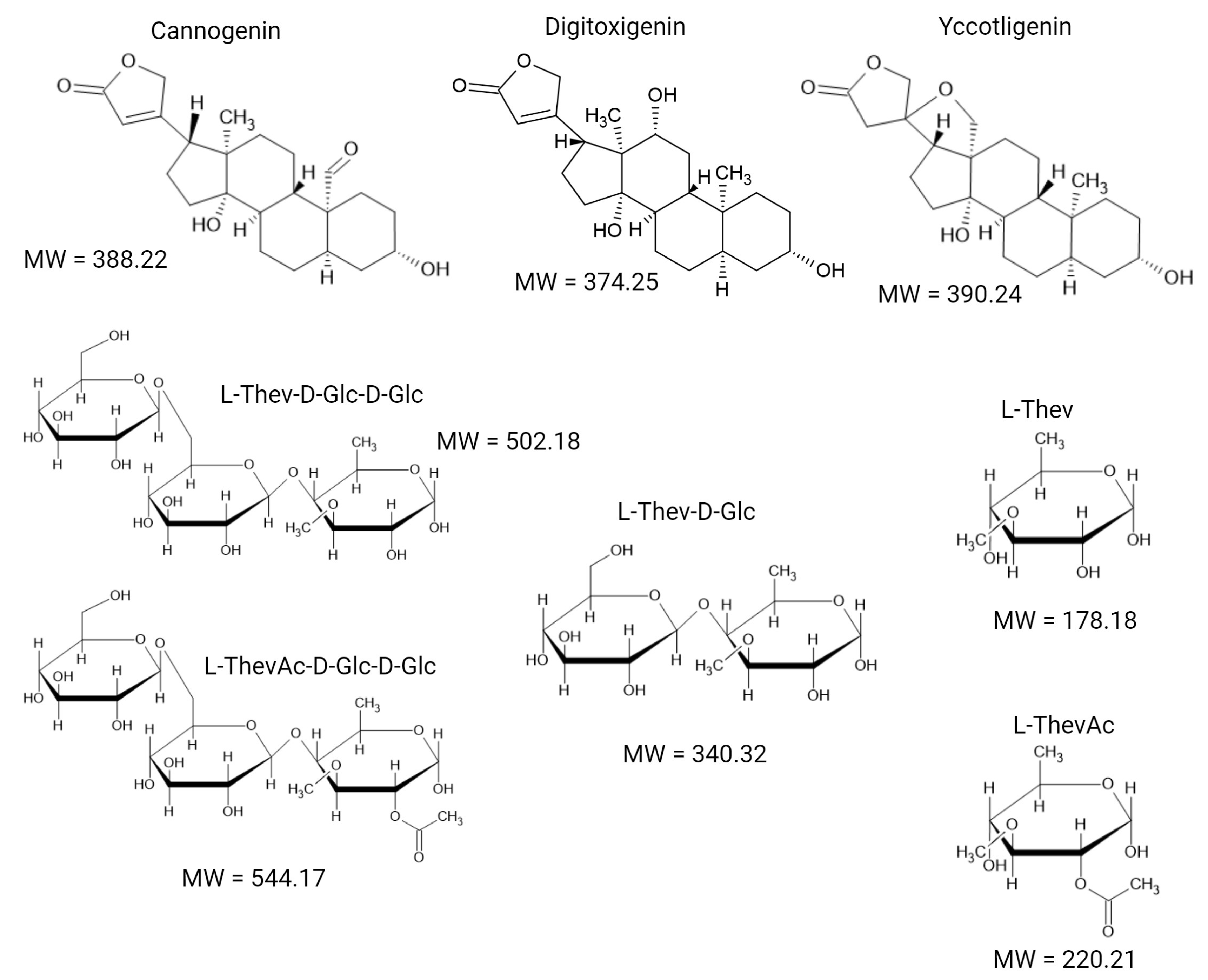
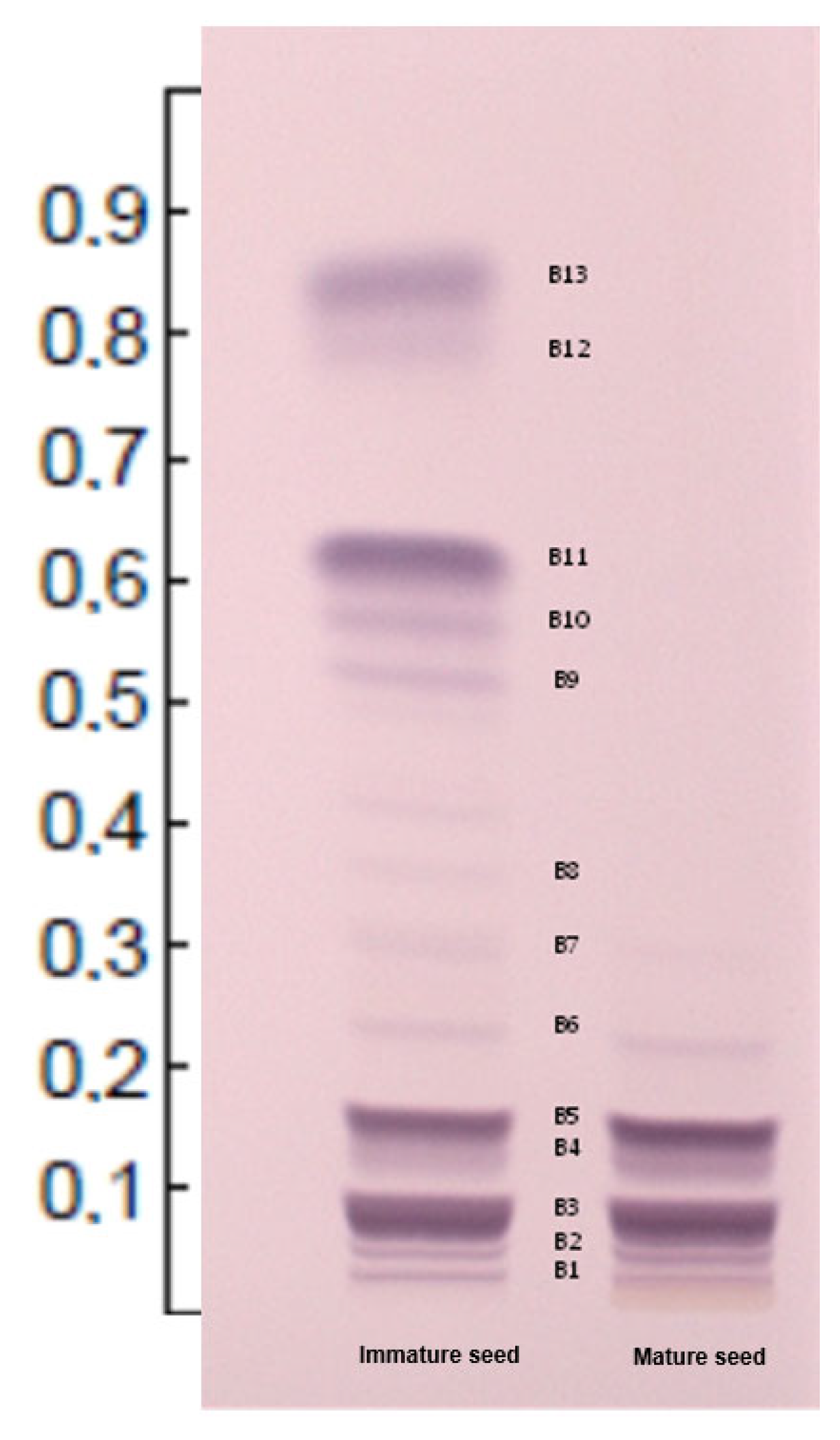
| ID | Seed | BAND | Rf | Aglycone | Carbohydrate | Previous Name | Proposed Name | ||
|---|---|---|---|---|---|---|---|---|---|
| C1 | I | B13 | 0.84 | Digitoxigenin | L-ThevAc | Peruvoside B acetate/Neriifolin acetate (Cerberin) | Digiperuvoside acetate or Digithevetoside acetate | Monoglycosides | Acetates |
| C2 | I | B12 | 0.78 | Yccotligenin | L-ThevAc | Peruvoside C acetate | Yccoperuvoside acetate or Yccothevetoside acetate | ||
| C3 | I | Cannogenin | L-ThevAc | Peruvoside A acetate | Canperuvoside acetate or Canthevetoside acetate | ||||
| C4 | I | B11 | 0.61 | Digitoxigenin | L-L-Thev | Peruvoside B (Neriifolin) | Digiperuvoside or Digithevetoside | ||
| C5 | I | B10 | 0.56 | Yccotligenin | L-Thev | Peruvoside C | Yccoperuvoside or Yccothevetoside | ||
| C6 | I | B9 | 0.51 | Cannogenin | L-Thev | Peruvoside A | Canperuvoside or Canthevetoside | ||
| C7 | I | B8 | 0.35 | Digitoxigenin | L-Thev-D-Glc | Thevebioside B | Digithevebioside | Di-glycosides | |
| C8 | M/I | B7 | 0.29 | Yccotligenin | L-Thev-D-Glc | Thevebioside C | Yccothevebioside | ||
| C9 | M/I | B6 | 0.22 | Cannogenin | L-Thev-D-Glc | Thevebioside A | Canthevebioside | ||
