Free Radical Production Induced by Nitroimidazole Compounds Lead to Cell Death in Leishmania infantum Amastigotes
Abstract
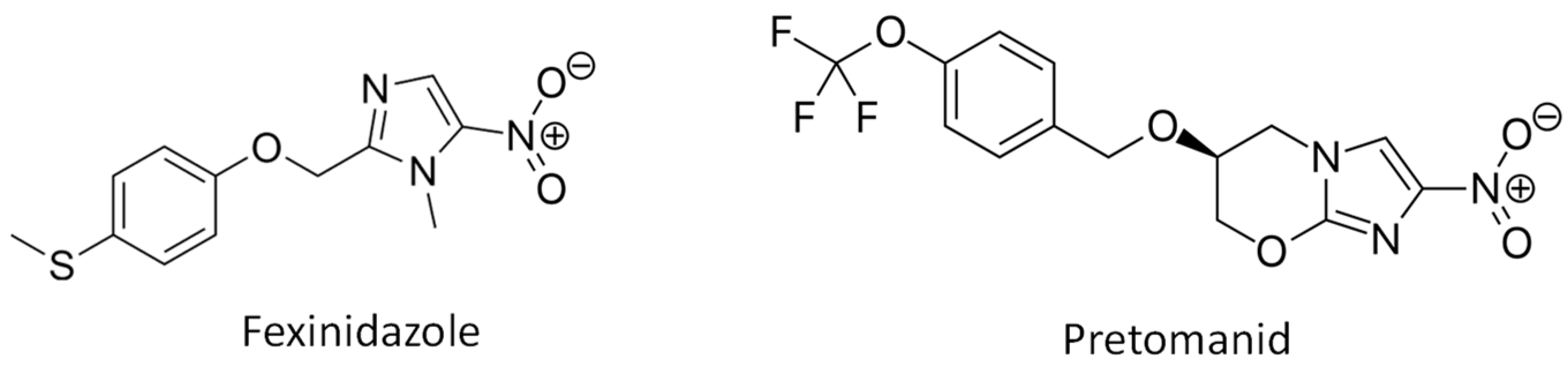
1. Introduction
2. Results
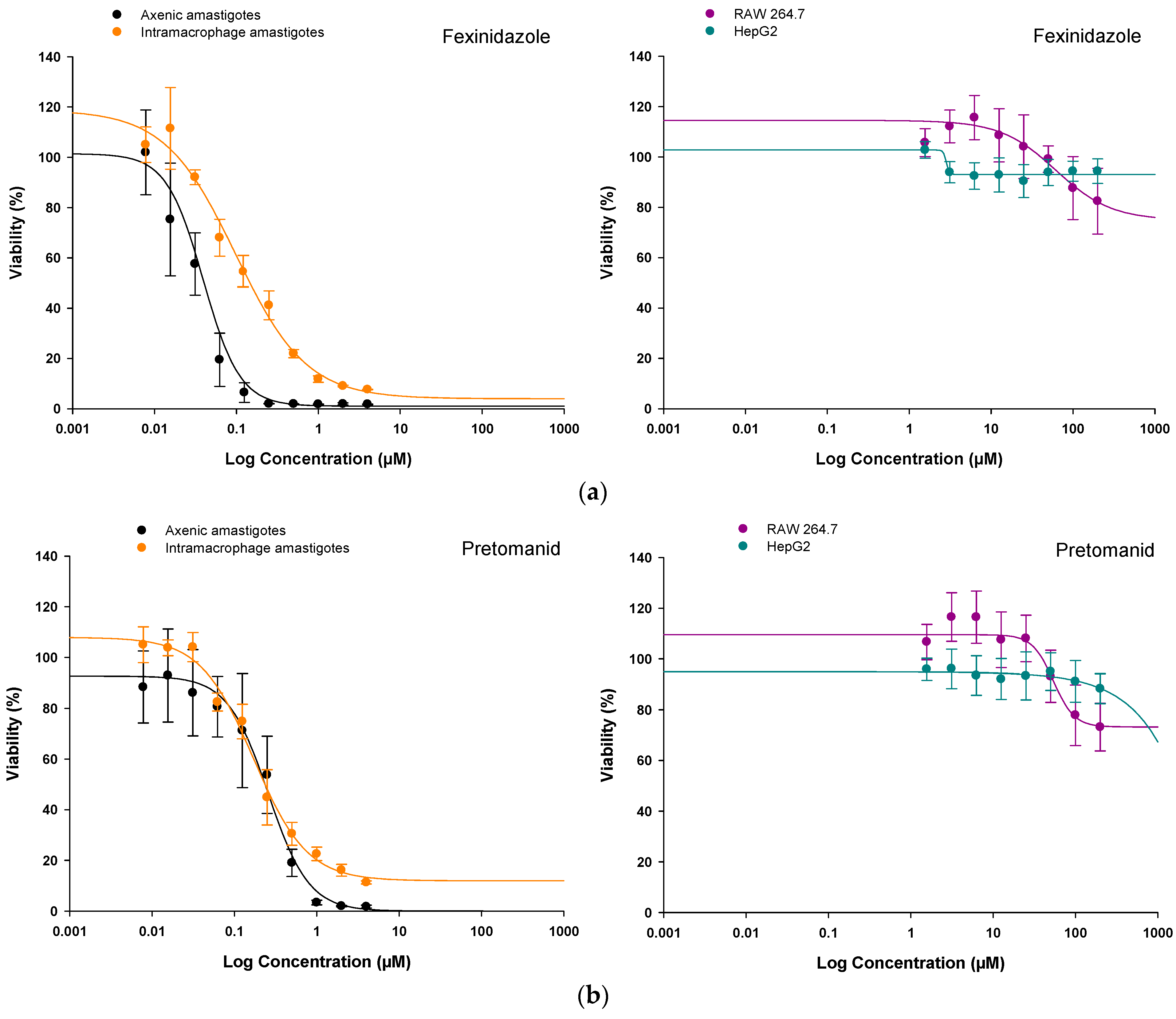
2.1. In Vitro Efficacy of Nitroimidazole Compounds on L. infantum-iRFP Amastigotes
2.2. In Vitro Safety of Nitroimidazole Compounds on HepG2 and RAW 264.7 Mammalian Cells
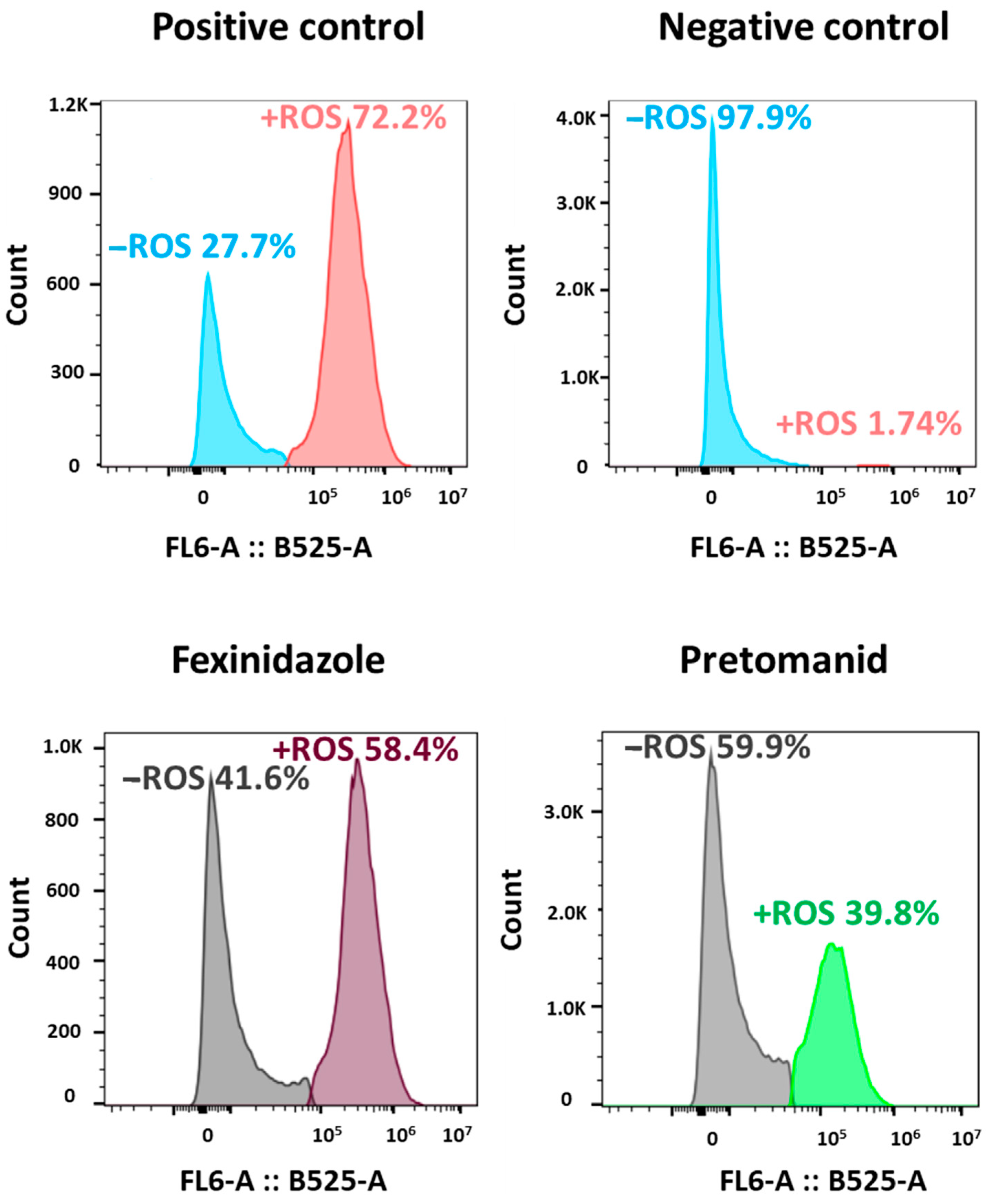
2.3. Induction of ROS Production in L. infantum by Fexinidazole and Pretomanid
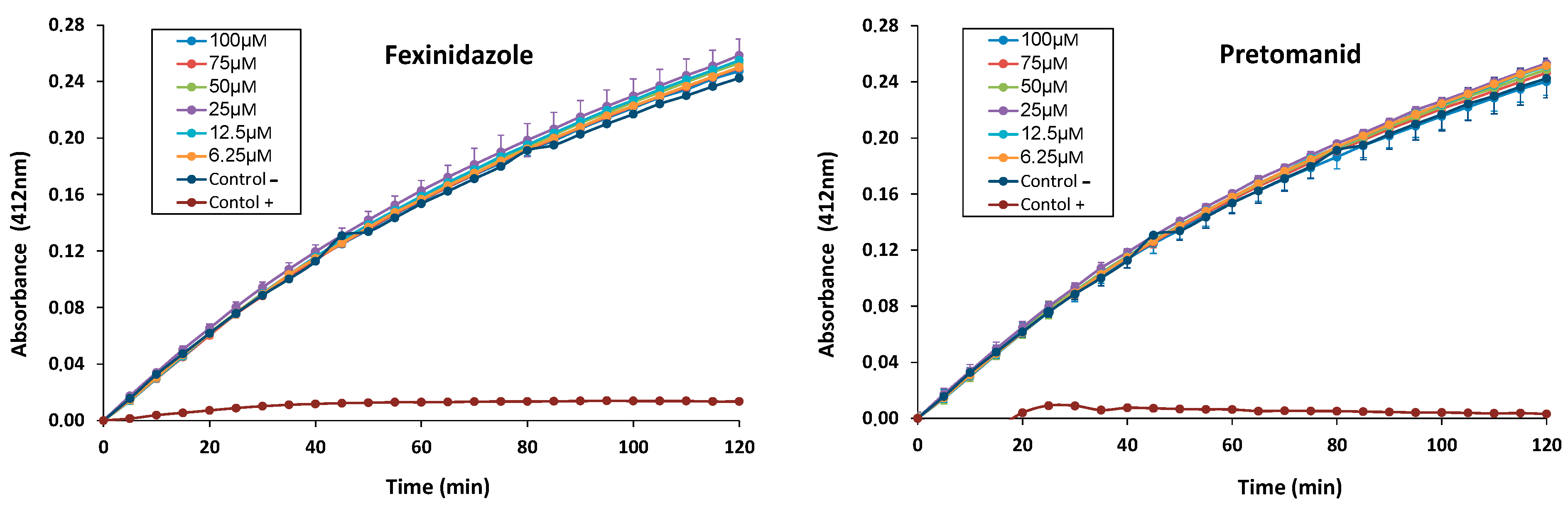
2.4. In Vitro Inhibitory Effect of Fexinidazole and Pretomanid on L. infantum TryR
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Ethical Statement
4.2. Nitroimidazole Compounds
4.3. Parasites and Mammalian Cell Lines
4.4. Assessment of In Vitro Cytotoxicity
4.5. Analysis of ROS Production by Axenic Amastigotes
4.6. Trypanothione Reductase Enzymatic Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 22 July 2024).
- Lukeš, J.; Mauricio, I.L.; Schönian, G.; Dujardin, J.-C.; Soteriadou, K.; Dedet, J.-P.; Kuhls, K.; Tintaya, K.W.Q.; Jirků, M.; Chocholová, E.; et al. Evolutionary and Geographical History of the Leishmania donovani Complex with a Revision of Current Taxonomy. Proc. Natl. Acad. Sci. USA 2007, 104, 9375–9380. [Google Scholar] [CrossRef] [PubMed]
- Engels, D.; Zhou, X.N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Clos, J.; Grünebast, J.; Holm, M. Promastigote-to-amastigote conversion in Leishmania spp.—A molecular view. Pathogens 2022, 11, 1052. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases Initiative (DNDi). Visceral Leishmaniasis. Available online: https://dndi.org/diseases/visceral-leishmaniasis/facts/ (accessed on 22 July 2024).
- World Health Organization. Control of the Leishmaniasis. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. Geneva, Switzerland: World Health Organization. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44412/WHO_TRS_949_eng.pdf?sequence=1&isAllowed=y (accessed on 22 July 2024).
- Van Griensven, J.; Diro, E. Visceral leishmaniasis, recent advances in diagnostic and treatment regimens. Infect. Dis. Clin. N. Am 2019, 33, 79–99. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef]
