Suppressed Ion Migration by Heterojunction Layer for Stable Wide-Bandgap Perovskite and Tandem Photovoltaics
Abstract
1. Introduction
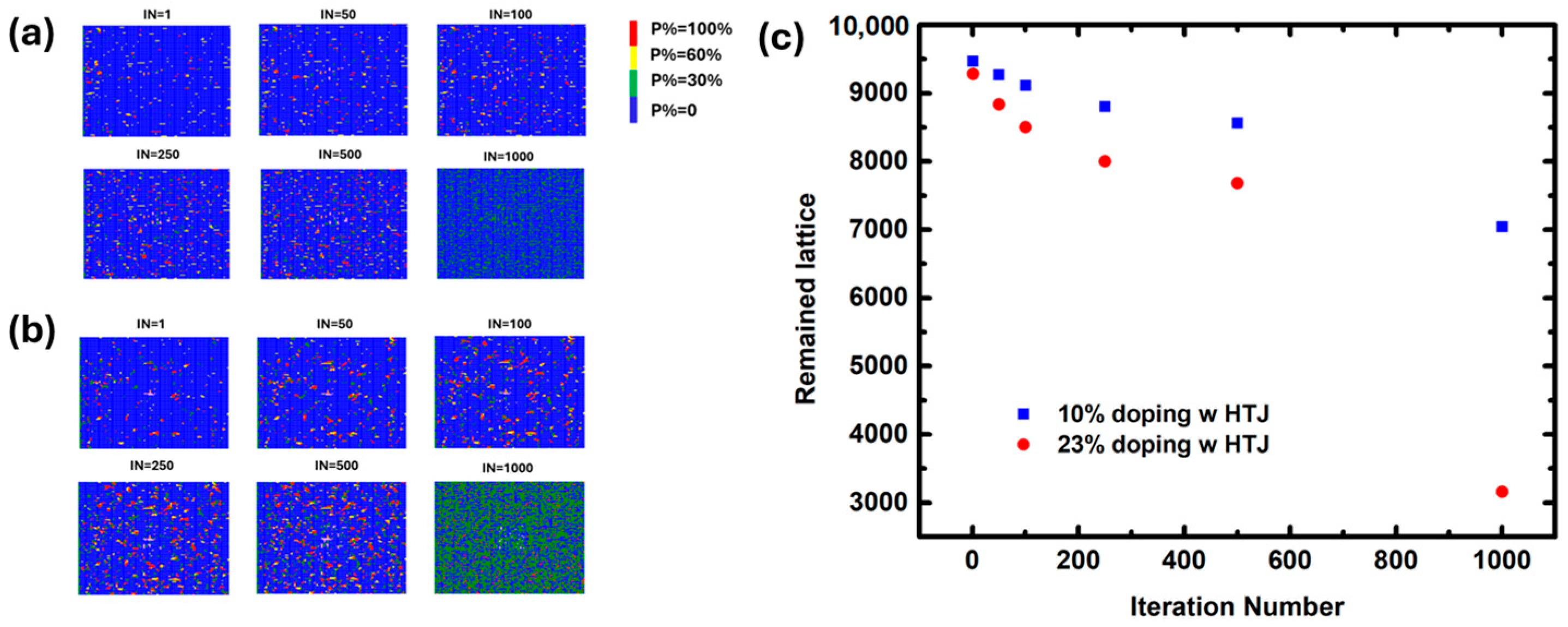
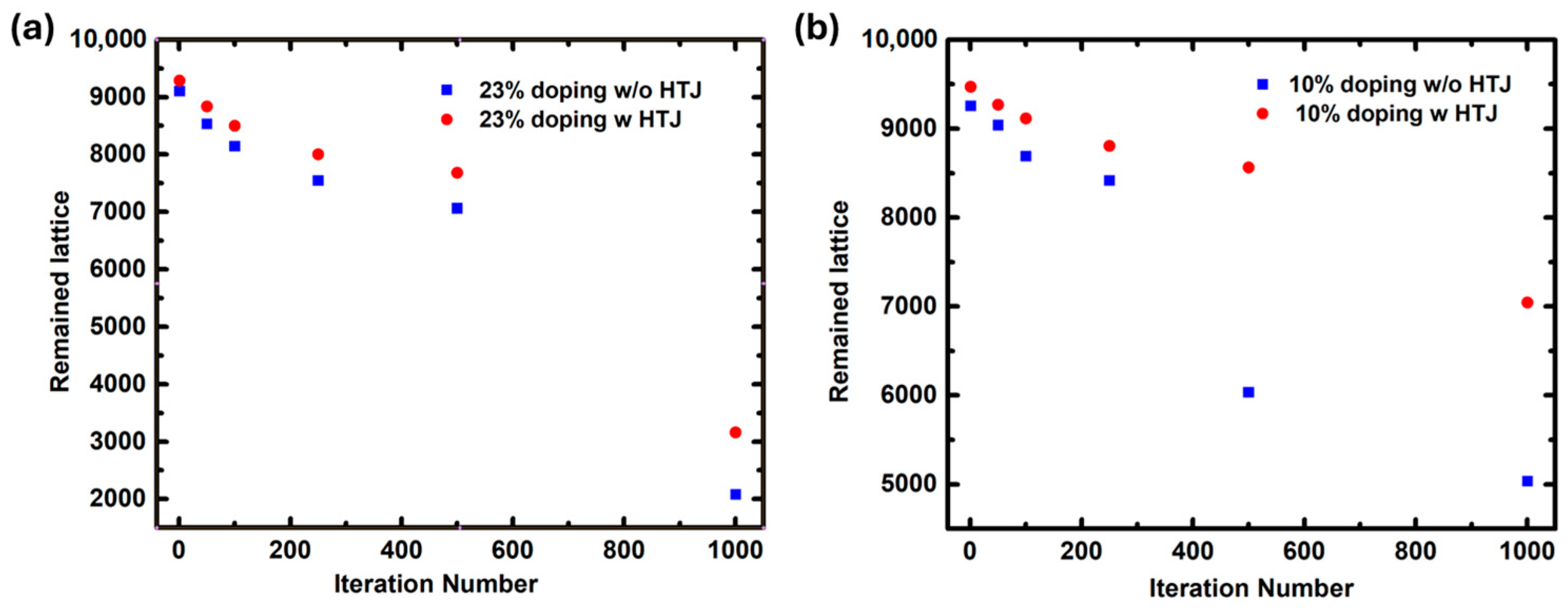
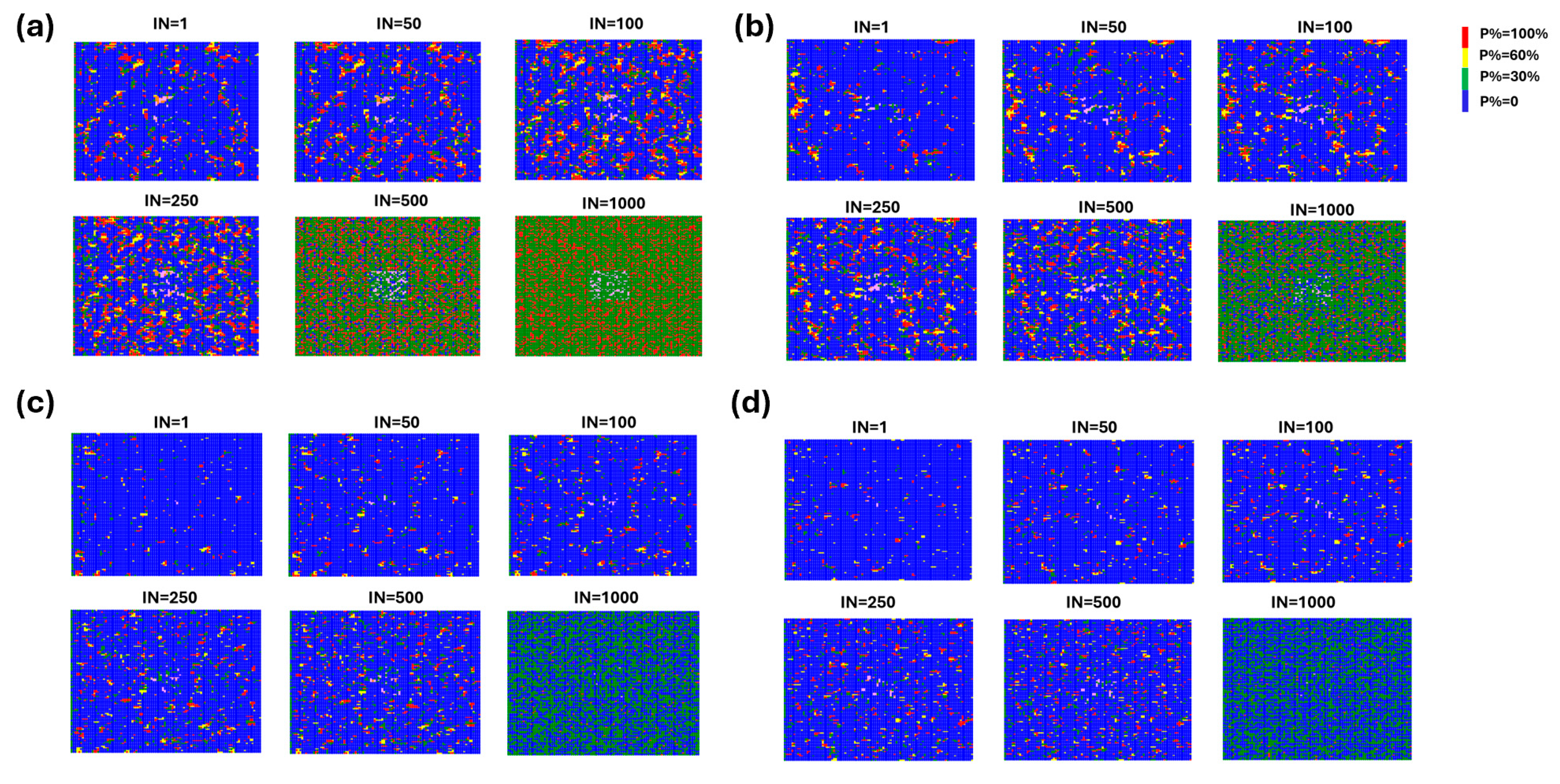
2. Results and Discussion
3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef]
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942. [Google Scholar] [CrossRef]
- Guan, H.; Zhou, S.; Fu, S.; Pu, D.; Chen, X.; Ge, Y.; Wang, S.; Wang, C.; Cui, H.; Liang, J. Regulating Crystal Orientation via Ligand Anchoring Enables Efficient Wide-Bandgap Perovskite Solar Cells and Tandems. Adv. Mater. 2024, 36, 2307987. [Google Scholar] [CrossRef]
- Tong, Y.; Najar, A.; Wang, L.; Liu, L.; Du, M.; Yang, J.; Li, J.; Wang, K.; Liu, S. Wide-bandgap organic–inorganic lead halide perovskite solar cells. Adv. Sci. 2022, 9, 2105085. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Walter, D.; Wu, Y.; Fong, K.C.; Jacobs, D.A.; Duong, T.; Peng, J.; Weber, K.; White, T.P.; Catchpole, K.R. Monolithic perovskite/Si tandem solar cells: Pathways to over 30% efficiency. Adv. Energy Mater. 2020, 10, 1902840. [Google Scholar] [CrossRef]
- Chin, X.Y.; Turkay, D.; Steele, J.A.; Tabean, S.; Eswara, S.; Mensi, M.; Fiala, P.; Wolff, C.M.; Paracchino, A.; Artuk, K. Interface passivation for 31.25%-efficient perovskite/silicon tandem solar cells. Science 2023, 381, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Allen, T.G.; De Bastiani, M.; Razzaq, A.; Xu, L.; Ugur, E.; Liu, J.; De Wolf, S. Pathways toward commercial perovskite/silicon tandem photovoltaics. Science 2024, 383, eadh3849. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.J.; Oliver, R.D.; Johnston, M.B.; Snaith, H.J. Methylammonium-free wide-bandgap metal halide perovskites for tandem photovoltaics. Nat. Rev. Mater. 2023, 8, 822–838. [Google Scholar] [CrossRef]
