Abstract
The effect of different methods of drying celery root enriched with beet juice by vacuum impregnation (VI) was studied. The process of convection drying, vacuum drying and freeze drying was carried out. Compared to dried indigenous celery, dry impregnated tissue was characterized by lower values of dry matter, L* and b* color parameters, as well as higher values of water activity, density and a* color parameter. In addition, VI reduced the drying time. Forty Volatile Organic Compounds (VOCs) were found in celery, while fifty-one VOCs were found in the profile of celery with beetroot juice. The innovative method of vacuum impregnation made it possible to produce a new type of product with changed properties and a variable VOCs profile. The best fit of the drying process kinetics was achieved by using the logistic model. Increasing the temperature during convection drying resulted in shorter drying time, increased values of dry matter, reduced the water activity value and altered VOCs.
1. Introduction
The consumption of vegetables is currently highly recommended in the diet, so it is desirable to develop new high-quality dried vegetables that are attractive to consumers, in order to expand the availability of products and diversify their market []. For this purpose, it is necessary to optimize drying conditions and use pretreatment to obtain specific properties related to color, water content, density, texture and volatile compounds, etc. [,]. Modern society is increasingly paying attention to a healthy lifestyle, placing importance on choosing conscious and functional food products []. Awareness of the effects of diet on overall health makes consumers care not only about taste but also about the nutritional value of the products they eat. In this context, the popularity of celery, which is rich in nutrients and has numerous health-promoting properties, is gaining ground [].
Celery root is a vegetable derived from the celery family (Apiaceae) and is widely used around the world [,]. There are many ways to consume celery root. Celery can be boiled, stewed, roasted or eaten raw. The most common color of celery root is white, yellow or green depending on the variety. It is low in calories, which makes it ideal for people who want to maintain or lose weight. Celery root is one of the few vegetables with a high fiber content, which aids in digestion and maintaining a healthy digestive system. It is rich in many nutrients (potassium—320 mg/100 g, vitamin C—6.2/8.0 mg/100 g, folate—12 mg/100 g, total sugars—2.25% and vitamin—K 100 mg/100 g) []. All parts of celery have medicinal properties, i.e., anti-inflammatory, antibacterial and able to lower serum lipids and blood glucose levels. In addition, celery prevents cardiovascular diseases and strengthens the heart [,]. In addition, due to its numerous volatile compounds, celery has a peculiar aroma and taste [].
The drying of vegetables and fruits is one of the prevailing methods for the manufacture and development of novel products, as it extends their shelf life and does not significantly degrade the quality of products with a high moisture content [,]. Extended drying time can effect undesirable physicochemical changes and low energy efficiency in food products. For this reason, innovative and cutting-edge approaches to the drying process and pretreatment prior to drying are key, which can improve the quality of biological materials, reduce drying time and increase the overall efficiency of the drying process []. Pretreatment plays an important role in accelerating the drying rate, oxidation, permeability and enzyme inactivation of many vegetables and fruits [].
Vacuum impregnation (VI) is a process that allows the development of new foods by changing their composition and affects the final characteristics of the product. During the vacuum impregnation process, there is an internal exchange of gas and liquid present in the pores with an external impregnating solution []. This occurs due to a mechanically induced pressure difference. The VI process consists of two stages: in the first stage the material is subjected to reduced pressure and in the second stage to atmospheric pressure. As a result of these phenomena, the intracellular capillaries are filled with the impregnating solution []. The process of vacuum impregnation can contribute to the modification of the properties of vegetables in terms of nutrition, sensory qualities, physicochemical values of color and water content, as well as changes in texture, antitussive and bioactive properties [,,,]. Therefore, the process is widely used in the functional food industry and can affect final product properties and drying characteristics [].
Research available in the literature on celery is mainly related to its breeding, cultivation and chemical composition. Celery is most commonly used in daily vegetable consumption. Despite numerous publications on the health-promoting properties of celery, there is a lack of other celery food products on the market []. Therefore, the purpose of this study was to investigate the effects of different drying techniques and parameters of celery root and vacuum-impregnated celery root on selected physical properties, volatile compounds and kinetics of the drying process.
2. Results and Discussion
The results and discussion section presents the results of the study of celery (C) and celery after the vacuum impregnation process (VI) using beetroot juice (CB). In the presented study, three drying methods were tested: freeze drying (FD), vacuum drying (VD) and convection drying at three temperatures of 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
2.1. Volatile Organic Compounds (VOCs) Profile
The beetroot impregnation procedure was carried out on celery, which was then subjected to different drying methods and the VOC profiles were compared with each other using the HS-SPME Arrow technique. The full VOC profiles of the samples are shown in Table 1 and Table 2. Forty VOCs were found in celery, while fifty-one VOCs were found in the profile of celery with beetroot juice.

Table 1.
HS-SPME Arrow VOCs profile for fresh celery. Celery (C) after freeze drying (FD), vacuum (VD) and convection drying at a temperature of 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).

Table 2.
HS-SPME Arrow VOCs profile for fresh celery impregnated with beetroot juice. Celery after vacuum impregnation with beetroot juice (CB) after freeze drying (FD), vacuum (VD) and convection drying at a temperature of 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
In the case of fresh celery, the highest amount of volatile compounds were limonene (48.54%), β-pinene (13.4%), p-cymene (7.53%), β-(E)-ocimene (7.42%) and pentyl cyclohexa-1,3-diene (6.37%). The same compounds were repeated in fresh beetroot-impregnated celery: limonene (42.72%), β-pinene (18.16%), p-cymene (9.01%) and β-(E)-ocimene (8.37%).