- Hung, C.T.; Lam, F.C.; Perrier, D.G.; Souter, A. A stability study of amphotericin B in aqueous media using factorial design. Int. J. Pharm. 1988, 44, 117–123. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A.; Rai, M.; Prajapati, V.K.; Singh, A.K.; Ostyn, B.; Boelaert, M.; Dujardin, J.C.; Chakravarty, J. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infect. Dis. 2012, 55, 543–550. [Google Scholar] [CrossRef]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Rijal, S.; Ostyn, B.; de Vries, P.J.; Singh, R.; Bhattarai, N.; Uranw, S.; Dujardin, J.C.; Boelaert, M.; Beijnen, J.H.; et al. Failure of miltefosine in visceral leishmaniasis is associated with low drug exposure. J. Infect. Dis. 2014, 210, 146–153. [Google Scholar] [CrossRef]
- Palić, S.; Beijnen, J.H.; Dorlo, T.P.C. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int. J. Antimicrob. Agents 2022, 59, 106459. [Google Scholar] [CrossRef]
- Omollo, R.; Alexander, N.; Edwards, T.; Khalil, E.A.; Younis, B.M.; Abuzaid, A.A.; Wasunna, M.; Njoroge, N.; Kinoti, D.; Kirigi, G.; et al. Safety and efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: Study protocol for a randomized controlled trial. Trials 2011, 12, 166. [Google Scholar] [CrossRef]
- Musa, A.; Khalil, E.; Hailu, A.; Olobo, J.; Balasegaram, M.; Omollo, R.; Edwards, T.; Rashid, J.; Mbui, J.; Musa, B.; et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: A randomised controlled trial. PLoS Negl. Trop. Dis. 2012, 6, e1674. [Google Scholar]
- Ashok, P.; Chander, S.; Tejería, A.; García-Calvo, L.; Balaña-Fouce, R.; Murugesan, S. Synthesis and anti-leishmanial evaluation of 1-phenyl-2,3,4,9-tetrahydro-1H-β-carboline derivatives against Leishmania infantum. Eur. J. Med. Chem. 2016, 123, 814–821. [Google Scholar] [CrossRef]
- Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Ordóñez, C.; Sepúlveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; García-Estrada, C.; Reguera, R.M.; et al. Screening marine natural products for new drug leads against trypanosomatids and malaria. Mar. Drugs 2020, 18, 187. [Google Scholar] [CrossRef]