- Huang, T.; Xu, F.; Hu, J.; Wu, J.; Li, S.; Chen, P.; Jia, X.; Yan, H.; Ji, Y.; Luo, D. Rational heterostructure stacking enables 23% wide-bandgap perovskite solar cells by side-reaction inhibition. Energy Environ. Sci. 2024, 17, 5984–5992. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Q.; Bai, Y.; Chen, Q. Issues of phase segregation in wide-bandgap perovskites. Mater. Chem. Front. 2023, 7, 1896–1911. [Google Scholar] [CrossRef]
- Wang, R.T.; Xu, A.F.; Yang, L.W.; Chen, J.Y.; Kitai, A.; Xu, G. Magnetic-field-induced energy bandgap reduction of perovskite KMnF3. J. Mater. Chem. C 2020, 8, 4164–4168. [Google Scholar] [CrossRef]
- El-Mellouhi, F.; Marzouk, A.; Bentria, E.T.; Rashkeev, S.N.; Kais, S.; Alharbi, F.H. Hydrogen bonding and stability of hybrid organic–inorganic perovskites. ChemSusChem 2016, 9, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.-H.; Kong, E.-H.; Jang, H.M. The nature of hydrogen-bonding interaction in the prototypic hybrid halide perovskite, tetragonal CH3NH3PbI3. Sci. Rep. 2016, 6, 21687. [Google Scholar] [CrossRef] [PubMed]
- Svane, K.L.; Forse, A.C.; Grey, C.P.; Kieslich, G.; Cheetham, A.K.; Walsh, A.; Butler, K.T. How strong is the hydrogen bond in hybrid perovskites? J. Phys. Chem. Lett. 2017, 8, 6154–6159. [Google Scholar] [CrossRef]
- Lin, L.; Jones, T.W.; Yang, T.C.-J.; Li, X.; Wu, C.; Xiao, Z.; Li, H.; Li, J.; Qian, J.; Lin, L. Hydrogen bonding in perovskite solar cells. Matter 2024, 7, 38–58. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A review of perovskites solar cell stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Xu, K.J.; Wang, R.T.; Xu, A.F.; Chen, J.Y.; Xu, G. Hysteresis and Instability Predicted in Moisture Degradation of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 48882–48889. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Wei, H.; Zheng, X.; Yu, Z.; Shao, Y.; Shield, J.E.; Huang, J. Strained hybrid perovskite thin films and their impact on the intrinsic stability of perovskite solar cells. Sci. Adv. 2017, 3, eaao5616. [Google Scholar] [CrossRef]