Our previous studies have shown that the issue of impregnation varies depending on the plant material. In the studies on sweet potato, we observed interesting changes in the profile [], but in this case, as with courgette and broccoli [], there were no significant alterations. In previous cases, we used the juices of onion, kale and celery stalks to impregnate celery [] and the effect was significant. Meanwhile, the use of beetroot juice did significantly change the volatile compound profile in comparison with fresh, pure celery, which was shown in Figure 1 presenting HCA results. The amounts of major compounds such as limonene (36.02%) and pentyl cyclohexa-1,3-diene (3.22%), decreased after impregnation. In celery after vacuum impregnation, an increase in the content of compounds such as Fenipentol (2.74), γ-Terpinene (3.71) and β-(E)-Ocimene (7.47) and the appearance of compounds such as (E)-Caryophyllene (0.41), trans-Geranylacetone (4.17), (2E)-Decenal (0.56), (5Z)-Octen-1-ol (15.86), Caryophyllene oxide (36.02), cis-9-Tetradecen-1-ol (7.47) and cis-Limonene oxide (3.71) were observed. Convection drying of celery at 50 °C and 60 °C had the same effect. For the main compounds, freeze-drying proved to be the worst method because limonene (9.80%), β-pinene (2.39%) or β-(E)-ocimene (1.95%) decreased significantly; however, compounds such as tetradecane (15.28%), fenipentol (7.28%), dodecane (31.98%) and decanal (8.54%) increased. In the case of freeze-drying, the method used has a greater impact on the dried raw material than the addition of beetroot juice.
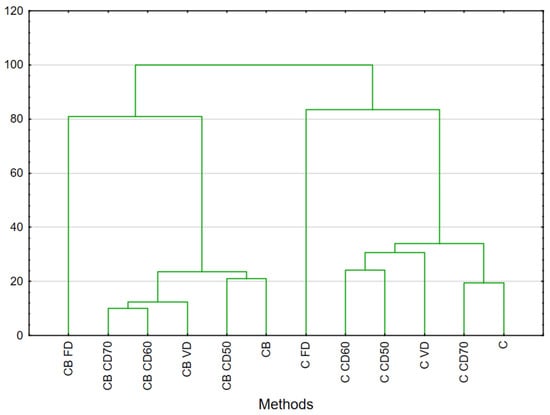
Figure 1.
HCA result analysis of celery and celery with beetroot juice. Celery (C), celery after vacuum impregnation with beetroot juice (CB), control (F), material after freeze drying (FD), vacuum (VD) and convection drying at a temperature of 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
Surprisingly, impregnation of celery with beetroot before convection drying at 50 °C brought a compound profile similar to fresh celery. This makes it possible to argue that its addition can maintain the natural flavor of celery.
The other methods for celery and celery with beetroot juice, such as vacuum drying at 60 °C or convection drying of celeriac after impregnation at 60 °C and 70 °C, were not significantly different, and the aroma profile was similar.
2.2. Drying Kinetics
The results of the statistical analysis and the constants and coefficients of the five proposed models used to describe the kinetics of the convection drying process of celery (C) and celery after vacuum impregnation (CB) are shown in Table 3. To match the experimental data with the selected drying models, the following statistical parameters were analyzed to determine the best model: R2, χ2, Ve and RMSE. It can be observed that a good fit to the experimental data was obtained for each of the analyzed models. The coefficient of determination (R2) of equations ranged from 0.9903 to 0.9994. The RMSE and reduced test (χ2) values were small, ranging from 0.0084 to 0.0370 and 0.0001 to 0.0016, respectively. The best fit to the experimental data was obtained for the logistic model, as the R2 was the highest, and RMSE, Ve and χ2 were the lowest.

Table 3.
Model parameters and statistics describing the drying kinetics of celery dried by convection at different temperatures. Celery (C), celery after vacuum impregnation with beetroot juice (CB), material after freeze drying (FD), vacuum (VD) and convection drying at a temperature of 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
The drying kinetics curves of celery root (C) and celery after vacuum impregnation with beet juice (CB) at different temperatures of the drying medium are shown in Figure 2 and Figure 3. Drying continued until a constant water content was reached. The moisture content of celery root decreased rapidly during the first 20 min. The rapid evaporation of water at the initial stage of drying may be due to the fact that the moisture in the samples at the early stage of drying was mainly on the surface of the sample, so it could evaporate quickly []. In subsequent stages of drying, the water loss time was extended. The water on the surface of the samples evaporated quickly, and the water inside the celery moved to the surface, which can occur through capillary movement, liquid diffusion, gas diffusion or surface diffusion [].
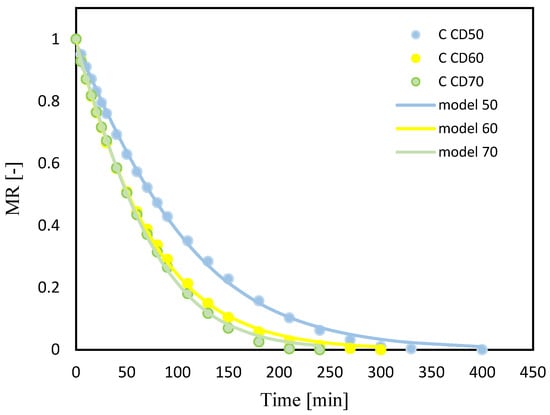
Figure 2.
Celery (C); drying kinetics for convective drying at 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70). MR—moisture ratio.
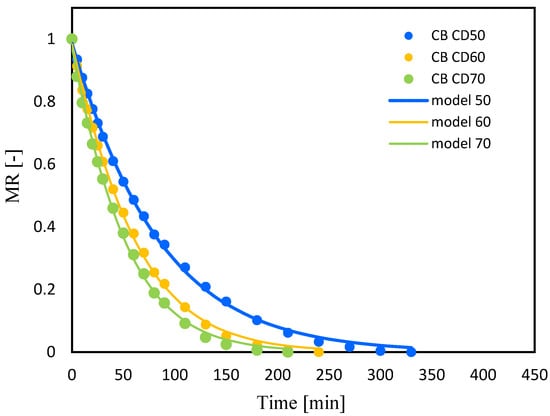
Figure 3.
Celery after VI with beetroot juice (CB); drying kinetics for convective drying at 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70). MR—moisture ratio.