- Reguera, R.M.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and promising novel drug candidates against visceral leishmaniasis. Pure Appl. Chem. 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Balaña-Fouce, R.; Reguera, R.M. Ex vivo phenotypic screening of two small repurposing drug collections identifies nifuratel as a potential new treatment against visceral and cutaneous leishmaniasis. ACS Infect. Dis. 2021, 7, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Balaña-Fouce, R.; Reguera, R.M. Further investigations of nitroheterocyclic compounds as potential antikinetoplastid drug candidates. Biomolecules 2023, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Kelly, J.M. Trypanocidal drugs: Mechanisms, resistance and new targets. Expert Rev. Mol. Med. 2009, 11, e31. [Google Scholar] [CrossRef]
- Wyllie, S.; Roberts, A.J.; Norval, S.; Patterson, S.; Foth, B.J.; Berriman, M.; Read, K.D.; Fairlamb, A.H. Activation of bicyclic nitro-drugs by a novel nitroreductase (NTR2) in Leishmania. PLoS Pathog. 2016, 12, e1005971. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, S.; Singh, R.; Vishwakarma, R.A.; Mignani, S.; Singh, P.P. Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview. Pharmaceuticals 2022, 15, 561. [Google Scholar] [CrossRef]
- Pal, C.; Bandyopadhyay, U. Redox-active antiparasitic drugs. Antioxid. Redox Signal. 2012, 17, 555–582. [Google Scholar] [CrossRef]
- Arias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bürgi, M.D.; Prieto, C.; Iglesias, A.A.; Cravero, R.M.; et al. Rational design of nitrofuran derivatives: Synthesis and valuation as inhibitors of Trypanosoma cruzi trypanothione reductase. Eur. J. Med. Chem. 2017, 125, 1088–1097. [Google Scholar] [CrossRef]
- Melcón-Fernández, E.; Galli, G.; García-Estrada, C.; Balaña-Fouce, R.; Reguera, R.M.; Pérez-Pertejo, Y. Miltefosine and nifuratel combination: A promising therapy for the treatment of Leishmania donovani visceral leishmaniasis. Int. J. Mol. Sci. 2023, 24, 1635. [Google Scholar] [CrossRef]
- González-Montero, M.C.; Andrés-Rodríguez, J.; García-Fernández, N.; Pérez-Pertejo, Y.; Reguera, R.M.; Balaña-Fouce, R.; García-Estrada, C. Targeting trypanothione metabolism in trypanosomatids. Molecules 2024, 29, 2214. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.C.; Pérez, J.G.; Cortés, O.L.; Riarte, A.; Pepper, M.; Marin-Neto, J.A.; Guyatt, G.H. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst. Rev. 2014, 2014, CD003463. [Google Scholar] [CrossRef]
