Characteristics of Indonesian Stingless Bee Propolis and Study of Metabolomic Properties Based on Region and Species
Abstract
1. Introduction
2. Results
2.1. Bee Species
2.2. Total Phenolic Content (TPC) Results
2.3. Total Flavonoid Content (TFC) Results
2.4. Antioxidant Activity Results
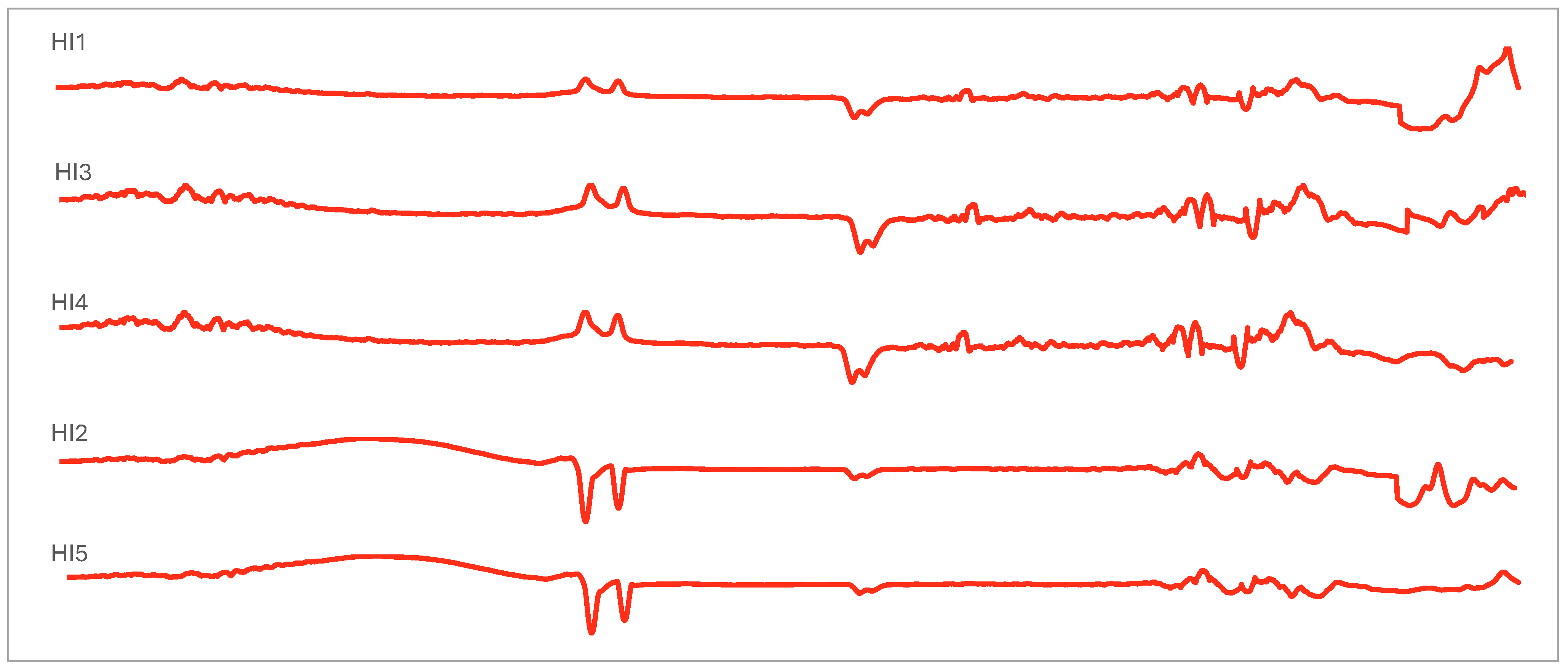
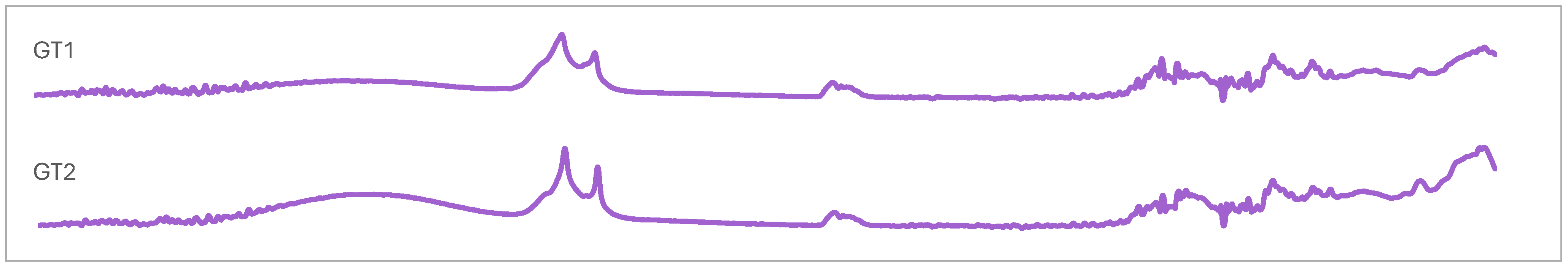
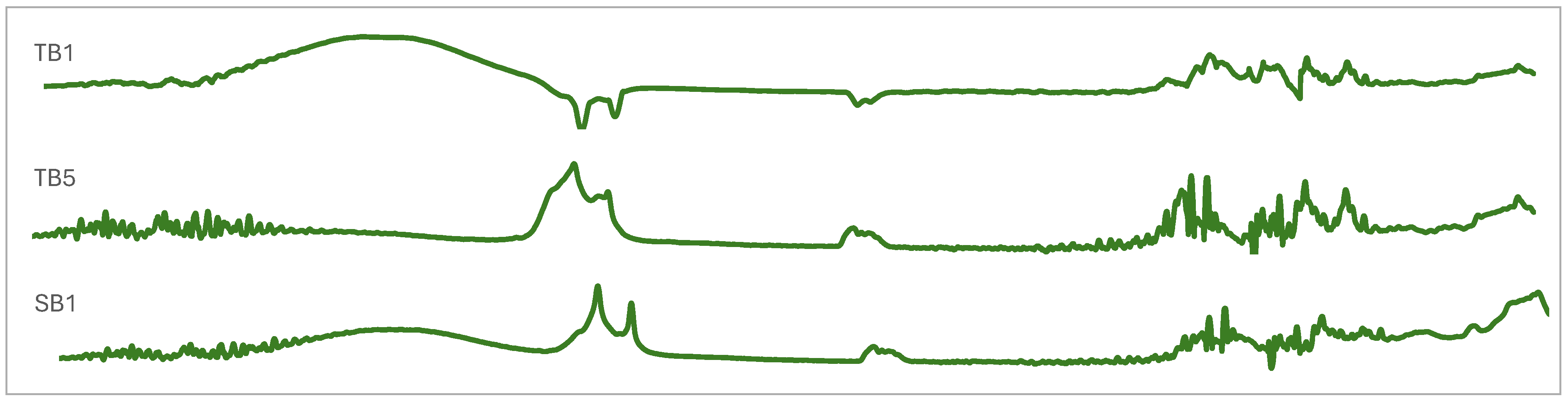
2.5. Chemometric Analysis of FTIR Spectroscopies
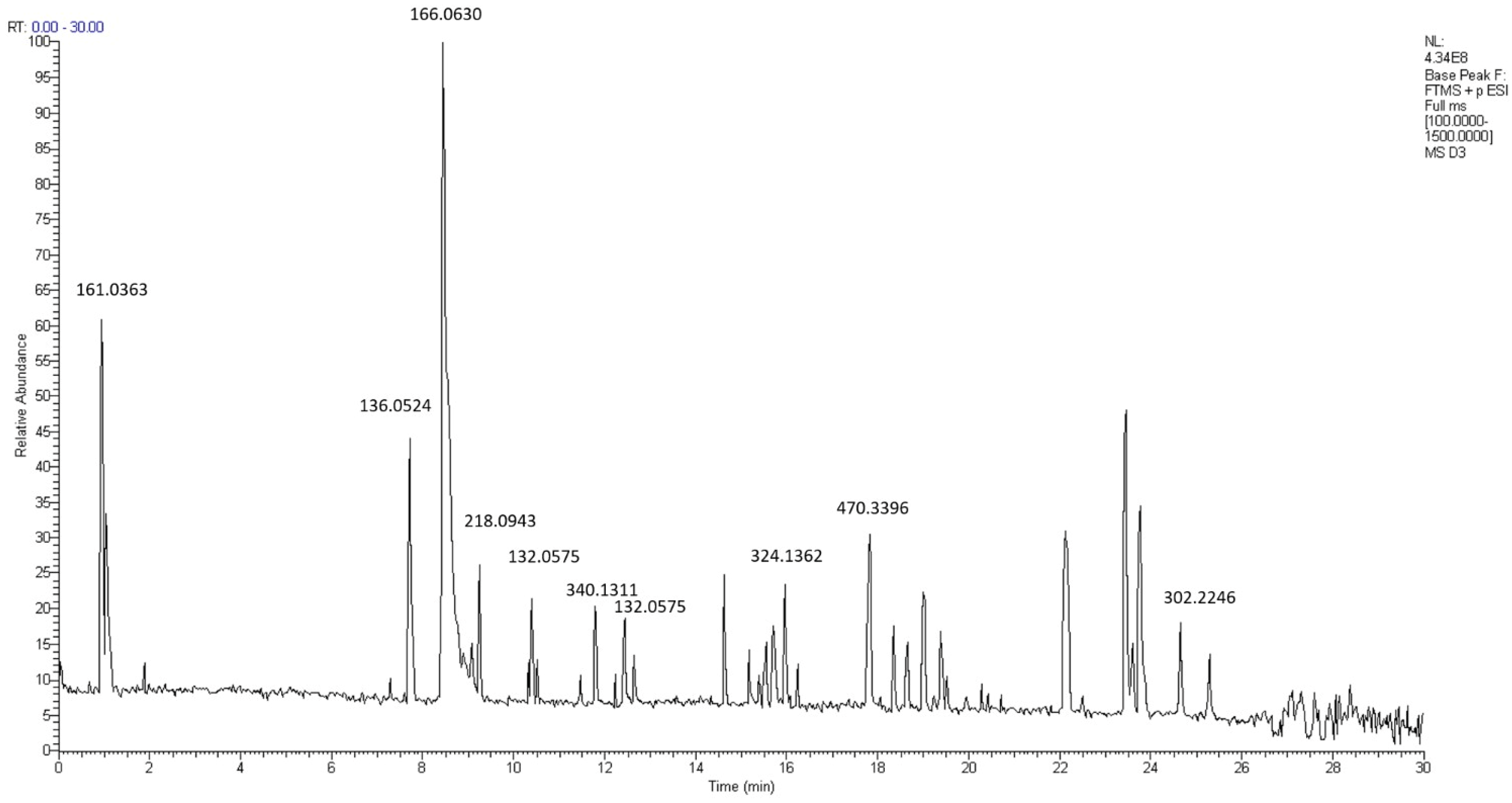
2.6. LC-MS/MS Analysis Result
2.7. Chemometric Analysis of LC-MS/MS
3. Discussion
3.1. TPC and TFC
3.2. Antioxidant Activity
3.3. Vibrational and Absorption Spectroscopies of Indonesian Propolis
3.4. Propolis Chemical Compounds and Potential Markers
4. Materials and Methods
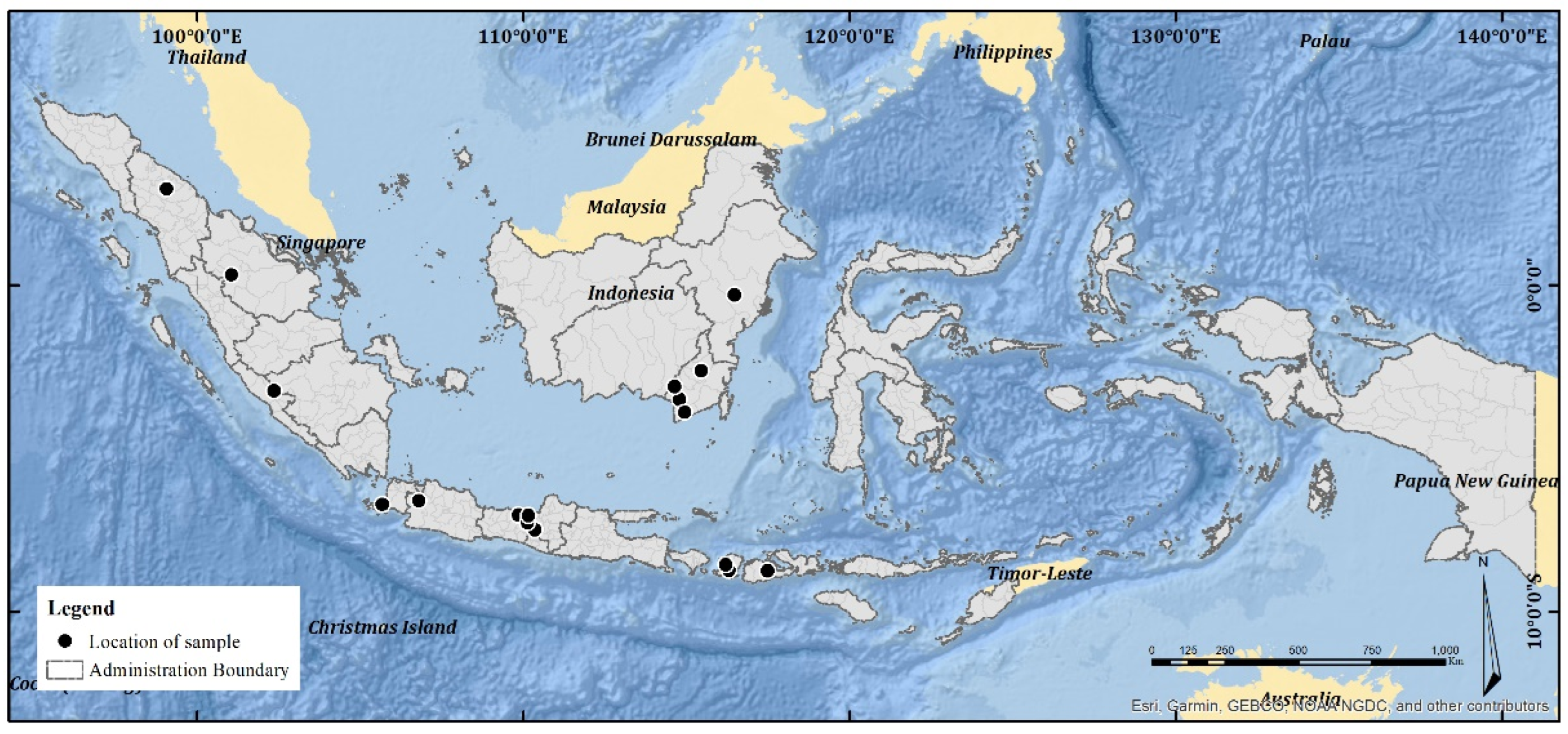
4.1. Samples
4.2. Sample Preparation
4.3. Propolis Extraction
4.4. Total Phenolic Content (TPC) Measurements
4.5. Total Flavonoid Content (TFC) Measurements
4.6. Antioxidant Activity with DPPH Assay
4.7. Antioxidant Activity with ABTS Assay
4.8. Fourier-Transform Infrared Analysis
4.9. LC-MS/MS Analysis
4.10. Chemometric Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyata, R.; Sahlan, M.; Ishikawa, Y.; Hashimoto, H.; Honda, S.; Kumazawa, S. Propolis Components from Stingless Bees Collected on South Sulawesi, Indonesia, and Their Xanthine Oxidase Inhibitory Activity. J. Nat. Prod. 2019, 82, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M. Biological Activity of Bee Propolis in Health and Disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.C.; Umsza-Guez, M.A.; Barbosa, J.D.V.; Portela, R.D.; Machado, B.A.S. Propolis: Types, Composition, Biological Activities, and Veterinary Product Patent Prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and Application in the Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2022, 127, 169–180. [Google Scholar] [CrossRef]
- Barros, K.B.N.T.; Neto, E.M.R.; de França Fonteles, M.M. Propolis and Its Cosmetic Applications: A Technological Prospection. J. Young Pharm. 2019, 11, 350. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M. Propolis: Harnessing Nature’s Hidden Treasure for Sustainable Agriculture. Agrochemicals 2023, 2, 581–597. [Google Scholar] [CrossRef]
- Pratami, D.K.; Mun’im, A.; Hermansyah, H.; Gozan, M.; Sahlan, M. Microencapsulation Optimization of Propolis Ethanolic Extract from Tetragonula spp. Using Response Surface Methodology. Int. J. App. Pharm 2020, 12, 197–206. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Mărghitaş, L.A.; Chirilă, F.; Copaciu, F.; Simonca, V.; Bobiş, O.; Erler, S. Influence of Geographic Origin, Plant Source and Polyphenolic Substances on Antimicrobial Properties of Propolis against Human and Honey Bee Pathogens. J. Apic. Res. 2017, 56, 588–597. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard Methods for Apis mellifera Propolis Research. J. Apic. Res. 2019, 58, 1–49. [Google Scholar] [CrossRef]
- Ali, J.R.; Heaney, L.R.; Alfred, R. Wallace’s Enduring Influence on Biogeographical Studies of the Indo-Australian Archipelago. J. Biogeogr. 2023, 50, 32–40. [Google Scholar] [CrossRef]