Increasing the temperature of the drying medium from 50 °C to 70 °C significantly reduced the drying time for both celery without pretreatment and celery impregnated with tomato juice, by 40% and 36%, respectively. Krzykowski et al. [], subjecting Wild Strawberry Fragaria vesca L. to drying, observed that increasing the convection drying temperature from 25 °C to 60 °C reduced drying time by 60%. Other authors also observed a reduction in drying time with an increase in the temperature of the convection process by subjecting jackfruit [], bitter gourds [] and coriander [] to drying.
In the case of celery drying, pretreatment affected the drying time at all three temperatures during convection drying (50, 60 and 70 °C). Thus, the drying period of celery subjected to vacuum impregnation with beet juice reduced the drying time, which is consistent with the results of studies of drying figs [] and cherry tomatoes []. Studying the effect of blanching on the drying process of mangoes, researchers observed a reduction in drying time from 14.46% to 19.95% depending on the drying method []. Similar observations were noted by other authors studying the effect of blanching on the drying time of celery [].
Figure 4 and Figure 5 show changes in water content (WC) with drying time during convection drying at 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70). It is clear that the moisture content of celery and celery samples after the vacuum impregnation process showed a general trend of decreasing with increasing drying time. It was noted that celery without pretreatment had a higher water content of 16.7 (g water/g dry weight). The application of the vacuum impregnation process reduced the water content of the samples after the vacuum impregnation process to 14 (g water/g dry weight). Kręcisz et al. [] recently showed that the moisture content of zucchini decreased after applying impregnating solutions such as onion juice, kale juice and a mixture of onion and kale juices (50:50), as well as with the addition of a 3% NaCl solution. Similar observations were noted in our previous studies on dried celery [].
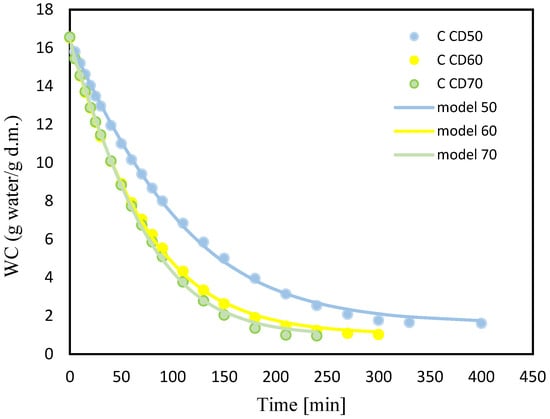
Figure 4.
Changes in water content (WC) for celery (C) with drying time for convective drying at 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
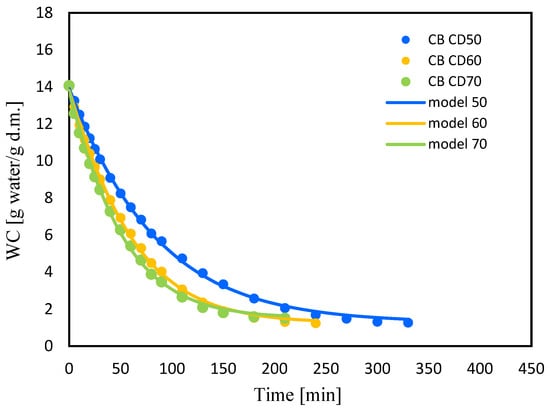
Figure 5.
Changes in water content (WC) for celery after VI with beetroot juice (CB) with drying time for convective drying at 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
2.3. Dry Matter (DM), Water Activity (AW) and Density (ρb)
The dry weight, water activity and density of fresh celery (C), vacuum-impregnated celery (CB) and the resulting dries are shown in Table 4. For dried celery, the lowest dry mass value was observed for CB CD50 (87.69%) and the highest was for C FD (99.26%). Theoretically, the growth of microorganisms is inhibited below the level of 20% moisture []. However, a study presented by Singh et al. [] shows that a moisture content of 15% in mangoes is considered safe. The use of the VI process significantly affected the dry weight of dried celery root, causing a decrease in the studied parameter from 1 to 5% depending on the drying method used. The drying method significantly affected DM. The highest values were recorded for dries obtained by the sublimation method (99.26%) and the lowest for dries obtained by the convection method at 50 °C. Increasing the drying temperature from 50 °C to 70 °C increased the dry weight from 8 to 9%, depending on the material used.

Table 4.
Dry matter (DM), water activity (AW), bulk density (ρb).
Water activity (Table 4) in dried celery root ranged from 0.110 to 0.557 (for C FD and CB CD50, respectively). Application of the vacuum impregnation process significantly increased AW in all tested samples compared to celery not subjected to VI. When stowing impregnating solutions containing more than 30 °Brix, a decrease in water activity is observed in the tested material []. The lowest AW values were recorded for FD for both C and CB. The lowest water activity values for freeze drying were recorded by other authors studying apples [], Increasing the process temperature yielded dries with lower water activity.
The bulk density for fresh materials ranged from 221.32 kg/m3 for celery without VI and 271.43 kg/m3 for celery after VI. On the other hand, the density of dried materials for celery without pretreatment and for celery after vacuum impregnation ranged from 34.28 kg/m3 to 72.04 kg/m3 and from 34.48 kg/m3 to 94.24 kg/m3, respectively. The lowest density value was recorded during freeze drying for C and CB, and it can also be noted that their difference is relatively not great, but the materials after vacuum impregnation have a higher value of the studied parameter. The lowest drying density after FD was also observed by other researchers who compared drying methods for apples []. Convection drying at 50, 60 and 70 °C temperatures showed a significant effect of temperature on the efficiency of the process. As the temperature of the drying medium increases, a reduction in the density of the product is observed, which can be beneficial, especially in the production of food or food materials for packaging. The vacuum impregnation process contributed to an increase in the density of celery in each of the tested materials and for each of the drying methods.
2.4. Color
Food color and its acceptability at the time of purchase have a direct impact on the identification and ultimately on the decision of consumers. Therefore, it is important that the darkening reaction and pigment damage do not occur during food processing []. Table 5 shows the color characteristics of fresh and dried samples.