| C10 | M/I | B5 | 0.15 | Digitoxigenin | L-ThevAc-D-Glc-D-Glc | Thevetin B acetate | Digithevetin acetate | Triglycosides | Acetates |
| C11 | M/I | B4 | 0.12 | Yccotligenin | L-ThevAc-D-Glc-D-Glc | Thevetin C acetate | Yccothevetin acetate | ||
| C12 | M/I | Cannogenin | L-ThevAc-D-Glc-D-Glc | Thevetin A acetate | Canthevetin acetate | ||||
| C13 | M/I | B3 | 0.07 | Digitoxigenin | L-Thev-D-Glc-D-Glc | Thevetin B (Cerberoside) | Digithevetin | ||
| C14 | M/I | B2 | 0.04 | Yccotligenin | L-Thev-D-Glc-D-Glc | Thevetin C | Yccothevetin | ||
| C15 | M/I | B1 | 0.02 | Cannogenin | L-Thev-D-Glc-D-Glc | Thevetin A | Canthevetin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Martínez, J.; Bravo-Villa, P.; Molina-Torres, J. Thevetia thevetioides Cardenolide and Related Cardiac Glycoside Profile in Mature and Immature Seeds by High-Resolution Thin-Layer Chromatography (HPTLC) and Quadrupole Time of Flight–Tandem Mass Spectrometry (Q-TOF MS/MS) Reveals Insights of the Cardenolide Biosynthetic Pathway. Molecules 2024, 29, 4083. https://doi.org/10.3390/molecules29174083