- Costa, A.G.; Yoshida, N.C.; Garcez, W.S.; Perdomo, R.T.; Matos, M.D.F.C.; Garcez, F.R. Metabolomics Approach Expands the Classification of Propolis Samples from Midwest Brazil. J. Nat. Prod. 2020, 83, 333–343. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Dembogurski, D.S.; Silva Trentin, D.; Boaretto, A.G.; Rigo, G.V.; da Silva, R.C.; Tasca, T.; Macedo, A.J.; Carollo, C.A.; Silva, D.B. Brown Propolis-Metabolomic Innovative Approach to Determine Compounds Capable of Killing Staphylococcus aureus Biofilm and Trichomonas Vaginalis. Food Res. Int. 2018, 111, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Margarita, G.E.; Wu, D.; Yuan, W.; Yan, S.; Qi, S.; Xue, X.; Wang, K.; Wu, L. Antibacterial Activity of Chinese Red Propolis against Staphylococcus aureus and MRSA. Molecules 2022, 27, 1693. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.L.F.; Ribeiro, P.R.; Franco, R.L.P.; Hilhorst, H.W.M.; de Castro, R.D.; Fernandez, L.G. Metabolite Profiling, Antioxidant and Antibacterial Activities of Brazilian Propolis: Use of Correlation and Multivariate Analyses to Identify Potential Bioactive Compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef]
- Zheng, X.; Al Naggar, Y.; Wu, Y.; Liu, D.; Hu, Y.; Wang, K.; Jin, X.; Peng, W. Untargeted Metabolomics Description of Propolis’s in Vitro Antibacterial Mechanisms against Clostridium perfringens. Food Chem. 2023, 406, 135061. [Google Scholar] [CrossRef]
- Azemin, A.; Md-Zin, N.B.; Mohd-Rodi, M.M.; Kim-Chee, A.S.; Zakaria, A.J.; Mohd, K.S. Application of Metabolite Profiling and Antioxidant Activity in Assessing the Quality of Processed and Unprocessed Stingless Bee’s Propolis. J. Fundam. Appl. Sci. 2018, 9, 637. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Gundu, S.S.; Bhatia, R.; Nandre, V.S.; Jagtap, S.D.; Mahajan, S.G.; Kulkarni, M.V. Chemical Profiling, Antioxidant, and Antimicrobial Activities of Indian Stingless Bees Propolis Samples. J. Apic. Res. 2019, 58, 617–625. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Hernández, I.M.; Fernández, M.C.; Rodríguez-Delgado, I.; De Oca Porto, R.M.; Piccinelli, A.L.; Celano, R.; Rastrelli, L. Chemical Characterization and Antioxidant Potential of Ecuadorian Propolis. Phytochemistry 2022, 203, 113415. [Google Scholar] [CrossRef]
- Alqarni, A.M.; Niwasabutra, K.; Sahlan, M.; Fearnley, H.; Fearnley, J.; Ferro, V.A.; Watson, D.G. Propolis Exerts an Anti-Inflammatory Effect on PMA-Differentiated THP-1 Cells via Inhibition of Purine Nucleoside Phosphorylase. Metabolites 2019, 9, 75. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, A.; Zhou, X.; Zhang, K.; Yuan, X.; Yuan, M.; He, J.; Pineda, M.A.; Li, K. Novel Ultrafiltrate Extract of Propolis Exerts Anti-inflammatory Activity through Metabolic Rewiring. Chem. Biodivers. 2024, 21, e202301315. [Google Scholar] [CrossRef] [PubMed]
- Alsherbiny, M.A.; Bhuyan, D.J.; Radwan, I.; Chang, D.; Li, C.-G. Metabolomic Identification of Anticancer Metabolites of Australian Propolis and Proteomic Elucidation of Its Synergistic Mechanisms with Doxorubicin in the MCF7 Cells. Int. J. Mol. Sci. 2021, 22, 7840. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Z.; Wu, Q.; Li, Z.; Yang, J.; Xuan, H. Integration with Transcriptomic and Metabolomic Analyses Reveals the In Vitro Cytotoxic Mechanisms of Chinese Poplar Propolis by Triggering the Glucose Metabolism in Human Hepatocellular Carcinoma Cells. Nutrients 2023, 15, 4329. [Google Scholar] [CrossRef] [PubMed]
- Saftić, L.; Peršurić, Ž.; Fornal, E.; Pavlešić, T.; Kraljević Pavelić, S. Targeted and Untargeted LC-MS Polyphenolic Profiling and Chemometric Analysis of Propolis from Different Regions of Croatia. J. Pharm. Biomed. Anal. 2019, 165, 162–172. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Q.; Wang, M.; Zhang, L. Metabolomics Reveals Discrimination of Chinese Propolis from Different Climatic Regions. Foods 2020, 9, 491. [Google Scholar] [CrossRef]
