Frustrated Alternative Approaches towards the Synthesis of a Thermally Stable 1,2-Diazacyclobutene
Abstract
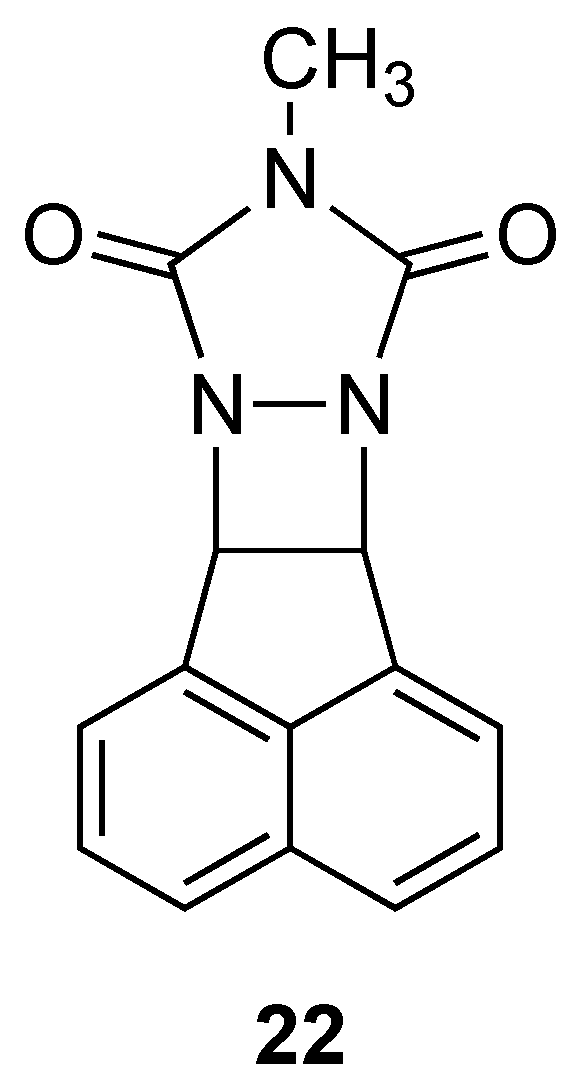
1. Introduction
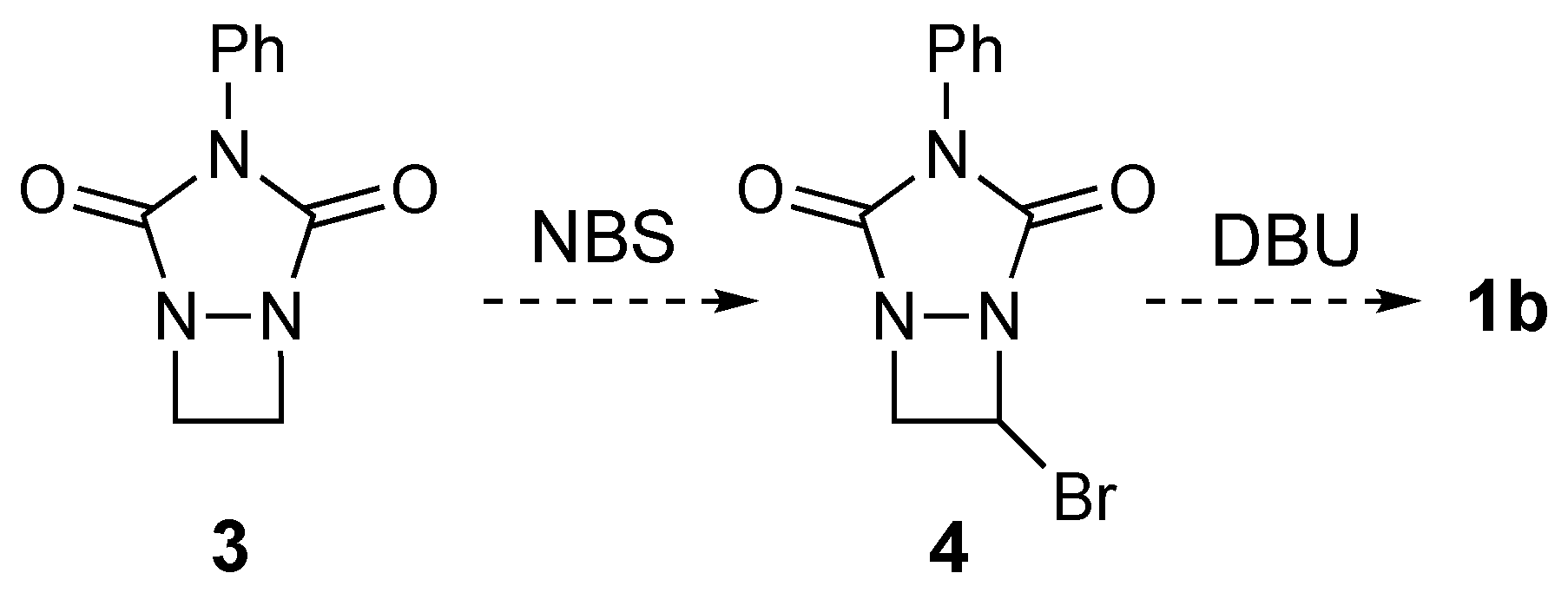
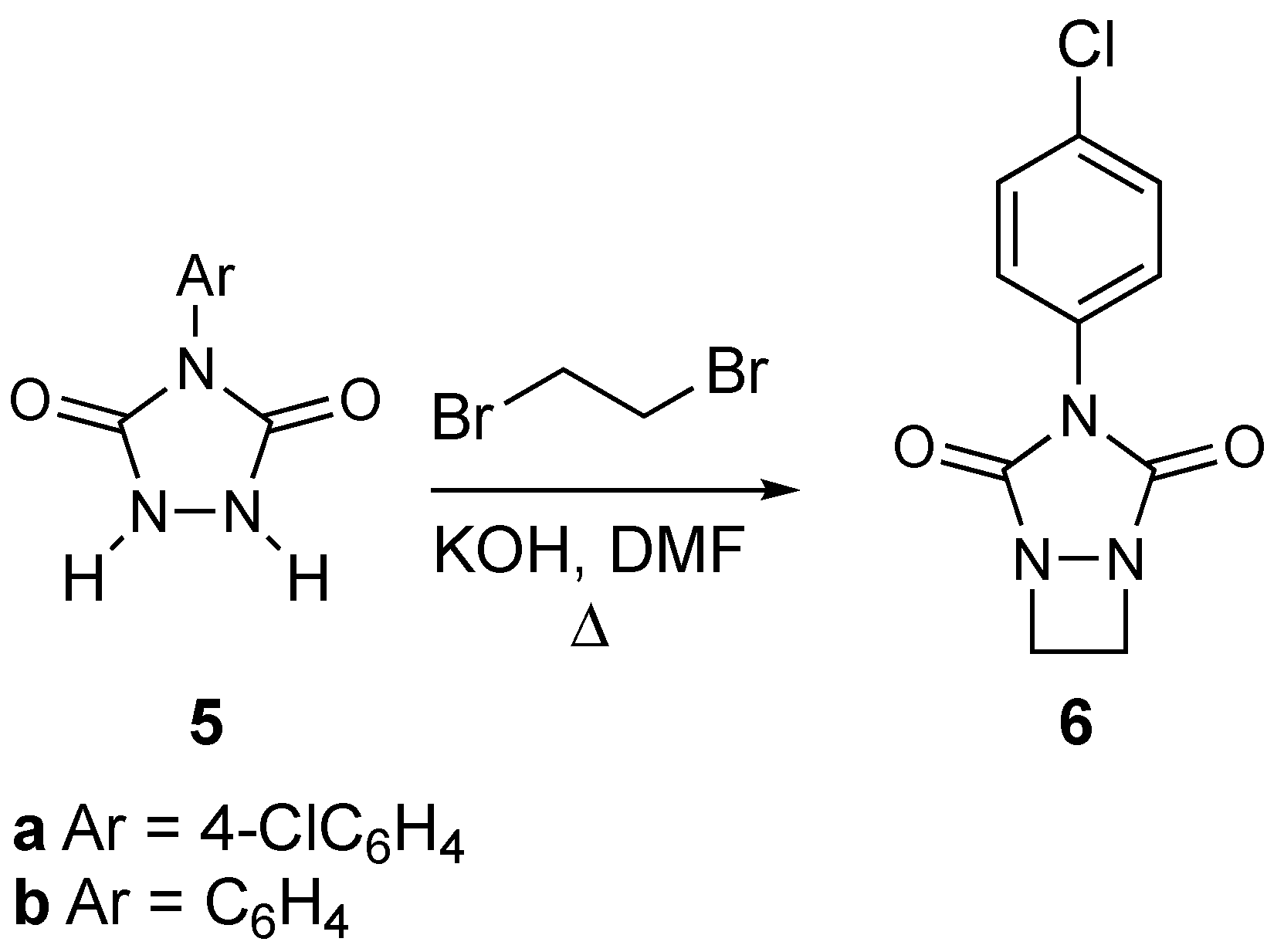
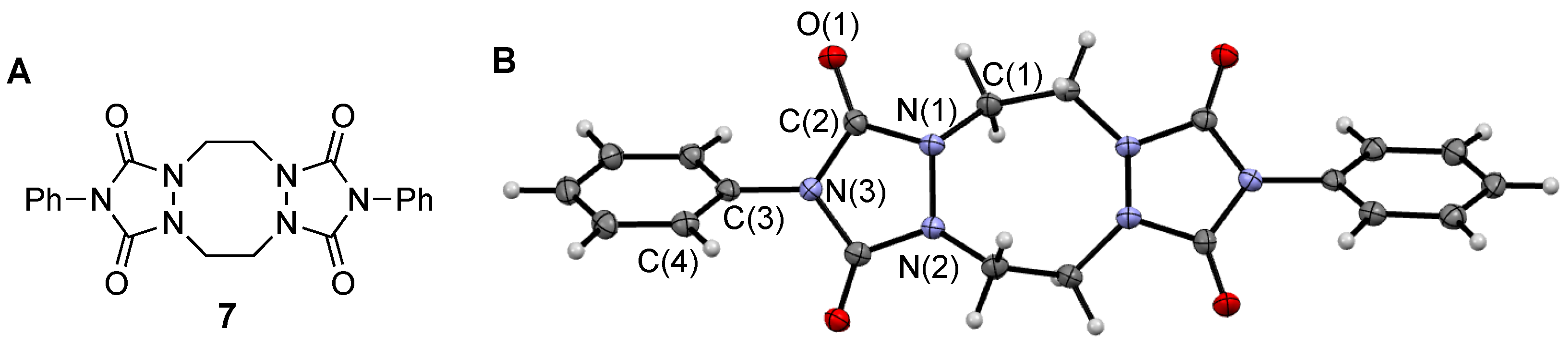
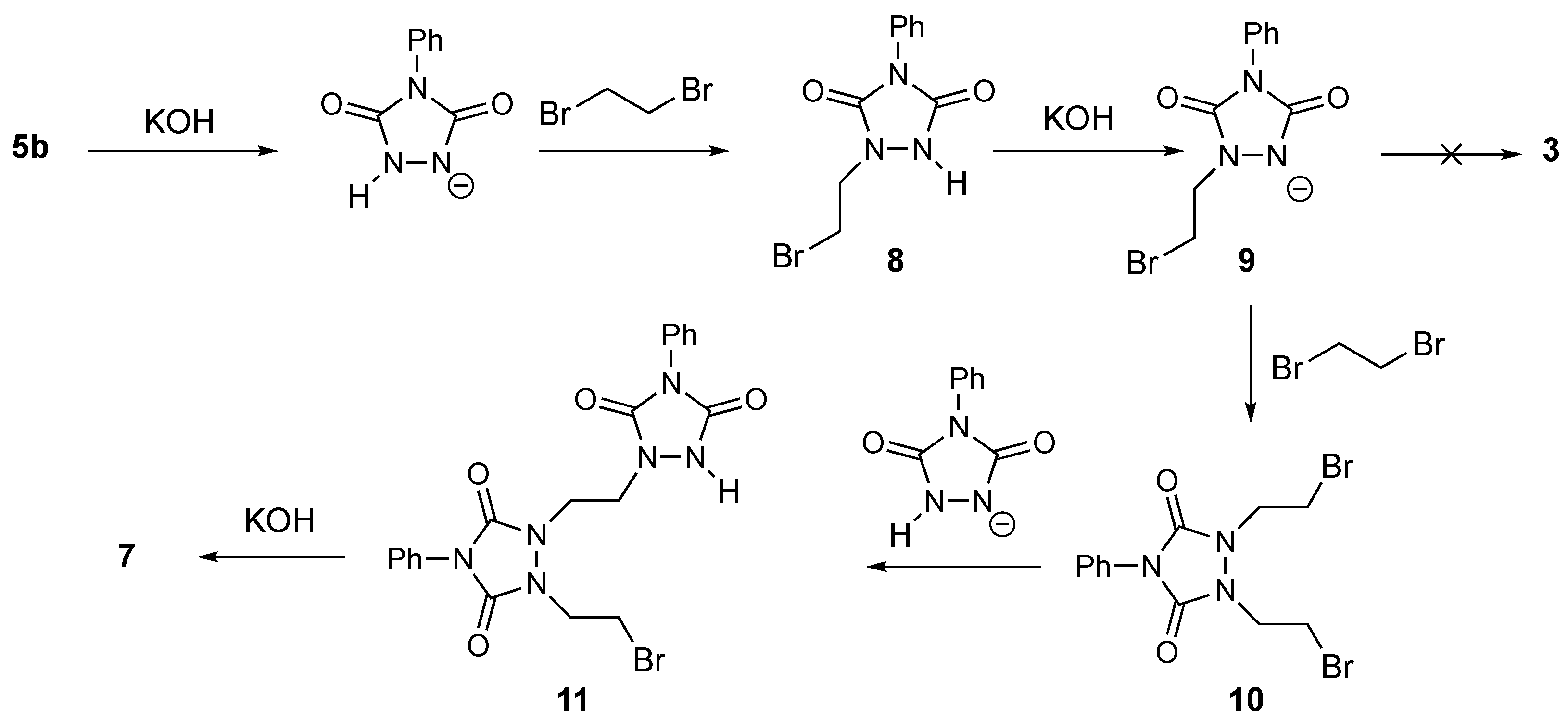
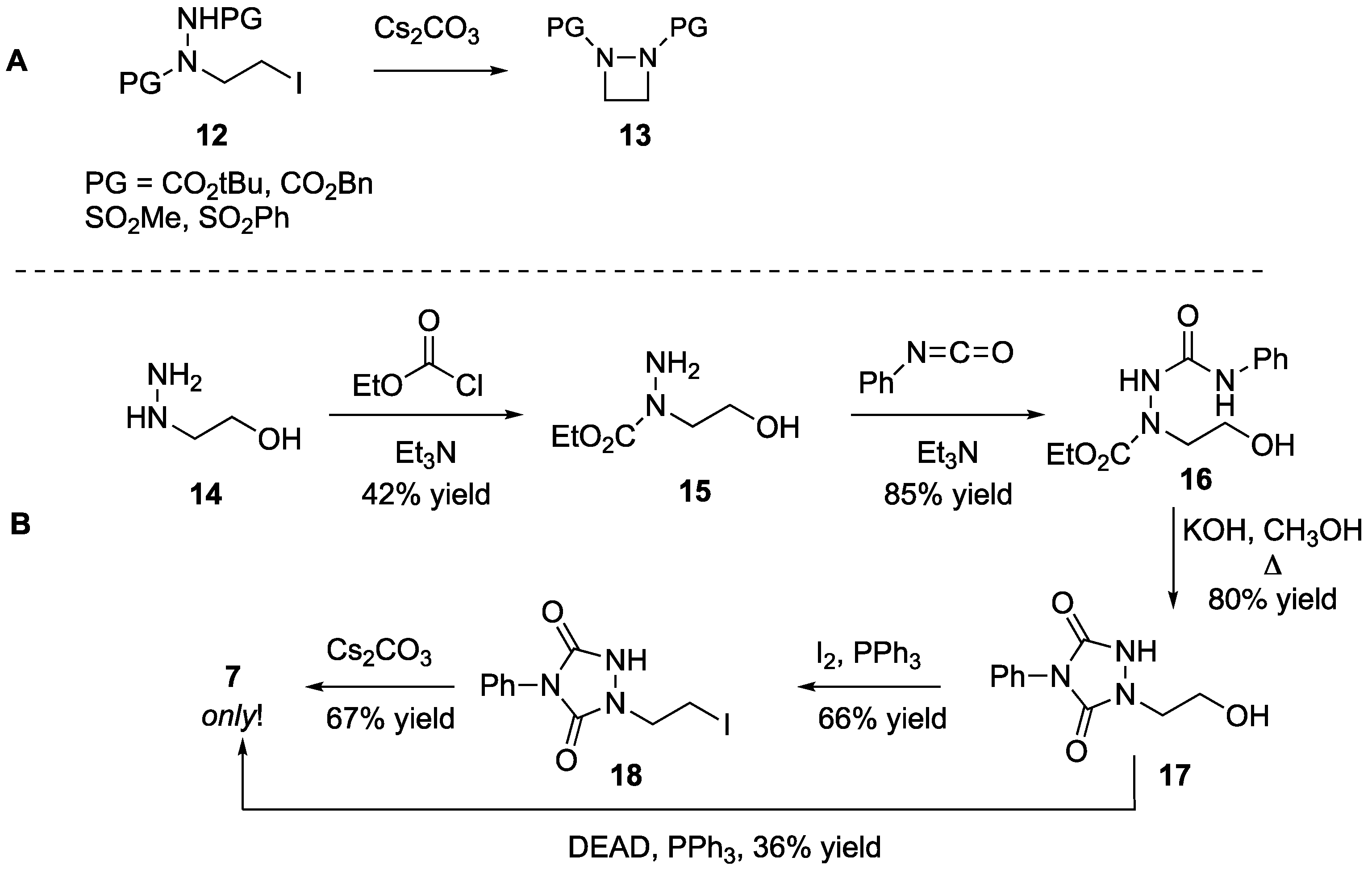
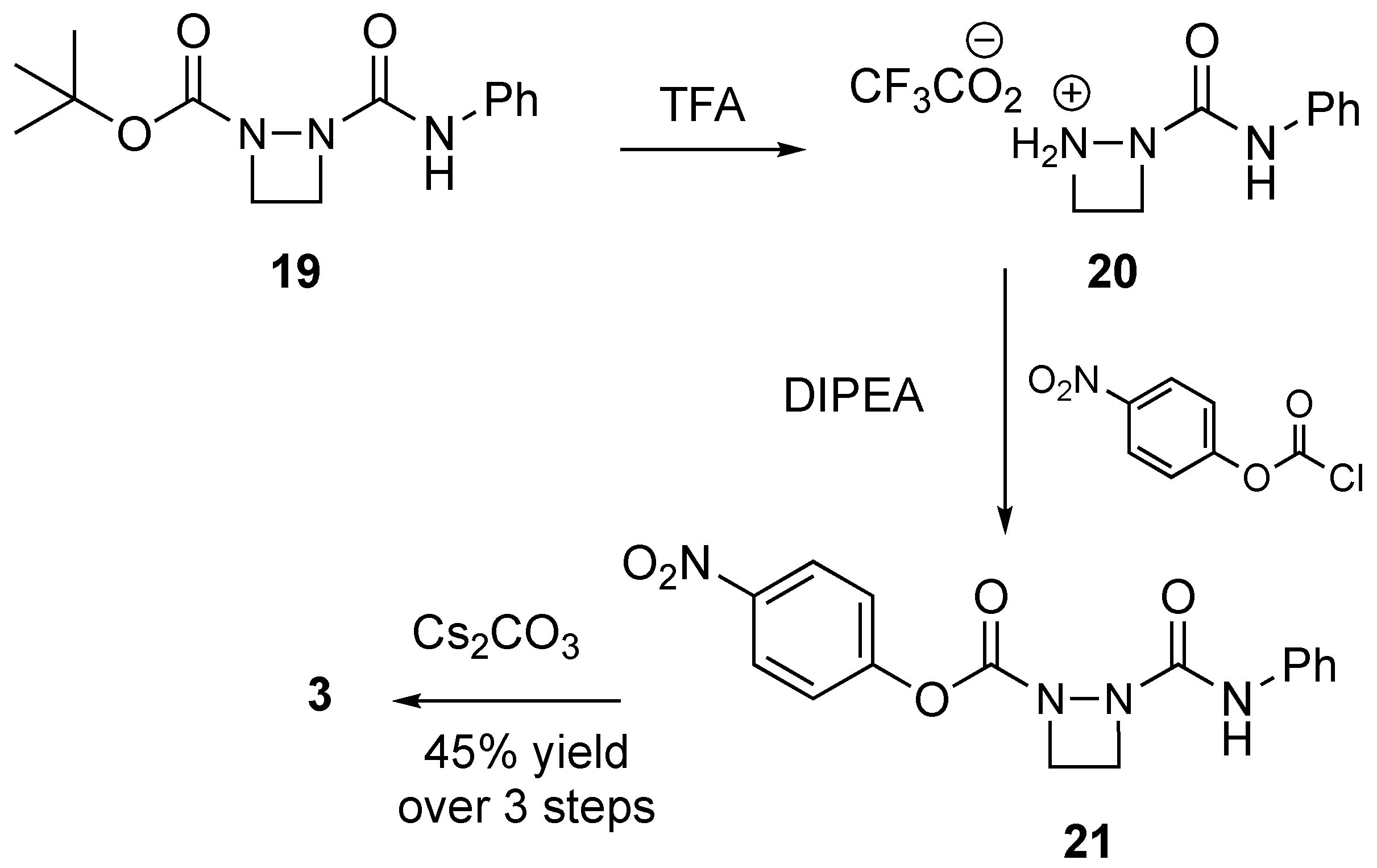
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Experimental Procedures
3.2.1. Synthesis of “Dimer” 7 from Urazole 5b
3.2.2. Synthesis of 11
3.2.3. Synthesis of 12
3.2.4. Synthesis of 13
3.2.5. Synthesis of 14
3.2.6. Attempted Cyclization of 14
3.2.7. Attempted Cyclization of 13
3.2.8. Synthesis of 3