- Levine, I.; Vera, O.G.; Kulbak, M.; Ceratti, D.-R.; Rehermann, C.; Márquez, J.A.; Levcenko, S.; Unold, T.; Hodes, G.; Balberg, I. Deep defect states in wide-band-gap ABX3 halide perovskites. ACS Energy Lett. 2019, 4, 1150–1157. [Google Scholar] [CrossRef]
- Zhou, Y.; Poli, I.; Meggiolaro, D.; De Angelis, F.; Petrozza, A. Defect activity in metal halide perovskites with wide and narrow bandgap. Nat. Rev. Mater. 2021, 6, 986–1002. [Google Scholar] [CrossRef]
- Yang, G.; Ni, Z.; Yu, Z.J.; Larson, B.W.; Yu, Z.; Chen, B.; Alasfour, A.; Xiao, X.; Luther, J.M.; Holman, Z.C. Defect engineering in wide-bandgap perovskites for efficient perovskite–silicon tandem solar cells. Nat. Photonics 2022, 16, 588–594. [Google Scholar] [CrossRef]
- Yao, Y.; Hang, P.; Li, B.; Hu, Z.; Kan, C.; Xie, J.; Wang, Y.; Zhang, Y.; Yang, D.; Yu, X. Phase-stable wide-bandgap perovskites for four-terminal perovskite/silicon tandem solar cells with over 30% efficiency. Small 2022, 18, 2203319. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; He, T.; Zhang, S.; Lei, X.; Jiang, Y.; Wang, D.; Sun, P.; Zhao, D.; Hsu, H.-Y.; Li, X. Halogen-halogen bonds enable improved long-term operational stability of mixed-halide perovskite photovoltaics. Chem 2021, 7, 3131–3143. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, Q.; Yang, Y.; Jiang, Y.; Dai, Z.; Huang, X.; Huo, J.; Guo, P.; Shen, H.; Liu, Z. Multifunctional molecule interface modification for high-performance inverted wide-bandgap perovskite cells and modules. J. Mater. Chem. A 2023, 11, 16871–16877. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, L.; Zhu, T.; Chen, H.; Chen, B.; Kubicki, D.J.; Balvanz, A.; Li, C.; Maxwell, A.; Ugur, E. Suppressed phase segregation for triple-junction perovskite solar cells. Nature 2023, 618, 74–79. [Google Scholar] [CrossRef]