- Saleh, K.; Zhang, T.; Fearnley, J.; Watson, D.G. A Comparison of the Constituents of Propolis from Different Regions of the United Kingdom by Liquid Chromatography-High Resolution Mass Spectrometry Using a Metabolomics Approach. Curr. Metabolomics 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Anđelković, B.; Vujisić, L.; Vučković, I.; Tešević, V.; Vajs, V.; Gođevac, D. Metabolomics Study of Populus Type Propolis. J. Pharm. Biomed. Anal. 2017, 135, 217–226. [Google Scholar] [CrossRef]
- Kamatou, G.; Sandasi, M.; Tankeu, S.; van Vuuren, S.; Viljoen, A. Headspace Analysis and Characterisation of South African Propolis Volatile Compounds Using GCxGC-ToF-MS. Rev. Bras. Farmacogn. 2019, 29, 351–357. [Google Scholar] [CrossRef]
- Fabio Turco, J.; Benhur Mokochinski, J.; Reyes Torres, Y. Lipidomic Analysis of Geopropolis of Brazilian Stingless Bees by LC-HRMS. Food Res. Int. 2023, 167, 112640. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Mohyeldin, M.M.; Shawky, E.; Metwally, A.M.; Ibrahim, R.S. Chemical Profiling of Egyptian Propolis and Determination of Its Xanthine Oxidase Inhibitory Properties Using UPLC–MS/MS and Chemometrics. LWT 2021, 136, 110298. [Google Scholar] [CrossRef]
- Stavropoulou, M.; Termentzi, A.; Kasiotis, K.M.; Cheilari, A.; Stathopoulou, K.; Machera, K.; Aligiannis, N. Untargeted Ultrahigh-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap Mass Spectrometry (UHPLC-HRMS) Metabolomics Reveals Propolis Markers of Greek and Chinese Origin. Molecules 2021, 26, 456. [Google Scholar] [CrossRef] [PubMed]
- Maraschin, M.; Somensi-Zeggio, A.; Oliveira, S.K.; Kuhnen, S.; Tomazzoli, M.M.; Raguzzoni, J.C.; Zeri, A.C.M.; Carreira, R.; Correia, S.; Costa, C.; et al. Metabolic Profiling and Classification of Propolis Samples from Southern Brazil: An NMR-Based Platform Coupled with Machine Learning. J. Nat. Prod. 2016, 79, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Elgin, E.S.; Çatav, S.S.; Babayeva, A.; Kim, H.; Dibek, E.; Çöl, B.; Chae, Y.K.; Klvrak, I. NMR Metabolomics Analysis of Escherichia coli Cells Treated with Turkish Propolis Water Extract Reveals Nucleic Acid Metabolism as the Major Target. J. Appl. Microbiol. 2023, 134, lxac031. [Google Scholar] [CrossRef]
- Stavropoulou, M.I.; Stathopoulou, K.; Cheilari, A.; Benaki, D.; Gardikis, K.; Chinou, I.; Aligiannis, N. NMR Metabolic Profiling of Greek Propolis Samples: Comparative Evaluation of Their Phytochemical Compositions and Investigation of Their Anti-Ageing and Antioxidant Properties. J. Pharm. Biomed. Anal. 2021, 194, 113814. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Ma, N.; Liu, G.; Wu, Q.; Su, S.; Wang, J.; Geng, Y. Ethanol Extract of Propolis Regulates Type 2 Diabetes in Mice via Metabolism and Gut Microbiota. J. Ethnopharmacol. 2023, 310, 116385. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Fu, H.-Y.; Hong, J.; Wan, X.; Yang, C.S.; Ho, C.-T. Comparison of Antioxidant Activities of Isoflavones from Kudzu Root (Pueraria lobata Ohwi). J. Food Sci. 2003, 68, 2117–2122. [Google Scholar] [CrossRef]
- Kim, G.-D.; Lee, J.Y.; Auh, J.-H. Metabolomic Screening of Anti-Inflammatory Compounds from the Leaves of Actinidia arguta (Hardy Kiwi). Foods 2019, 8, 47. [Google Scholar] [CrossRef]