Table 5.
Color parameters of raw and dried celery. Color parameters of raw and dried celery: L*—lightness, a* for (+) redness/(−) greenness, and b* for (+) yellowing, BI—browning index, C*—saturation, ΔE—total color of vegetables.
The value of the L* parameter ranged from 63.56 to 91.90 for celery without pretreatment, for celery after vacuum impregnation from 37.02 to 58.59. A decrease in the studied parameter was observed in samples after the vacuum impregnation process. This is due to the addition of beet juice, which has a characteristic dark color. Similar observations were noted by other authors who studied apples subjected to osmotic dehydration with chokeberry juice [] and zucchini and broccoli subjected to vacuum impregnation with beet juice []. During the process of vacuum impregnation, atmospheric pressure is lowered, and therefore, the capillaries expand and deform. By lowering the pressure, the capillaries are partially filled with an impregnating solution. In the next stage, VI, the pressure returns to the atmospheric pressure level, and there is a narrowing of the vessels and an intensive flow of the impregnating solution into their interior []. For both celery without pretreatment and celery after VI after freeze drying, the highest values of the L* parameter were recorded, demonstrating the brightening of the tested materials in the case of this drying method. These results are in accordance with the studies of other authors []. During FD, the temperature of dried vegetables is lowered below the freezing point. Therefore, water is removed from vegetables by sublimation of ice to water vapor, which allows a direct transition from a solid state to a gaseous state, and the liquid phase is skipped. During sublimation drying, the pressure is lower than the triple point of water. Sublimation drying, combining low temperatures and pressure, is considered the best dehydration method, thanks to which it is possible to maintain a very good color, shape and taste of dried products [,]. Dries obtained by using the vacuum method for both tested materials were characterized by obtaining the lowest values of the L* parameter (C VD—63.56; CB VD—37.02), as can be seen in Figure 6. Other researchers drying celery at 60 °C observed higher L* values for vacuum-dried celery than convection-dried celery. Differences in color range may probably be due to prolonged drying time by the VD method []. Much literature confirms that increasing the drying time causes a decrease in the brightness of vegetables [,].
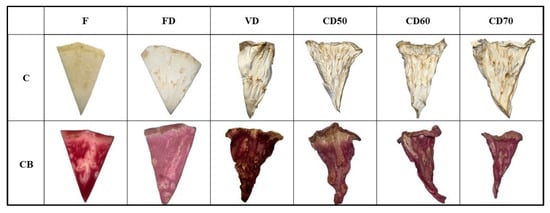
Figure 6.
Celery (C), celery after vacuum impregnation with beetroot juice (CB), control (F), material after freeze drying (FD), vacuum (VD) and convection drying at a temperature of 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
The use of the vacuum impregnation process resulted in a significant increase in the color index a*, which is related to the red shade, as well as an increase in the browning value (BI), saturation (C*) and the color difference ∆E. This is caused by the use of beetroot juice, which is characterized by an intense dark burgundy color. These results are consistent with the observations of other authors who examined the effect of vacuum impregnation using beetroot juice on the properties of zucchini and broccoli [].
2.5. Pearson Correlation of Drying Kinetics and Selected Celery Quality Attributes
As shown in Figure 7, Pearson’s correlation was used to examine the association between selected celery properties. The data showed a significant (p < 0.05) positive correlation between vacuum impregnation and drying methods (r = 0.87), 1-Hexanol (r = 0.94), 1-Heptanol (r = 0.87), Decane (r = 0.91), unknown sesquiterpene or sesquiterpenoid (r = 0.88) and a negative correlation with color (r = −0.91), Limonene (r = −0.89), γ-Terpinene (r = −0.89) and β-Pinene (r = −0.85). The results also showed a significant negative correlation of drying methods with color (r = −0.89) and a significantly positive correlation with 1-Hexanol (r = 0.89), 1-Heptanol (r = 0.84) and Decane (r = 0.88). The data obtained indicate that vacuum impregnation had a greater impact on VOCs than drying methods. Moreover, a significant negative correlation was observed between dry matter and water activity (r = −0.92) and bulk density (r = −96). Water activity positively correlates with density (r = 0.88). The results also showed a positive correlation of color with Limonene (r = 0.72), γ-Terpinene (r = 0.71) and β-Pinene (r = 0.72) and a significant negative correlation with 1-Hexanol (r = −0.90), 1-Heptanol (r = −0.87) and Decane (r = −0.92).
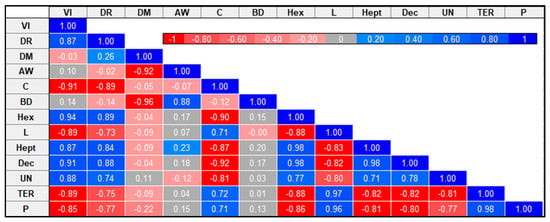
Figure 7.
Correlation matrix for selected celery parameters. VI: vacuum impregnation; DR: drying methods; DM: dry matter; AW: water activity; C: color; BD: bulk density; Hex: 1-Hexanol; L: Limonene; Hept: 1-Heptanol; Dec: Decane; UN: unknown sesquiterpene or sesquiterpenoid; TER: γ-Terpinene; P: β-Pinene.
2.6. Principal Component Analysis (PCA)
Approximately 84% of the sample data variance was explained by the first two principal components: PC1 (50.37%) and PC2 (33.81%). PC1 was negatively correlated with the bulk density, water activity, dry mass, Decane, 1-Hexanol and Tetradec-1-ene, and positively with β-Pinnene, Limonene and color of celery. PC2 was negatively correlated with Tetradec-1-ene, color and dry mass and positively correlated with bulk density, water activity, Decane, 1-Hexanol, β-Pinnene and Limonene of celery (Figure 8).