3.2.9. Attempted Bromination of 3
3.3. X-ray Data Collection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breton, G.W.; Shugart, J.J.; Hughey, C.A.; Perala, S.M.; Hicks, A.D. Synthesis of D1-1,2-Diazetines via a Diels-Alder Cycloaddition Approach. Org. Lett. 2001, 3, 3185–3187. [Google Scholar] [CrossRef] [PubMed]
- De Bruycker, K.; Billiet, S.; Houck, H.A.; Chattopadhyay, S.; Winne, J.M.; Du Prez, F.E. Triazolinediones as Highly Enabling Synthetic Tools. Chem. Rev. 2016, 116, 3919–3974. [Google Scholar] [CrossRef] [PubMed]
- Narangoda, C.J.; Lex, T.R.; Moore, M.A.; McMillen, C.D.; Kitaygorodskiy, A.; Jackson, J.E.; Whitehead, D.C. Accessing the Rare Diazacyclobutene Motif. Org. Lett. 2018, 20, 8009–8013. [Google Scholar] [CrossRef] [PubMed]
- Nunn, E.E.; Warrener, R.N. Dimethyl D3-1,2-Diazetine-1,2-dicarboxylate: A New Four-membered 6p-Ring System. J. Chem. Soc. Chem. Comm. 1972, 14, 818–819. [Google Scholar] [CrossRef]
- Wakabayashi, O.; Matsuya, K.; Ohta, H.; Tetsuo, J.; Suzuki, S. 1,2-Alkylene-4-substituted urazole herbicides. U.S. Patent 4,249,934, 19 September 1978. [Google Scholar]
- Brown, M.J.; Clarkson, G.J.; Fox, D.J.; Inglis, G.G.; Shipman, M. Critical importance of leaving group ‘softness’ in nucleophilic ring closure reactions of ambident anions to 1.2-diazetidines. Tetrahedron Lett. 2010, 51, 382–384. [Google Scholar] [CrossRef]