- Pratami, D.K.; Mun’im, A.; Sundowo, A.; Sahlan, M. Phytochemical Profile and Antioxidant Activity of Propolis Ethanolic Extract from Tetragonula Bee. Pharmacogn. J. 2018, 10, 73–80. [Google Scholar] [CrossRef]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic Constituents, Antioxidant and Antimicrobial Activity and Clustering Analysis of Propolis Samples Based on PCA from Different Regions of Anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic Composition and Antioxidant Activity of Propolis from Various Regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Syed Salleh, S.N.A.; Mohd Hanapiah, N.A.; Ahmad, H.; Wan Johari, W.L.; Osman, N.H.; Mamat, M.R. Determination of Total Phenolics, Flavonoids, and Antioxidant Activity and GC-MS Analysis of Malaysian Stingless Bee Propolis Water Extracts. Scientifica 2021, 2021, 3789351. [Google Scholar] [CrossRef]
- Sulaeman, A.; Marliyati, S.A.; Fahrudin, M. Antioxidant Activity and Total Phenolic Content of Stingless Bee Propolis from Indonesia. J. Apic. Sci. 2019, 63, 139–147. [Google Scholar]
- Osés, S.M.; Marcos, P.; Azofra, P.; de Pablo, A.; Fernández-Muíño, M.Á.; Sancho, M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yuan, X.; Pineda, M.; Liang, Z.; He, J.; Sun, S.; Pan, T.; Li, K. A Comparative Study between Chinese Propolis and Brazilian Green Propolis: Metabolite Profile and Bioactivity. Food Funct. 2020, 11, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Zainal, W.N.H.W.; Azian, N.A.A.M.; Albar, S.S.; Rusli, A.S. Effects of Extraction Method, Solvent and Time on the Bioactive Compounds and Antioxidant Activity of Tetrigona apicalis Malaysian Propolis. J. Apic. Res. 2021, 61, 264–270. [Google Scholar] [CrossRef]
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Giménez, A.; Schmeda-Hirschmann, G. Chemical Profiling and Antioxidant Activity of Bolivian Propolis. J. Sci. Food Agric. 2016, 96, 2142–2153. [Google Scholar] [CrossRef]
- Araújo, K.S.d.S.; dos Santos Júnior, J.F.; Sato, M.O.; Finco, F.D.B.A.; Soares, I.M.; Barbosa, R.d.S.; Alvim, T.D.C.; Ascêncio, S.D.; Mariano, S.M.B. Physicochemical Properties and Antioxidant Capacity of Propolis of Stingless Bees (Meliponinae) and Apis from Two Regions of Tocantins, Brazil. Acta Amaz. 2016, 46, 61–68. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhang, C.; Hu, F. Anti-Inflammatory Effects of Ethanol Extracts of Chinese Propolis and Buds from Poplar (Populus canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef]
- Asem, N.; Abdul Gapar, N.A.; Abd Hapit, N.H.; Omar, E.A. Correlation between Total Phenolic and Flavonoid Contents with Antioxidant Activity of Malaysian Stingless Bee Propolis Extract. J. Apic. Res. 2020, 59, 437–442. [Google Scholar] [CrossRef]
- Ding, Q.; Sheikh, A.R.; Gu, X.; Li, J.; Xia, K.; Sun, N.; Wu, R.A.; Luo, L.; Zhang, Y.; Ma, H. Chinese Propolis: Ultrasound-Assisted Enhanced Ethanolic Extraction, Volatile Components Analysis, Antioxidant and Antibacterial Activity Comparison. Food Sci. Nutr. 2021, 9, 313–330. [Google Scholar] [CrossRef]
- Zawawi, N.; Chong, P.J.; Mohd Tom, N.N.; Saiful Anuar, N.S.; Mohammad, S.M.; Ismail, N.; Jusoh, A.Z. Establishing Relationship between Vitamins, Total Phenolic and Total Flavonoid Content and Antioxidant Activities in Various Honey Types. Molecules 2021, 26, 4399. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Da Silva Moreira Thiré, R.M.; McGuinness, G.B. FTIR Analysis and Quantification of Phenols and Flavonoids of Five Commercially Available Plants Extracts Used in Wound Healing. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Zullkiflee, N.; Zaini, S.N.Z.; Taha, H.; Hashim, F.; Usman, A. Phytochemicals, Mineral Contents, Antioxidants, and Antimicrobial Activities of Propolis Produced by Brunei Stingless Bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci. 2020, 27, 2902–2911. [Google Scholar] [CrossRef]