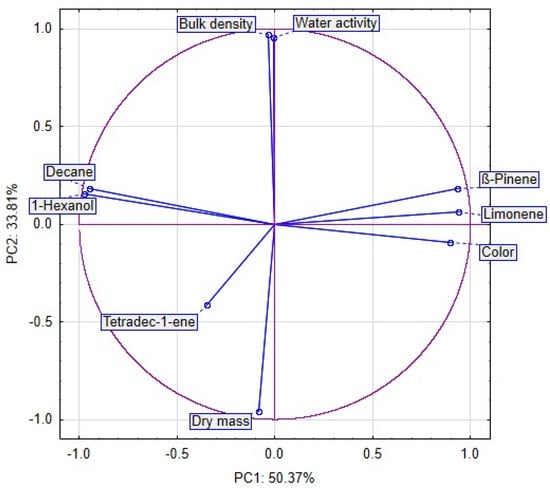
Figure 8.
PCA analysis of dried selery.
The PCA plots reveal that the first principal component (PC1) refers to the use of vacuum impregnation process at 50.37%. In the figure, positive values of PC1 describe the results obtained for celery without vacuum impregnation process (Figure 9). The negative values of PC1 describe the celery with vacuum impregnation process. The second principal component (PC2) refers to the use of drying method at 33.81%. In the figure, negative values of PC2 describe the celery after freeze drying. The positive values of PC2 describe the results obtained for fresh celery without drying. Values from 1 to −1 of PC2 describe celery after convection and vacuum drying. Placing the samples in the space of the first two factors showed that the samples were different from each other (Figure 8). Moreover, significant differences can be observed between clusters. The first cluster included sample C F and CB F (fresh celery). The second cluster included sample celery after freeze drying. The next cluster included samples celery without VI and after vacuum and convection drying (3), and the last cluster included samples after VI and vacuum and convection drying (4).
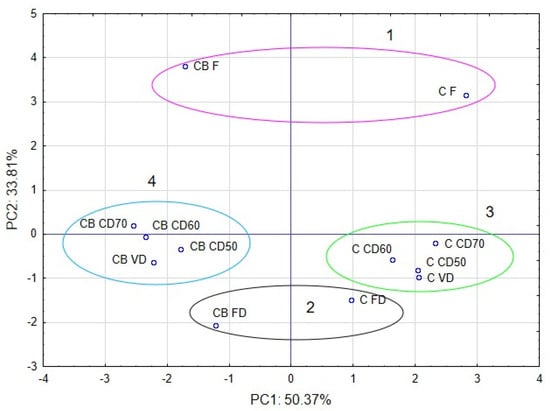
Figure 9.
The celery objects in space of first two major components by PCA (principal component analysis).
3. Materials and Methods
3.1. Plant Materials
Celery was purchased from a local vegetable market (Wrocław, Poland) in 2023. The raw material was stored at 4 ± 2 °C in a RL58GRGIH refrigerator (Samsung Electronics Polska sp. z o.o., Wronki, Poland) until testing. Celery with an initial moisture content of 90.11% was washed and peeled, then sliced into 6.5 mm thick slices. Samples for testing were prepared in the shape of wedges measuring 5 × 5 × 3 cm. All recipes are presented in Table 6.

Table 6.
Samples codes for raw and dried celery.
Beet juice (Multi-Smak Sp. z o.o., Warsaw, Poland)—7.9 °Brix—was used as an impregnating solution. The nutritional value of beet juice per 100 mL was as follows: energy value 176 kJ/42 kcal, fat 0.1 g including saturated fatty acids 0.0 g, carbohydrates 7.3 g including sugars 6.9 g, fiber 2.2 g, protein 1.8 g, salt 0.1 g, vitamin C 10 mg/12.5% RWS*.
3.2. Vacuum Impregnation (VI)
Vacuum impregnation of celery was performed in a prototype (Figure 10) device located in the laboratory of the Wrocław University of Life Sciences []. The prepared celery weighing 125 g was placed in a perforated vessel made of stainless steel. The vacuum phase of the impregnation process was carried out in a vacuum chamber connected to a vacuum pump at a pressure of 0.06 MPa for 2 min. Then 700 mL of the impregnation solution was added while maintaining a constant pressure of 0.06 MPa for 4 min. During this part of the process, the samples were completely immersed in the impregnating solution, allowing effective saturation of the material. The celery was then left in the impregnating solution for 15 min under atmospheric pressure. After VI, the material was drained using filter paper.
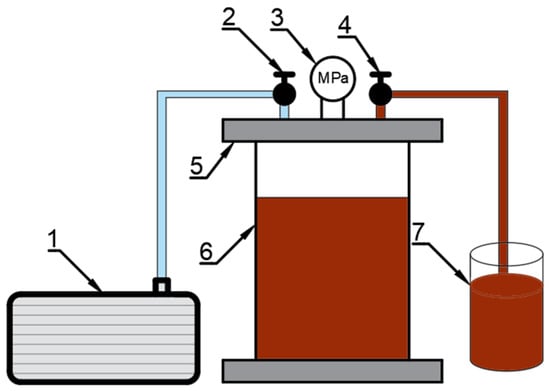
Figure 10.
Schematic of the vacuum impregnation apparatus. 1—vacuum pump, 2,4—valves, 3—vacuum gauge, 5—lid, 6—vacuum chamber, 7—impregnation liquid.
3.3. Drying Methods
After the vacuum impregnation process, the material was subjected to drying by using three methods: the freeze drying method (FD), the vacuum method (VD) and the convection method (CD).
3.3.1. Freeze Drying (FD)
After the vacuum impregnation process, the material was immediately frozen at −20 °C for 24 h. The freezing rate was 1 °C·min−1 in the RL58GRGIH freezer (Samsung Electronics Polska sp. z o.o., Wronki, Poland). FD was performed in the device (Free-Zone 4.5 L, Labconco, Fort Scott, KS, USA). The drying process lasted 24 h at 5 Pa pressure at −50 °C. The heating shelves reached a temperature of 22 °C.