- Zhao, Y.; Yavuz, I.; Wang, M.; Weber, M.H.; Xu, M.; Lee, J.-H.; Tan, S.; Huang, T.; Meng, D.; Wang, R. Suppressing ion migration in metal halide perovskite via interstitial doping with a trace amount of multivalent cations. Nat. Mater. 2022, 21, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, X.; Xie, H.; Cai, X.; Zheng, B.; Yu, H.; Liu, E.; Hao, X.; Zhang, M. Unraveling the mechanism of ion-migration suppression by interstitial doping for operationally stable CsPbI2Br perovskite solar cells. Chem. Mater. 2022, 34, 1010–1019. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Hou, T.; Sun, X.; Hao, X. Extrinsic interstitial ions in metal halide perovskites: A review. Small 2023, 19, 2303060. [Google Scholar] [CrossRef] [PubMed]
- Zai, H.; Ma, Y.; Chen, Q.; Zhou, H. Ion migration in halide perovskite solar cells: Mechanism, characterization, impact and suppression. J. Energy Chem. 2021, 63, 528–549. [Google Scholar] [CrossRef]
- Chen, C.; Song, Z.; Xiao, C.; Awni, R.A.; Yao, C.; Shrestha, N.; Li, C.; Bista, S.S.; Zhang, Y.; Chen, L. Arylammonium-assisted reduction of the open-circuit voltage deficit in wide-bandgap perovskite solar cells: The role of suppressed ion migration. ACS Energy Lett. 2020, 5, 2560–2568. [Google Scholar] [CrossRef]
- Wang, R.T.; Xu, A.F.; Zhang, W.; Xu, G. The influence of compression on the lattice stability of α-FAPbI3 revealed by numerical simulation. New J. Chem. 2022, 46, 16130–16137. [Google Scholar] [CrossRef]
- Wang, T.; Xu, F.; Wang, Q.; Tai, L.; Xu, G. Improved Perovskite Structural Stability by Halogen Bond from Excessive Lead Iodide via Numerical Simulation. Crystals 2022, 12, 1073. [Google Scholar] [CrossRef]
- Wang, R.T.; Jin, X.; Tan, W.; Zhang, Y.; Zhang, W.; Abbas, A.; Lyu, B.; Xu, F. Hindered Phase Transition Kinetics of α-CsPbI3 by External Tension. Energy Technol. 2023, 11, 2300523. [Google Scholar] [CrossRef]
- Tai, L.; Wang, Q.; Wang, R.T.; Gu, X.; Xu, F. Controlled formation of ball-milled carbon quantum dots via optimized graphite structures by numerical simulation. New J. Chem. 2024, 48, 10087–10092. [Google Scholar] [CrossRef]