- Barud, H.d.S.; de Araújo Júnior, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; de Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M. de O.L.L.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef]
- Kačuráková, M.; Smith, A.C.; Gidley, M.J.; Wilson, R.H. Molecular Interactions in Bacterial Cellulose Composites Studied by 1D FT-IR and Dynamic 2D FT-IR Spectroscopy. Carbohydr. Res. 2002, 337, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Rodrigues, M.R.; Bueno, P.C.P.; de Mello Costa-Machado, A.R.; de Oliveira Lima Leite Vaz, M.M.; Nascimento, A.P.; Barud, H.S.; Berretta-Silva, A.A. Preparation and Thermal Characterization of Inclusion Complex of Brazilian Green Propolis and Hydroxypropyl-β-Cyclodextrin: Increased Water Solubility of the Chemical Constituents and Antioxidant Activity. J. Therm. Anal. Calorim. J Therm Anal Calorim 2012, 108, 87–94. [Google Scholar] [CrossRef]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and Effective Evaluation of the Antioxidant Capacity of Propolis Extracts Using DPPH Bleaching Kinetic Profiles, FT-IR and UV–Vis Spectroscopic Data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Fleschin, S. Recent Applications of Fourier Transform Infrared Spectrophotometry in Herbal Medicine Analysis. Appl. Spectrosc. Rev. 2011, 46, 251–260. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of Stingless Bees: A Phytochemist’s Guide through the Jungle of Tropical Biodiversity. Phytomedicine 2021, 86, 153098. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Yuliana, N.D.; Wijaya, C.H.; Nasrullah, N. Classification of Trigona spp. Bee Propolis from Four Regions in Indonesia Using FTIR Metabolomics Approach. In Proceedings of the 13th Asean Food Conference, Singapore, 9–11 September 2013; pp. 9–11. [Google Scholar]
- Basari, N.; Ramli, S.N.; Abdul-Mutalid, N.A.; Shaipulah, N.F.M.; Hashim, N.A. Flowers Morphology and Nectar Concentration Determine the Preferred Food Source of Stingless Bee, Heterotrigona itama. J. Asia. Pac. Entomol. 2021, 24, 232–236. [Google Scholar] [CrossRef]
- Benedick, S.; Gansau, J.A.; Ahmad, A.H. Foraging Behaviour of Heterotrigona itama (Apidae: Meliponini) in Residential Areas. Pertanika J. Trop. Agric. Sci. 2021, 44, 485–502. [Google Scholar] [CrossRef]
- Mulyawan, L.Z.; Rachmawarifa, C.M.; Sudaryadi, I. Unraveling the Floral Preference: Bee Pollen Identification and Characterization of Tetragonula laeviceps. In Proceedings of the BIO Web of Conferences, EDP Sciences, Baku, Azerbaijan, 5–6 November 2024; Volume 94, p. 3004. [Google Scholar]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid Chromatographic–Mass Spectrometric Analysis of Anthocyanin Composition of Dark Blue Bee Pollen from Echium Plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef]
- Webby, R.; Bloor, S. Pigments in the Blue Pollen and Bee Pollen of Fuchsia excorticata. Z. Für Naturforsch. C 2000, 55, 503–505. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H. Bin Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Ma, Z.; Ye, L.; Liu, B.; Qiu, S.-X. N-Containing Phytochemicals from the Seeds of Brucea javanica. Chem. Nat. Compd. 2017, 53, 799–801. [Google Scholar] [CrossRef]