3.3.2. Vacuum Drying (VD)
Vacuum drying was carried out in a V0101 dryer (Memmert, Schwabach, Germany) at 60 °C under a pressure of 10 kPa. The drying time was 24 h.
3.3.3. Convection Drying (CD)
Convection drying was performed in a dryer designed and built at the Institute of Agricultural Engineering (Wroclaw, Poland) []. Samples weighing 40 g were placed in a single layer in baskets. The air flow velocity was 0.5 M·s−1. Drying was performed at three temperatures: 50 °C (CD50), 60 °C (CD60) and 70 °C (CD70).
3.4. Mathematical Modeling
The moisture ratio was calculated using Equation (1) []:
where: Mt—water content after time t (g water/g dry weight), Me—equilibrium water content (g water/g dry weight), M0—initial water content (g water/g dry weight)
In order to select the best mathematical model to describe the convection drying of celery root, five equations commonly cited in the literature were analyzed (Table 7).

Table 7.
Models taken advantage for description of course convective drying.
3.5. Volatile Organic Compounds (VOCs)
Headspace Solid-Phase Microextraction (HS-SPME Arrow)—GC-MS
Volatile organic compound (VOC) analysis was performed with HS-SPME technique. Amounts of 0.2 ± 0.005 g of dried and 2 ± 0.010 g of fresh material (previously homogenized) were weighed in sequence and put into 20 mL headspace glass vials. Then, 2-undecanone (Sigma-Aldrich, Steinheim, Germany) was added as an internal standard in the amount of 5 µL (1 mg/mL). HS-SPME extraction was performed using a 1.10 mm DVB/C-WR/PDMS SPME Arrow fiber (Shimadzu, Kyoto, Japan). Before each analysis, the fiber was pre-conditioned at 250 °C for 5 min. Extraction was preceded by sample incubation for 5 min in 60 °C, and thereafter the VOC extraction was carried out for 30 min at 60 °C. Desorption of the sample lasted 3 min in the GC injection port. Analyte separation and identification were performed on a Shimadzu QP 2020 Plus (Shimadzu, Kyoto, Japan) equipped with a ZB-5Msi column (Phenomenex, Torrance, CA, USA) (30 m × 0.25 mm × 0.25 µm). The injector temperature was 250 °C, and the carrier gas was helium at a flow rate of 1.0 mL·min−1 with a linear velocity of 36.3 cm·s−1 and a split of 20. The temperature program was 50 °C, then 130 °C at a rate of 4 °C min−1; then 180 °C at a rate of 10 °C min−1; then 280 °C at a rate of 20 °C min−1. The ion source temperature and interface temperature were 220 and 250 °C, respectively. The MS mode was scan 40–400 m/z. The analyses were carried out in triplicate.
A comparison of the obtained mass spectra (at least 90%) and a comparison of the calculated linear retention indices (LRI) (±15) with the NIST 20 (National Institute of Standards and Technology, Gaithersburg, MD, USA) and FFNSC (Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds) databases were used to identify the analyses. The quantification was obtained by area normalization method against the internal standard peak.
3.6. Physical Properties
3.6.1. Dry Weight, Water Activity, Bulk Density
Dry weight of celery samples was determined gravimetrically by drying at 70 °C for 24 h under reduced pressure (5 kPa) in a vacuum dryer (Memmert, VO101, Schwabach, Germany). The measurement was performed in triplicate, and the average of the measurements was taken as the result.
The water activity of the tested samples was determined using AquaLab 4TE ± 0.003 (AquaLab, Warsaw, Poland). Measurements were made at a constant temperature of 25 °C. The result obtained was the average of four measurements.
Bulk density was taken using a measuring cylinder, which was filled with the material []. The measurement was made in six repetitions and calculated according to the formula:
where: ρb—bulk density [kg · m−3], ws—weight of samples [kg], V—volume [m3].
3.6.2. Color
Using a colorimeter (Minolta Chroma Meter CR-200 colorimeter (Minolta Corp., Osaka, Japan)), the color indices L* (brightness), a* (red-green) and b* (yellow-blue) were obtained. And also, the overall color change (ΔE), the browning index (BI) and color saturation (C*) were calculated using the following equations []
Total color change (ΔE) was calculated according to equation:
where:
L*control, a*control, and b*control—color of fresh celery
L*sample, a*sample, and b*sample—color of dried celery samples
The color saturation (C*):
The browning Index (BI):
where:
3.7. Statistical Analysis
Statistical analyses were performed using Statistica version 13.1 (StatSoft, Tulsa, OK, USA). One-way analysis of variance (ANOVA) using Duncan’s test was used to compare the mean values. Differences were considered to be significant at p < 0.05. The significant differences in major VOCs (abundance in fresh samples at least 1%) were verified by hierarchical cluster analysis (HCA). Numerical data used before the analysis were standardized according to software algorithm. Assumptions for HCA included Ward’s linkage, Euclidean distance and the strict (33%) Sneath’s criterium. Principal component analysis (PCA) was also performed for the tested selected physical properties and volatile compounds of celery depending on the drying method and vacuum impregnation applied. A dendrogram plot was then prepared to analyze the clustered data.
4. Conclusions
In this study, we investigated the effect of pretreatment, such as vacuum impregnation, and various drying process methods and parameters on the drying properties and selected quality characteristics and VOCs of dried celery. The results showed that pretreatment significantly reduced the drying time of celery and increased their drying rate, regardless of the temperature of the drying medium used. In addition, the dries that were obtained using VI were characterized by lower dry matter and increased water activity and density. The use of beet juice as an impregnating solution significantly reduced the brightness and increased the shade of red in the samples tested. Moreover, the vacuum impregnation process influenced the content of volatile compounds, which is related to the addition of beetroot juice. The amounts of major compounds such as limonene (36.02%), pentyl cyclohexa-1,3-diene (3.22%) and Terpinene <gamma-> (3.71%) decreased after impregnation. The effect of different drying conditions on the quality of the dried samples was also studied. Drying methods had a significant effect on water activity, density, color, drying time and VOCs. Increasing the temperature of the drying medium during convection drying reduced the drying time and increased the values of dry weight and water activity. Of all the mathematical models tested, the logistic model had the best fit. Droughts obtained by using the sublimation method had the lowest water activity values and the lowest bulk density. In addition, the study confirmed that dries obtained by using the sublimation method retained their color and shape better than those obtained by using other methods.