- Dean, C.; Roesner, S.; Rajkumar, S.; Clarkson, G.J.; Jones, M.; Shipman, M. Synthesis of sp3-rich chemical libraries based upon 1,2-diazetidines. Tetrahedron 2021, 79, 131836. [Google Scholar] [CrossRef]
- Bausch, M.J.; David, B.; Dobrowolski, P.; Guadalupe-Fasano, C.; Gostowski, R.; Selmarten, D.; Prasad, V.; Vaughn, A.; Wang, L.H. Proton-Transfer Chemistry of Urazoles and Related Imides, Amides, and Diacyl Hydrazides. J. Org. Chem. 1991, 56, 5463–5651. [Google Scholar] [CrossRef]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, USA, 2004; pp. 568–569. [Google Scholar]
- Mitsunobu, O. The Use of Diethyl Azodicarboxylate and Triphenylphosphine in Synthesis and Transformation of Natural Products. Synthesis 1981, 1981, 1–28. [Google Scholar] [CrossRef]
- Breton, G.W.; Martin, K.L. Are 1,2-Dihydrodiazetes Aromatic? An Experimental and Computational Investigation. J. Org. Chem. 2002, 67, 6699–6704. [Google Scholar] [CrossRef] [PubMed]
- Breton, G.W.; Hughes, J.S.; Pitchko, T.J.; Martin, K.L.; Hardcastle, K.J. Unexpected Sigma Bond Rupture during the Reaction of N-Methyl-1,2,4-triazoline-3,5-dione with Acenaphthylene and Indene. Org. Chem. 2014, 79, 8212–8220. [Google Scholar] [CrossRef] [PubMed]
- Djerassi, C. Brominations with N-Bromosuccinimide and Related Compounds. The Wohl-Ziegler Reaction. Chem. Rev. 1948, 43, 271–317. [Google Scholar] [CrossRef] [PubMed]