- Barman, M.; Tenhaken, R.; Dötterl, S. A Review on Floral Scents and Pigments in Cucurbits: Their Biosynthesis and Role in Flower Visitor Interactions. Sci. Hortic. 2023, 322, 112402. [Google Scholar] [CrossRef]
- Akšić, M.F.; Čolić, S.; Meland, M.; Natić, M. Sugar and Polyphenolic Diversity in Floral Nectar of Cherry. Co-Evol. Second Metab. 2020, 2020, 755–773. [Google Scholar] [CrossRef]
- Drescher, N.; Klein, A.-M.; Schmitt, T.; Leonhardt, S.D. A Clue on Bee Glue: New Insight into the Sources and Factors Driving Resin Intake in Honeybees (Apis mellifera). PLoS ONE 2019, 14, e0210594. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays—A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef] [PubMed]
- Nurhidayati, L.; Abdillah, S.; Mumpuni, E.; Rafi, M. Characterization, FTIR Spectra Profile, and Plate Anti-Aggregation Activity of Crude Fucoidan from Sargassum crassifolium. Int. J. Appl. Pharm. 2022, 14, 45–50. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]


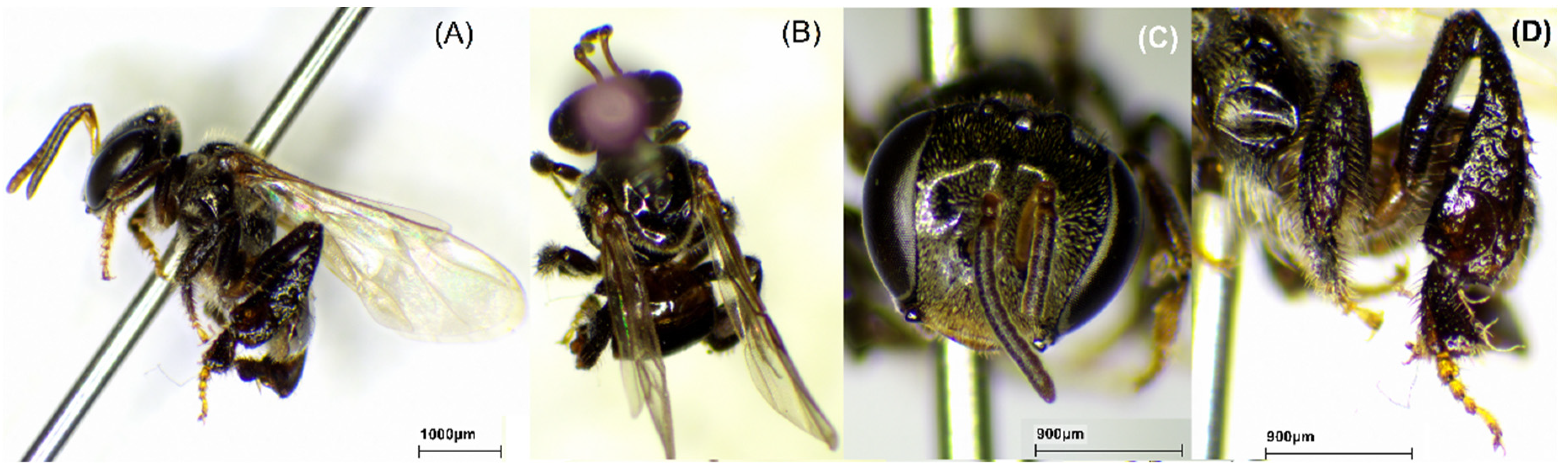





| Sample name | Bee Species | Region | TPC ± SD (mg GAE/g) | TFC ± SD (mg QE/g) |
|---|---|---|---|---|
| Jav_HI1 | H. itama | Magelang | 61.42 ± 5.13 | 63.74 ± 7.47 |
| Jav_HI2 | H. itama | Kendal | 69.72 ± 4.89 | 52.22 ± 5.27 |
| Kal_HI3 | H. itama | Banjarbaru | 278.78 ± 15.79 | 74.40 ± 3.63 |
| Kal_HI4 | H. itama | Kutai Kartanegara | 208.8 ± 10.12 | 112.8 ± 5.24 |
| Sum_HI5 | H. itama | Kampar | 67.56 ± 2.84 | 70.11 ± 2.73 |
| NT_TB1 | T. biroi | Sumbawa | 336.96 ± 0.64 | 111.40 ± 0.81 |
| Kal_TB2 | T. biroi | Tanah Laut | 97.61 ± 1.96 | 59.49 ± 3.22 |
| Jav_TB3 | T. biroi | Batang | 83.66 ± 2.21 | 60.74 ± 3.95 |
| Jav_TB4 | T. biroi | Bogor | 114.48 ± 5.35 | 48.5 ± 0.57 |
| Kal_TB5 | T. biroi | Barito Kuala | 71.50 ± 0.42 | 39.30 ± 0.73 |
| Jav_TB6 | T. biroi | Magelang | 80.34 ± 0.87 | 25.46 ± 0.84 |
| Jav_TL1 | T. laeviceps | Pandeglang | 63.08 ± 0.68 | 44.56 ± 2.81 |
| Jav_TL2 | T. laeviceps | Batang | 55.40 ± 0.93 | 45.04 ± 1.15 |
| Jav_TL3 | T. laeviceps | Magelang | 33.08 ± 4.27 | 30.74 ± 3.95 |
| Kal_TL4 | T. laeviceps | Hulu Sungai Tengah | 114.92 ± 15.26 | 74.40 ± 3.63 |
| Kal_TL5 | T. laeviceps | Barito Kuala | 32.99 ± 0.66 | 20.66 ± 4.08 |
| Kal_GT1 | G. thoracica | Kutai Kartanegara | 174.2 ± 12.09 | 94.18 ± 5.02 |
| Sum_GT2 | G. thoracica | Lebong | 81.34 ± 2.61 | 49.58 ± 9.4 |
| Kal_GT3 | G. thoracica | Hulu Sungai Tengah | 58.10 ± 4.89 | 43.52 ± 5.27 |
| Sum_GT4 | G. thoracica | Pematang Siantar | 374.20 ± 0.51 | 194.18 ± 0.98 |
| NT_SB1 | T. clypearis | West Lombok | 144.2 ± 0.75 | 81.02 ± 0.67 |
| NT_SB2 | T. clypearis | Central Lombok | 134.86 ± 7.43 | 57.32 ± 1.61 |
| Jav_SB3 | T. drescheri | Batang | 79.44 ± 0.42 | 43.5 ± 0.73 |
| Sample Name | IC50 DPPH (ppm) | IC50 ABTS (ppm) | Activity |
|---|---|---|---|
| Jav_HI1 | 65.4 ± 4.15 | 56.66 ± 9.64 | Strong |
| Jav_HI2 | 57.78 ± 3.32 | 56.71 ± 0.47 | Strong |
| Kal_HI3 | 28.87 ± 6.05 | 39.71 ± 0.79 | Very strong |
| Kal_HI4 | 25.64 ± 0.55 | 38.79 ± 0.65 | Very strong |
| Sum_HI5 | 59.45 ± 3.23 | 60.24 ± 3.69 | Strong |
| NT_TB1 | 19.03 ± 0.08 | 26.34 ± 3.64 | Very strong |
| Kal_TB2 | 47.73 ± 4.02 | 46.46 ± 5.61 | Very strong |
| Jav_TB3 | 115.28 ± 2.30 | 117.21 ± 10.76 | Moderate |
| Jav_TB4 | 70.30 ± 1.40 | 64.96 ± 4.43 | Strong |
| Kal_TB5 | 61.69 ± 0.25 | 63.78 ± 0.29 | Strong |
| Jav_TB6 | 50.79 ± 0.25 | 52.78 ± 0.45 | Strong |
| Jav_TL1 | 116.82 ± 2.34 | 108.59 ± 1.97 | Moderate |
| Jav_TL2 | 118.10 ± 2.36 | 110.21 ± 10.27 | Moderate |
| Jav_TL3 | 83.37 ± 6.07 | 93.13 ± 4.00 | Strong |