5. Patents
Patent Poland, no. 421913. Vacuum impregnating machine and method for initial processing of material. Wrocław University of Environmental and Life Sciences, Wrocław, PL. Authors: Bogdan Stępień, Radosław Maślankowski, Leszek Rydzak, and Marta Pasławska.
Author Contributions
Conceptualization, M.K. (Magdalena Kręcisz); methodology, M.K. (Magdalena Kręcisz) and M.K. (Marta Klemens), A.L. and B.S.; validation, M.K. (Magdalena Kręcisz) and M.K. (Marta Klemens); investigation, M.K. (Magdalena Kręcisz) and M.K. (Marta Klemens); writing—original draft preparation, M.K. (Magdalena Kręcisz) and M.K. (Marta Klemens); writing—review and editing, M.K. (Magdalena Kręcisz); visualization, M.K. (Magdalena Kręcisz) and M.K. (Marta Klemens); supervision, M.K. (Magdalena Kręcisz); project administration, M.K. (Magdalena Kręcisz);. All authors have read and agreed to the published version of the manuscript.
Funding
The APC is financed by Wrocław University of Environmental and Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Betoret, N.; Puente, L.; Díaz, M.; Pagán, M.; García, M.; Gras, M.; Martínez-Monzó, J.; Fito, P. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Kręcisz, M.; Stępień, B.; Łyczko, J.; Kamiński, P. The Influence of the Vacuum Impregnation, Beetroot Juice, and Various Drying Methods on Selected Properties of Courgette and Broccoli Snacks. Foods 2023, 12, 4294. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Martín, M.E.; Martínez-Navarrete, N.; Chiralt, A. Effect of vacuum impregnation and microwave application on structural changes which occurred during air-drying of apple. LWT—Food Sci. Technol. 2005, 38, 471–477. [Google Scholar] [CrossRef]
- Bylok, F. “Healthy” lifestyle as a determinant of consumer behavior. Econ. Probl. (PL). 2011, 78, 33–46. [Google Scholar]
- Bronkowska, M.; Orzeł, D.; Wyka, J.; Habánová, M.; Jarossova, M.A. Organic food—A way to have a “healthy” diet? In Biopotraviny, Tradičné a Regionálne Potraviny na Slovensku av Zahraničí; Slovenskej Ekonomickej Knižnice: Bratislava, Slovakia, 2017; p. 16. [Google Scholar]
- Wang, N.; Xu, Y.; Chao, H.; Zhang, M.; Zhou, Y.; Wang, M. Effects of celery powder on wheat dough properties and textural, antioxidant and starch digestibility properties of bread. J. Food Sci. Technol. 2020, 57, 1710–1718. [Google Scholar] [CrossRef]
- Kosson, R.; Anyszka, Z.; Grzegorzewska, M.; Golian, J.; Kohut, M.; Badełek, E. Postharvest quality of celeriac (Apium graveolens L. var. rapaceum (Mill.) Gaud.) depending on weed control methods. Prog. Plant Prot. 2014, 54, 77–83. [Google Scholar] [CrossRef]
- Kooti, W.; Daraei, N. A Review of the Antioxidant Activity of Celery (Apium graveolens L.). J. Evid.—Based Integr. Med. 2017, 22, 1029–1034. [Google Scholar] [CrossRef]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the factors that influence the aroma profile of Apium graveolens: A review. Food Chem. 2021, 345, 128673. [Google Scholar] [CrossRef]
- Bassey, E.J.; Cheng, J.H.; Sun, D.W. Novel nonthermal and thermal pretreatments for enhancing drying performance and improving quality of fruits and vegetables. Trends Food Sci. Technol. 2021, 112, 137–148. [Google Scholar] [CrossRef]
- Su, W.H.; Bakalis, S.; Sun, D.W. Chemometric determination of time series moisture in both potato and sweet potato tubers during hot air and microwave drying using near/mid-infrared (NIR/MIR) hyperspectral techniques. Dry. Technol. 2020, 38, 806–823. [Google Scholar] [CrossRef]
- Srimagal, A.; Mishra, S.; Pradhan, R.C. Effects of ethyl oleate and microwave blanching on drying kinetics of bitter gourd. J. Food Sci. Technol. 2017, 54, 1192–1198. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Kidoń, M. Applicability of Vacuum Impregnation to Modify Phys-ico-Chemical, Sensory and Nutritive Characteristics of Plant Origin Products—A Review. Int. J. Mol. Sci. 2014, 15, 16577–16610. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, M.; Stępień, B.; Pasławska, M.; Popłoński, J.; Dulak, K. Physicochemical and Quality Properties of Dried Courgette Slices: Impact of Vacuum Impregnation and Drying Methods. Molecules 2021, 26, 4597. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, M.; Kolniak-Ostek, J.; Łyczko, J.; Stępień, B. Evaluation of bioactive compounds, volatile compounds, drying process kinetics and selected physical properties of vacuum impregnation celery dried by different methods. Food Chem. 2023, 413, 135490. [Google Scholar] [CrossRef]
- Kręcisz, M.; Kolniak-Ostek, J.; Stępień, B.; Łyczko, J.; Pasławska, M.; Musiałowska, J. Influence of Drying Methods and Vacuum Impregnation on Selected Quality Factors of Dried Sweet Potato. Agriculture 2021, 11, 858. [Google Scholar] [CrossRef]
- Chungcharoen, T.; Prachayawarakorn, S.; Soponronnarit, S.; Tungtrakul, P. Effect of drying temperature on drying characteristics and quality of germinated rices prepared from paddy and brown rice. Dry. Technol. 2012, 30, 1844–1853. [Google Scholar] [CrossRef]