- Zipse, H. Radical Stability–A Theoretical Perspective. Top. Curr. Chem. 2006, 263, 163–189. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment—Olex2 Dissected. Acta Cryst. 2015, 71, 59–75. [Google Scholar]

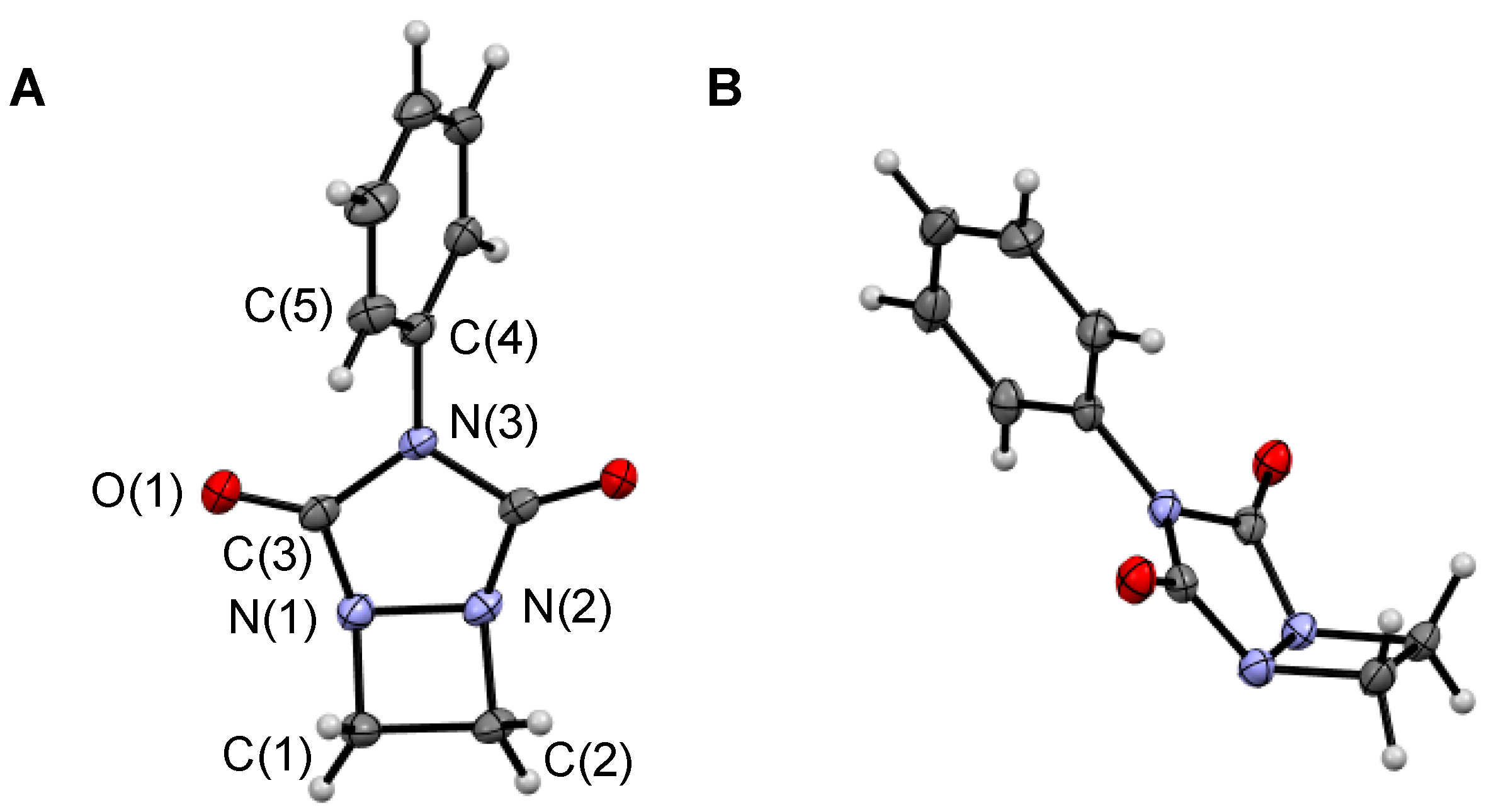
| 3 | 7 | |
| Chemical formula | C10H9N3O2 | C20H18N6O4 |
| Mr | 203.20 | 406.40 |
| Deposition number | 2373853 | 2373840 |
| Crystal system and space group | orthorhombic, P212121 | monoclinic, P21 |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 6.00967(7), 11.74365(12), 13.52523(14) | 7.8071(3), 7.1290(3), 16.3695(6) |
| β (°) | 90 | 92.298(3) |
| V (Å3) | 954.549(17) | 910.34(6) |
| Z | 4 | 2 |
| Radiation type | Cu Kα | Cu Kα |
| μ (mm−1) | 0.85 | 0.89 |
| Crystal size (mm) | 0.14 × 0.10 × 0.09 | 0.36 × 0.23 × 0.20 |