Vázquez-Martínez J, Bravo-Villa P, Molina-Torres J. Thevetia thevetioides Cardenolide and Related Cardiac Glycoside Profile in Mature and Immature Seeds by High-Resolution Thin-Layer Chromatography (HPTLC) and Quadrupole Time of Flight–Tandem Mass Spectrometry (Q-TOF MS/MS) Reveals Insights of the Cardenolide Biosynthetic Pathway. Molecules. 2024; 29(17):4083. https://doi.org/10.3390/molecules29174083
Chicago/Turabian StyleVázquez-Martínez, Juan, Paulina Bravo-Villa, and Jorge Molina-Torres. 2024. "Thevetia thevetioides Cardenolide and Related Cardiac Glycoside Profile in Mature and Immature Seeds by High-Resolution Thin-Layer Chromatography (HPTLC) and Quadrupole Time of Flight–Tandem Mass Spectrometry (Q-TOF MS/MS) Reveals Insights of the Cardenolide Biosynthetic Pathway" Molecules 29, no. 17: 4083. https://doi.org/10.3390/molecules29174083
APA StyleVázquez-Martínez, J., Bravo-Villa, P., & Molina-Torres, J. (2024). Thevetia thevetioides Cardenolide and Related Cardiac Glycoside Profile in Mature and Immature Seeds by High-Resolution Thin-Layer Chromatography (HPTLC) and Quadrupole Time of Flight–Tandem Mass Spectrometry (Q-TOF MS/MS) Reveals Insights of the Cardenolide Biosynthetic Pathway. Molecules, 29(17), 4083. https://doi.org/10.3390/molecules29174083