- Kaushal, P.; Sharma, H.K. Osmo-convective dehydration kinetics of jackfruit (Artocarpus heterophyllus). J. Saudi Soc. Agric. Sci. 2016, 15, 118–126. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Gawlik-Dziki, U.; Janiszewska-Turak, E.; Biernacka, B. Wild Strawberry Fragaria vesca L.: Kinetics of Fruit Drying and Quality Characteristics of the Dried Fruits. Processes 2020, 8, 1265. [Google Scholar] [CrossRef]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Iwiński, H.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Coriandrum sativum L.—Effect of Multiple Drying Techniques on Volatile and Sensory Profile. Foods 2021, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Şahin, U.; Öztürk, H.K. Effects of pulsed vacuum osmotic dehydration (PVOD) on drying kinetics of figs (Ficus carica L.). Innov. Food Sci. Emerg. Technol. 2016, 36, 104–111. [Google Scholar] [CrossRef]
- An, K.; Li, H.; Zhao, D.; Ding, S.; Tao, H.; Wang, Z. Effect of osmotic dehydration with pulsed vacuum on hot-air drying kinetics and quality attributes of cherry tomatoes. Dry. Technol. 2013, 31, 698–706. [Google Scholar] [CrossRef]
- Singh, S.; Kawade, S.; Dhar, a.; Powar, S. Analysis of mango drying methods and effect of blanching process based on energy consumption, drying time using multi-criteria decision-making. Clean. Eng. Technol. 2022, 8, 100500. [Google Scholar] [CrossRef]
- Kian-Pour, N.; Akdeniz, E.; Toker, O.S. Influence of coating-blanching in starch solutions, on the drying kinetics, transport properties, quality parameters, and microstructure of celery root chips. LWT—Food Sci. Technol. 2022, 160, 113262. [Google Scholar] [CrossRef]
- Drying and Dehydration of Fruits and Vegetabeles Indian Institute of Food Processing Technology. Ministry of Food Processing Industries, Govt. of India Thanjavur 2020 Tamil Nadu. Available online: https://niftem-t.ac.in/curmetmg.pdf (accessed on 28 December 2023).
- Schulze, B.; Hubbermann, E.M.; Schwarz, K. Stability of quercetin derivatives in vacuum impregnated apple slices after drying (microwave vacuum drying, air drying, freeze drying) and storage. LWT—Food Sci. Technol. 2014, 57, 426–433. [Google Scholar] [CrossRef]
- da Cunha, R.M.C.; Brandão, S.C.R.; de Medeiros, R.A.B.; da Silva Júnior, E.V.; Fernandes da Silva, J.H.; Azoubel, P.M. Effect of ethanol pretreatment on melon convective drying. Food Chem. 2020, 333, 127502. [Google Scholar] [CrossRef]
- Masztalerz, K.; Łyczko, J.; Lech, K. Effect of filtrated osmotic solution based on concentrated chokeberry juice and mint extract on the drying kinetics, energy consumption and physicochemical properties of dried apples. Molecules 2021, 26, 3274. [Google Scholar] [CrossRef] [PubMed]
- Rudy, S.; Dziki, D.; Biernacka, B.; Polak, R.; Krzykowski, A.; Krajewska, A.; Stanisławczyk, R.; Rudy, M.; Żurek, J.; Rudzki, G. Impact of Drying Process on Grindability and Physicochemical Properties of Celery. Foods 2024, 13, 2585. [Google Scholar] [CrossRef]
- Feng, Y.; Tan, C.P.; Yagoub, A.E.A.; Xu, B.; Sun, Y.; Ma, H.; Xu, X.; Yu, X. Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chem. 2020, 324, 126883. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, A.; Dziki, D.; Yilmaz, M.A.; Özdemir, F.A. Physicochemical Properties of Dried and Powdered Pear Pomace. Molecules 2024, 29, 742. [Google Scholar] [CrossRef]
- Pasławska, M.; Nawirska-Olszańska, A.; Stępień, B.; Klim, A. The Influence of Vacuum Impregnation on Nutritional Properties of Fluidized Bed Dried Kale (Brassica oleracea L. Var. Acephala) Leaves. Molecules 2018, 23, 2764. [Google Scholar] [CrossRef]
- Jałoszyński, K.; Szarycz, M.; Jarosz, B. The effect of convective and microwave-vacuum drying on the activity of aromatic substances in parsley (PL). Inżynieria Rol. 2006, 12, 209–215. [Google Scholar]
- Sarimeseli, A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers. Manag. 2011, 52, 1449–1453. [Google Scholar] [CrossRef]
- Henderson, S.M.; Pabis, S. Grain Drying Theory II: Temperature Effect on Drying Coefficient. J. Agric. Eng. Res. 1961, 6, 169–174. [Google Scholar]
- Demir, V.; Gunhan, T.; Yagcioglu, A.K.; Degrmencioglu, A. Mathematical modeling and the determination of some quality parameters of air-dried bay leaves. Biosyst. Eng. 2004, 88, 325–335. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M.; Okyay Menges, H. Evaluation of drying methods with respect to drying parameters, some nutritional and colour characteristics of peppermint (Mentha x piperita L.). Energy Convers. Manag. 2010, 51, 2769–2775. [Google Scholar] [CrossRef]
- Soysal, Y.; Öztekin, S.; Eren, Ö. Microwave drying of parsley: Modeling, kinetics, and energy aspects. Biosyst. Eng. 2006, 93, 403–413. [Google Scholar] [CrossRef]
- ASAE S269.3; ASAE Standard. Wafers, Pellet, and Crumbles—Definitions and Methods for Determining Density, Dura-Bility and Moisture Content. ASTM International: West Conshohocken, PA, USA, 1989.
- Li, M.; Wang, B.; Lv, W.; Zhao, D. Effect of ultrasound pretreatment on the drying kinetics and characteristics of pregelatinized kidney beans based on microwave-assisted drying. Food Chem. 2022, 397, 133806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).