| Diffractometer | XtaLAB AFC11 (RCD3) | XtaLAB Synergy-S |
| Absorption correction | Numerical and empirical | Numerical and empirical |
| Tmin, Tmax | 1.000, 0.823 | 1.000, 0.585 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 13,937, 1858, 1858 | 15,905, 3762, 3762 |
| Rint | 0.0234 | 0.0533 |
| (sin θ/λ)max (Å−1) | 0.615 | 0.633 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0233, 0.0553, 1.166 | 0.0344, 0.0854, 1.095 |
| No. of reflections | 1858 | 3762 |
| No. of parameters | 172 | 345 |
| Δρmax, Δρmin (e Å−3) | 0.12, −0.19 | 0.20, −0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breton, G.W.; Martin, K.L. Frustrated Alternative Approaches towards the Synthesis of a Thermally Stable 1,2-Diazacyclobutene. Molecules 2024, 29, 4068. https://doi.org/10.3390/molecules29174068
Breton GW, Martin KL. Frustrated Alternative Approaches towards the Synthesis of a Thermally Stable 1,2-Diazacyclobutene. Molecules. 2024; 29(17):4068. https://doi.org/10.3390/molecules29174068
Chicago/Turabian StyleBreton, Gary W., and Kenneth L. Martin. 2024. "Frustrated Alternative Approaches towards the Synthesis of a Thermally Stable 1,2-Diazacyclobutene" Molecules 29, no. 17: 4068. https://doi.org/10.3390/molecules29174068
APA StyleBreton, G. W., & Martin, K. L. (2024). Frustrated Alternative Approaches towards the Synthesis of a Thermally Stable 1,2-Diazacyclobutene. Molecules, 29(17), 4068. https://doi.org/10.3390/molecules29174068