- Mansoldo, F.R.P.; Carta, F.; Angeli, A.; Cardoso, V.D.S.; Supuran, C.T.; Vermelho, A.B. Chagas Disease: Perspectives on the past and present and challenges in drug discovery. Molecules 2020, 25, 5483. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.J.; Nevis, I.; Fernández, E.; Sánchez, A. A rapid review on the efficacy and safety of pharmacological treatments for Chagas disease. Trop. Med. Infect. Dis. 2021, 6, 128. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alqahtani, A.M.; Alanazi, T.A.; Kamal, M.; Jawaid, T.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Discovery, development, inventions and patent review of fexinidazole: The first all-oral therapy for human African trypanosomiasis. Pharmaceuticals 2022, 15, 128. [Google Scholar] [CrossRef]
- Fernando da Silva Santos-Júnior, P.; Rocha Silva, L.; José Quintans-Júnior, L.; Ferreira da Silva-Júnior, E. Nitro compounds against trypanosomatidae parasites: Heroes or villains? Bioorg. Med. Chem. Lett. 2022, 75, 128930. [Google Scholar] [CrossRef]
- Wyllie, S.; Patterson, S.; Stojanovski, L.; Simeons, F.R.; Norval, S.; Kime, R.; Read, K.D.; Fairlamb, A.H. The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci. Transl. Med. 2012, 4, 119re1. [Google Scholar] [CrossRef]
- De Morais-Teixeira, E.; Rabello, A.; Aguiar, M.M.G. In vitro activity and in vivo efficacy of fexinidazole against New World Leishmania species. J. Antimicrob. Chemother. 2019, 74, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Damasio, D.S.D.N.; Antunes, P.A.; Lages, E.B.; Morais-Teixeira, E.; Vital, K.D.; Cardoso, V.N.; Fernandes, S.O.A.; Aguiar, M.G.; Ferreira, L.A.M. A new oral self-emulsifying drug delivery system improves the antileishmania efficacy of fexinidazole in vivo. Int. J. Pharm. 2023, 631, 122505. [Google Scholar] [CrossRef]