- Slotcavage, D.J.; Karunadasa, H.I.; McGehee, M.D. Light-induced phase segregation in halide-perovskite absorbers. ACS Energy Lett. 2016, 1, 1199–1205. [Google Scholar] [CrossRef]
- Ye, T.; Hou, Y.; Nozariasbmarz, A.; Yang, D.; Yoon, J.; Zheng, L.; Wang, K.; Wang, K.; Ramakrishna, S.; Priya, S. Cost-effective high-performance charge-carrier-transport-layer-free perovskite solar cells achieved by suppressing ion migration. ACS Energy Lett. 2021, 6, 3044–3052. [Google Scholar] [CrossRef]
- Choe, H.; Jeon, D.; Lee, S.J.; Cho, J. Mixed or segregated: Toward efficient and stable mixed halide perovskite-based devices. ACS Omega 2021, 6, 24304–24315. [Google Scholar] [CrossRef]
- Lian, X.; Zuo, L.; Chen, B.; Li, B.; Wu, H.; Shan, S.; Wu, G.; Yu, X.; Chen, Q.; Chen, L. Light-induced beneficial ion accumulation for high-performance quasi-2D perovskite solar cells. Energy Environ. Sci. 2022, 15, 2499–2507. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhang, W.; Yang, W.; Yu, Z.; Xu, G.; Xu, F. Suppressed Ion Migration by Heterojunction Layer for Stable Wide-Bandgap Perovskite and Tandem Photovoltaics. Molecules 2024, 29, 4030. https://doi.org/10.3390/molecules29174030
Wang T, Zhang W, Yang W, Yu Z, Xu G, Xu F. Suppressed Ion Migration by Heterojunction Layer for Stable Wide-Bandgap Perovskite and Tandem Photovoltaics. Molecules. 2024; 29(17):4030. https://doi.org/10.3390/molecules29174030
Chicago/Turabian StyleWang, Taoran, Weiwei Zhang, Wenjuan Yang, Zeyi Yu, Gu Xu, and Fan Xu. 2024. "Suppressed Ion Migration by Heterojunction Layer for Stable Wide-Bandgap Perovskite and Tandem Photovoltaics" Molecules 29, no. 17: 4030. https://doi.org/10.3390/molecules29174030
APA StyleWang, T., Zhang, W., Yang, W., Yu, Z., Xu, G., & Xu, F. (2024). Suppressed Ion Migration by Heterojunction Layer for Stable Wide-Bandgap Perovskite and Tandem Photovoltaics. Molecules, 29(17), 4030. https://doi.org/10.3390/molecules29174030