| Kal_TL4 | 70.03 ± 0.92 | 64.75 ± 1.84 | Strong |
| Kal_TL5 | 83.16 ± 3.28 | 89.93 ± 1.77 | Strong |
| Kal_GT1 | 46.20 ± 1.65 | 37.78 ± 1.42 | Very strong |
| Sum_GT2 | 57.28 ± 0.48 | 67.35 ± 2.24 | Strong |
| Kal_GT3 | 69.34 ± 5.13 | 68.29 ± 3.08 | Strong |
| Sum_GT4 | 11.06 ± 0.55 | 17.29 ± 0.34 | Very strong |
| NT_SB1 | 31.12 ± 0.99 | 45.14 ± 4.25 | Very strong |
| NT_SB2 | 41.75 ± 0.96 | 43.63 ± 0.54 | Very strong |
| Jav_SB3 | 51.69 ± 0.35 | 53.77 ± 0.9 | Strong |
| Group | Sample | Species | Region | Island |
|---|---|---|---|---|
| Group 1 | HI2 | H. itama | Kendal | Java |
| HI1 | H. itama | Magelang | Java | |
| HI3 | H. itama | Banjar Baru | Kalimantan | |
| HI4 | H. itama | Kutai Kartanegara | Kalimantan | |
| HI5 | H. itama | Kampar | Sumatra | |
| Group 2 | GT1 | G. thoracica | Kutai Kartanegara | Kalimantan |
| GT2 | G. thoracica | Lebong | Sumatra | |
| Group 3 | TB2 | T. biroi | Tanah Laut | Kalimantan |
| TB3 | T. biroi | Batang | Java | |
| TB4 | T. biroi | Bogor | Java | |
| TB6 | T. biroi | Magelang | Java | |
| TL1 | T. laeviceps | Pandeglang | Java | |
| TL2 | T. laeviceps | Batang | Java | |
| TL3 | T. laeviceps | Magelang | Java | |
| TL4 | T. laeviceps | Hulu Sungai Tengah | Kalimantan | |
| TL5 | T. laeviceps | Barito Kuala | Kalimantan | |
| SB3 | T. drescheri | Batang | Java | |
| GT3 | G. thoracica | Hulu Sungai Tengah | Kalimantan | |
| Group 4 | TB1 | T. biroi | Sumbawa | Nusa Tenggara |
| TB5 | T. biroi | Barito Kuala | Kalimantan | |
| SB1 | T. clypearis | West Lombok | Nusa Tenggara | |
| Group 5 | SB2 | T. clypearis | Central Lombok | Nusa Tenggara |
| GT4 | G. thoracica | Pematang Siantar | Sumatra |
| Wave Number (cm−1) | Vibrational Modes | Position Band Sample | ||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | ||
| 3500–3200 | O-H of alcohols, phenols, and carboxylic acids | - | √ | √ | - | √ |
| 3400–3250 | N-H of amines | - | √ | - | - | √ |
| 3000–2850 | CH3, CH2, and CH of alkenes | √ | √ | √ | √ | √ |
| 1810–1640 | C=O | √ | √ | √ | √ | √ |
| 1680–1600 | C=C of conjugated aromatic rings | - | √ | √ | √ | √ |
| 1550–1475 | N-O | √ | √ | √ | - | - |
| 1500–1400 | CH of aromatic rings | √ | √ | √ | √ | √ |
| 1470–1450 | C-H | - | √ | - | √ | - |
| 1360–1290 | N-O | - | - | √ | - | √ |
| 1335–1250 | C-N | √ | - | √ | √ | √ |
| 1320–1000 | C-O of carboxylic acids, phenols, esters | √ | √ | √ | √ | √ |
| 1300–1150 | CH2 of alkenes | √ | √ | √ | √ | √ |
| 1250–1020 | C-N | √ | √ | √ | √ | √ |
| No. | Compound | RT (min) | Formula | Molecular Weight |
|---|---|---|---|---|
| 1 | Unknown compound | 0.99 | C6H8O5 | 161.0363 |
| 2 | 4-Methoxybenzaldehyde | 7.76 | C8H8O2 | 136.0524 |
| 3 | Apocynin | 8.52 | C9H10O3 | 166.0630 |
| 4 | Eupatoriochromene | 9.12 | C13H14O3 | 218.0943 |
| 5 | Unknown compound | 10.37 | C9H8O | 132.0575 |
| 6 | (-)-8-Prenylnaringenin | 11.84 | C20H20O5 | 340.1311 |
| 7 | trans-Cinnamaldehyde | 12.47 | C9H8O | 132.0575 |
| 8 | Glabridin | 16.01 | C20H20O4 | 324.1362 |
| 9 | (1R,2R,5S,8R,10R,14R)-20-hydroxy-1,2,14,18,18-pentamethyl-17-oxo-8-(prop-1-en-2-yl) pentacyclohenicosane-5-carboxylic acid | 17.86 | C30H46O4 | 470.3396 |
| 10 | Abietic acid | 24.70 | C20H30O2 | 302.2246 |
| No. | Compound Name | RT (min) | Formula | Molecular Weight | HI | TL |
|---|---|---|---|---|---|---|
| 1 | Petunidin | 8.12 | C16H13O7 | 318.0758 | High | Low |
| 2 | Gingerone C | 10.35 | C20H22O4 | 327.1682 | High | Low |
| 3 | L-β-aspartyl-L-leucine | 10.97 | C10H18N2O5 | 247.1304 | High | Low |