- Silva, R.A.; Damasio, D.S.; Coelho, L.D.; de Morais-Teixeira, E.; Queiroz-Junior, C.M.; Souza, P.E.; Azevedo, R.B.; Tedesco, A.; Ferreira, L.A.; Oliveira, M.C.; et al. Combination of the Topical Photodynamic Therapy of Chloroaluminum Phthalocyanine Liposomes with Fexinidazole Oral Self-Emulsifying System as a New Strategy for Cutaneous Leishmaniasis Treatment. Pharmaceutics 2024, 16, 509. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S.; Stojanovski, L.; Perry, M.R.; Simeons, F.R.; Norval, S.; Osuna-Cabello, M.; De Rycker, M.; Read, K.D.; Fairlamb, A.H. The R enantiomer of the antitubercular drug PA-824 as a potential oral treatment for visceral leishmaniasis. Antimicrob. Agents Chemother. 2013, 57, 4699–4706. [Google Scholar] [CrossRef]
- Calvo-Álvarez, E.; Stamatakis, K.; Punzón, C.; Álvarez-Velilla, R.; Tejería, A.; Escudero-Martínez, J.M.; Pérez-Pertejo, Y.; Fresno, M.; Balaña-Fouce, R.; Reguera, R.M. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003666. [Google Scholar] [CrossRef] [PubMed]
- Drugs for Neglected Diseases Initiative (DNDi). Fexinidazole/Miltefosine Combination (VL). Available online: https://dndi.org/research-development/portfolio/fexinidazole-vl/ (accessed on 22 July 2024).
- Keam, S.J. Pretomanid: First approval. Drugs 2019, 79, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; O’Connor, P.D.; Marshall, A.J.; Yardley, V.; Maes, L.; Gupta, S.; Launay, D.; Braillard, S.; Chatelain, E.; Wan, B.; et al. Heteroaryl ether analogues of an antileishmanial 7-substituted 2-nitroimidazooxazine lead afford attenuated hERG risk: In vitro and in vivo appraisal. Eur. J. Med. Chem. 2021, 209, 112914. [Google Scholar] [CrossRef] [PubMed]
- Koniordou, M.; Patterson, S.; Wyllie, S.; Seifert, K. Snapshot Profiling of the Antileishmanial Potency of Lead Compounds and Drug Candidates against Intracellular Leishmania donovani Amastigotes, with a Focus on Human-Derived Host Cells. Antimicrob. Agents Chemother. 2017, 61, e01228-16. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.Y.; Wyllie, S.; Patterson, S.; Oza, S.L.; Read, K.D.; Fairlamb, A.H. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 2893–2900. [Google Scholar] [CrossRef]
- Voak, A.A.; Gobalakrishnapillai, V.; Seifert, K.; Balczo, E.; Hu, L.; Hall, B.S.; Wilkinson, S.R. An essential type I nitroreductase from Leishmania major can be used to activate leishmanicidal prodrugs. J. Biol. Chem. 2013, 288, 28466–28476. [Google Scholar] [CrossRef]
- Bot, C.; Hall, B.S.; Alvarez, G.; Di Maio, R.; González, M.; Cerecetto, H.; Wilkinson, S.R. Evaluating 5-nitrofurans as trypanocidal agents. Antimicrob. Agents Chemother. 2013, 57, 1638–1647. [Google Scholar] [CrossRef]
- Wyllie, S.; Patterson, S.; Fairlamb, A.H. Assessing the essentiality of Leishmania donovani nitroreductase and its role in nitro drug activation. Antimicrob. Agents Chemother. 2013, 57, 901–906. [Google Scholar] [CrossRef]
- Docampo, R. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem. Biol. Interact. 1990, 73, 1–27. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Blackburn, P.; Ulrich, P.; Chait, B.T.; Cerami, A. Trypanothione: A novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science 1985, 227, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Manta, B.; Comini, M.; Medeiros, A.; Hugo, M.; Radi, R. Trypanothione: A unique bis-glutathionyl derivative in trypanosomatids. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3199–3216. [Google Scholar] [CrossRef] [PubMed]
- Krauth-Siegel, R.L.; Comini, M.A. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.B.; Ulrich, P.; Fairlamb, A.H.; Rosenberg, I.; Pereira, M.; Sela, M.; Cerami, A. “Subversive” substrates for the enzyme trypanothione disulfide reductase: Alternative approach to chemotherapy of Chagas disease. Proc. Natl. Acad. Sci. USA 1988, 85, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Maya, J.D.; Bollo, S.; Nuñez-Vergara, L.J.; Squella, J.A.; Repetto, Y.; Morello, A.; Périé, J.; Chauvière, G. Trypanosoma cruzi: Effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 2003, 65, 999–1006. [Google Scholar] [CrossRef]
- Filonov, G.S.; Piatkevich, K.D.; Ting, L.M.; Zhang, J.; Kim, K.; Verkhusha, V.V. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 2012, 29, 757–761. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar]
- Lo Presti, M.S.; Bazán, P.C.; Strauss, M.; Báez, A.L.; Rivarola, H.W.; Paglini-Oliva, P.A. Trypanothione reductase inhibitors: Overview of the action of thioridazine in different stages of Chagas disease. Acta Trop. 2015, 145, 79–87. [Google Scholar] [CrossRef]

| Tested Compound | Axenic Amastigotes | HepG2 Cells | RAW 264.7 Cells | SI1 | SI2 | |||
|---|---|---|---|---|---|---|---|---|
| EC50 Values (μM) | p | CC50 Values (μM) | p | CC50 Values (μM) | p | |||