| 4 | Flazine | 10.67 | C17H12N2O4 | 309.0859 | High | Low |
| 5 | Pinusolide | 18.93 | C21H30O4 | 347.2195 | Low | High |
| 6 | Prostaglandine F2a | 12.93 | C20H34O5 | 355.2441 | High | Low |
| 7 | Mannitol 1-phosphate | 1.51 | C6H15O9P | 263.0660 | High | Low |
| 8 | Hydroxyprolyl-Isoleucine | 1.59 | C11H20N2O4 | 245.1441 | Low | High |
| 9 | Cucurbic acid | 9.23 | C12H20O3 | 213.1611 | High | Low |
| 10 | Galacticol | 1.09 | C6H14O6 | 183.0830 | High | Low |
| No. | Sample Code | Location (City and Province) | Bee Species |
|---|---|---|---|
| 1 | Jav_HI1 | Magelang, Central Java | H. itama |
| 2 | Jav_HI2 | Kendal, Central Java | H. itama |
| 3 | Kal_HI3 | Banjar Baru, South Kalimantan | H. itama |
| 4 | Kal_HI4 | Kutai Kartanegara, East Kalimantan | H. itama |
| 5 | Sum_HI5 | Kampar, Riau | H. itama |
| 6 | NT_TB1 | Sumbawa, West Nusa Tenggara | T. biroi |
| 7 | Kal_TB2 | Tanah Laut, South Kalimantan | T. biroi |
| 8 | Jav_TB3 | Batang, Central Java | T. biroi |
| 9 | Jav_TB4 | Bogor, West Java | T. biroi |
| 10 | Kal_TB5 | Barito Kuala, South Kalimantan | T. biroi |
| 11 | Jav_TB6 | Magelang, Central Java | T. biroi |
| 12 | Jav_TL1 | Pandeglang, Banten | T. laeviceps |
| 13 | Jav_TL2 | Batang, Central Java | T. laeviceps |
| 14 | Jav_TL3 | Magelang, Central Java | T. laeviceps |
| 15 | Kal_TL4 | Hulu Sungai Tengah, East Kalimantan | T. laeviceps |
| 16 | Kal_TL5 | Barito Kuala, South Kalimantan | T. laeviceps |
| 17 | Kal_GT1 | Kutai Kartanegara, East Kalimantan | G. thoracica |
| 18 | Sum_GT2 | Lebong, Bengkulu | G. thoracica |
| 19 | Kal_GT3 | Hulu Sungai Tengah, East Kalimantan | G. thoracica |
| 20 | Sum_GT4 | Pematang Siantar, Nort Sumatra | G. thoracica |
| 21 | NT_SB1 | West Lombok, West Nusa Tenggara | T. clypearis |
| 22 | NT_SB2 | Central Lombok, West Nusa Tenggara | T. clypearis |
| 23 | Jav_SB3 | Batang, Central Java | T. drescheri |
| Reagent | Volume (μL) | ||
|---|---|---|---|
| Blank | Control | Sample | |
| EEP | - | - | 20 |
| DPPH 150 μmol/L | - | 180 | 180 |
| Methanol | 200 | 20 | - |
| Reagent | Volume (μL) | ||
|---|---|---|---|
| Blank | Control | Sample | |
| EEP | - | - | 50 |
| ABTS solution | - | 50 | 50 |
| Ethanol | 100 | 50 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratami, D.K.; Sahlan, M.; Bayu, A.; Putra, M.Y.; Ibrahim, B.; Siswadi; Qodriah, R.; Mun’im, A. Characteristics of Indonesian Stingless Bee Propolis and Study of Metabolomic Properties Based on Region and Species. Molecules 2024, 29, 4037. https://doi.org/10.3390/molecules29174037
Pratami DK, Sahlan M, Bayu A, Putra MY, Ibrahim B, Siswadi, Qodriah R, Mun’im A. Characteristics of Indonesian Stingless Bee Propolis and Study of Metabolomic Properties Based on Region and Species. Molecules. 2024; 29(17):4037. https://doi.org/10.3390/molecules29174037
Chicago/Turabian StylePratami, Diah Kartika, Muhamad Sahlan, Asep Bayu, Masteria Yunovilsa Putra, Baharudin Ibrahim, Siswadi, Rahmatul Qodriah, and Abdul Mun’im. 2024. "Characteristics of Indonesian Stingless Bee Propolis and Study of Metabolomic Properties Based on Region and Species" Molecules 29, no. 17: 4037. https://doi.org/10.3390/molecules29174037
APA StylePratami, D. K., Sahlan, M., Bayu, A., Putra, M. Y., Ibrahim, B., Siswadi, Qodriah, R., & Mun’im, A. (2024). Characteristics of Indonesian Stingless Bee Propolis and Study of Metabolomic Properties Based on Region and Species. Molecules, 29(17), 4037. https://doi.org/10.3390/molecules29174037