| Fexinidazole | 0.04 ± 0.00 | *** | >200.00 | N/A | >200.00 | N/A | >5000.00 | >5000.00 |
| Pretomanid | 0.28 ± 0.02 | *** | >200.00 | N/A | >200.00 | N/A | >714.29 | >714.29 |
| Amp B | 0.27 ± 0.02 | *** | 69.75 ± 13.69 | *** | 6.70 ± 0.67 | *** | 258.33 | 24.82 |
| Tested Compound | Intramacrophage Amastigotes | HepG2 Cells | RAW 264.7 Cells | SI1 | SI2 | |||
|---|---|---|---|---|---|---|---|---|
| EC50 Values (μM) | p | CC50 Values (μM) | p | CC50 Values (μM) | p | |||
| Fexinidazole | 1.32 ± 0.06 | *** | >200.00 | N/A | >200.00 | N/A | >151.52 | >151.52 |
| Pretomanid | 0.66 ± 0.08 | *** | >200.00 | N/A | >200.00 | N/A | >303.03 | >303.03 |
| Amp B | 0.32 ± 0.02 | *** | 69.75 ± 13.69 | *** | 6.70 ± 0.67 | *** | 217.97 | 20.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés-Rodríguez, J.; González-Montero, M.-C.; García-Fernández, N.; Calvo-Álvarez, E.; Pérez-Pertejo, M.-Y.; Reguera-Torres, R.-M.; Balaña-Fouce, R.; García-Estrada, C. Free Radical Production Induced by Nitroimidazole Compounds Lead to Cell Death in Leishmania infantum Amastigotes. Molecules 2024, 29, 4041. https://doi.org/10.3390/molecules29174041
Andrés-Rodríguez J, González-Montero M-C, García-Fernández N, Calvo-Álvarez E, Pérez-Pertejo M-Y, Reguera-Torres R-M, Balaña-Fouce R, García-Estrada C. Free Radical Production Induced by Nitroimidazole Compounds Lead to Cell Death in Leishmania infantum Amastigotes. Molecules. 2024; 29(17):4041. https://doi.org/10.3390/molecules29174041
Chicago/Turabian StyleAndrés-Rodríguez, Julia, María-Cristina González-Montero, Nerea García-Fernández, Estefanía Calvo-Álvarez, María-Yolanda Pérez-Pertejo, Rosa-María Reguera-Torres, Rafael Balaña-Fouce, and Carlos García-Estrada. 2024. "Free Radical Production Induced by Nitroimidazole Compounds Lead to Cell Death in Leishmania infantum Amastigotes" Molecules 29, no. 17: 4041. https://doi.org/10.3390/molecules29174041
APA StyleAndrés-Rodríguez, J., González-Montero, M.-C., García-Fernández, N., Calvo-Álvarez, E., Pérez-Pertejo, M.-Y., Reguera-Torres, R.-M., Balaña-Fouce, R., & García-Estrada, C. (2024). Free Radical Production Induced by Nitroimidazole Compounds Lead to Cell Death in Leishmania infantum Amastigotes. Molecules, 29(17), 4041. https://doi.org/10.3390/molecules29174041







