Molecular Dynamics Simulation Combined with Neural Relationship Inference and Markov Model to Reveal the Relationship between Conformational Regulation and Bioluminescence Properties of Gaussia Luciferase
Abstract
1. Introduction
2. Results
2.1. Protein Preparation and Structure Stability
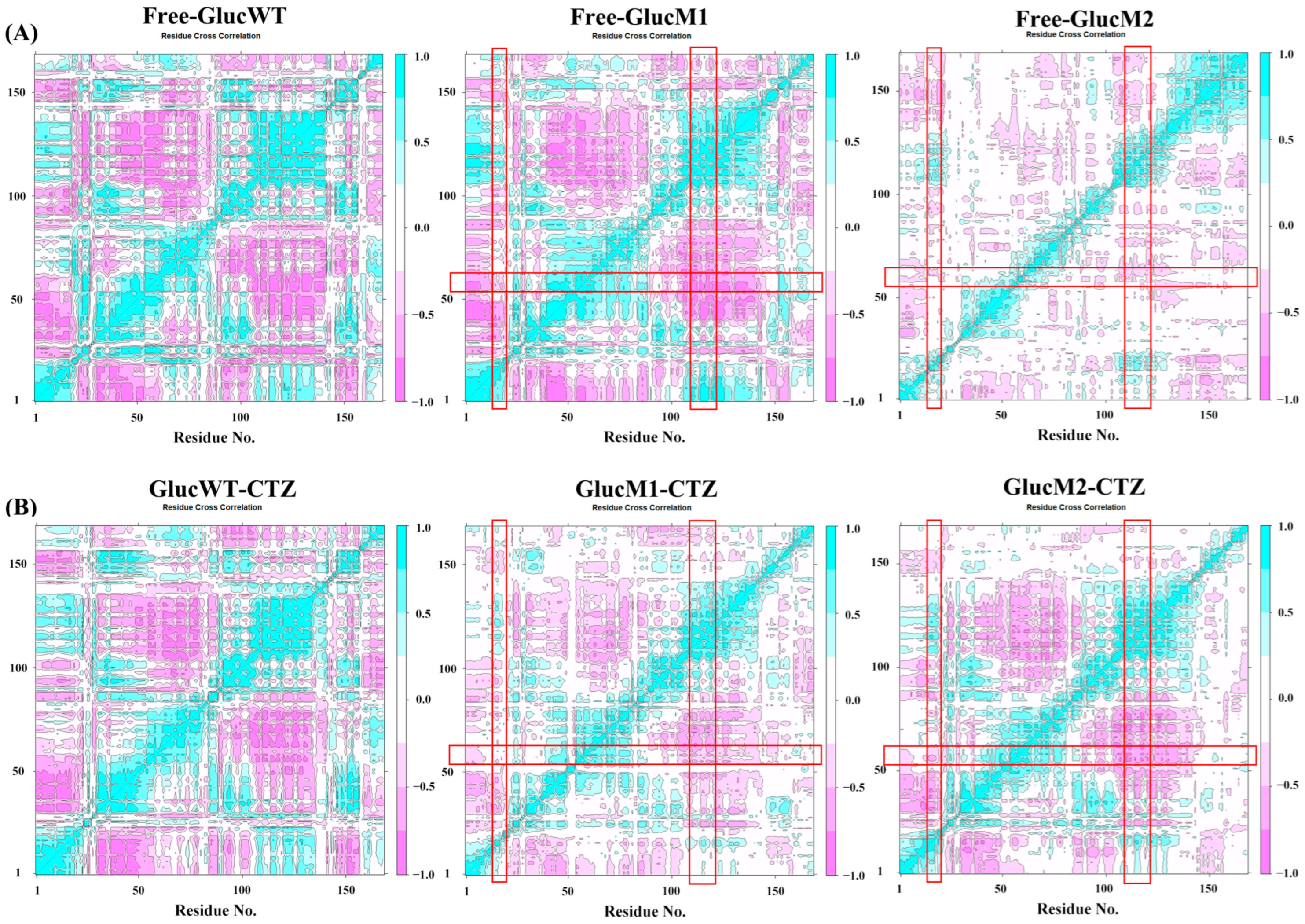
2.2. Dynamical Cross-Correlation Matrix Analysis
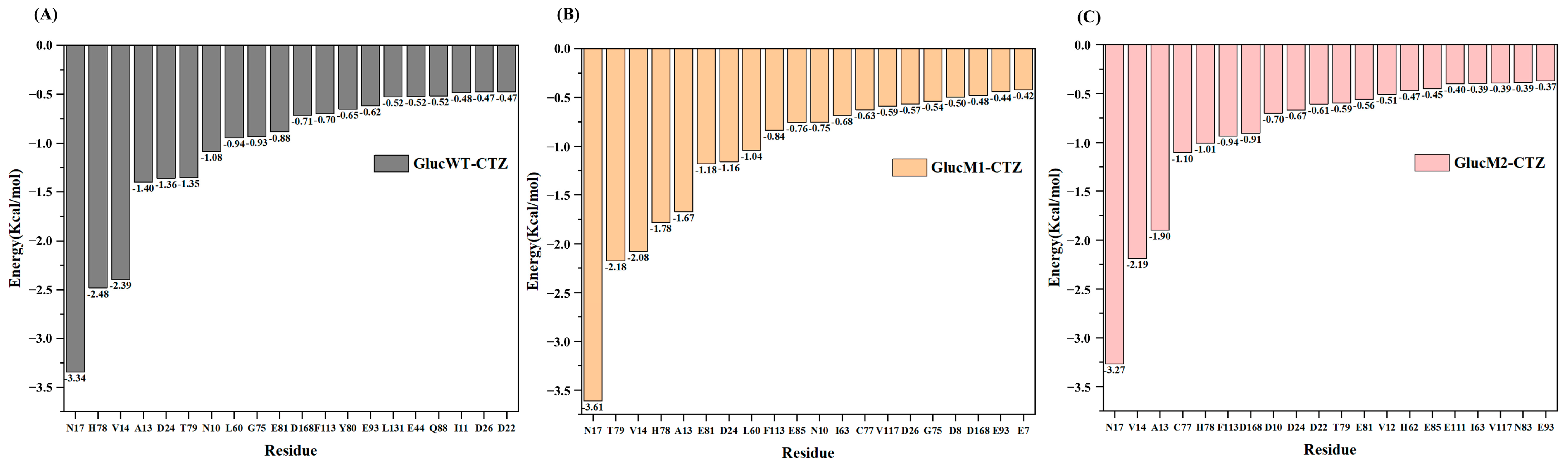
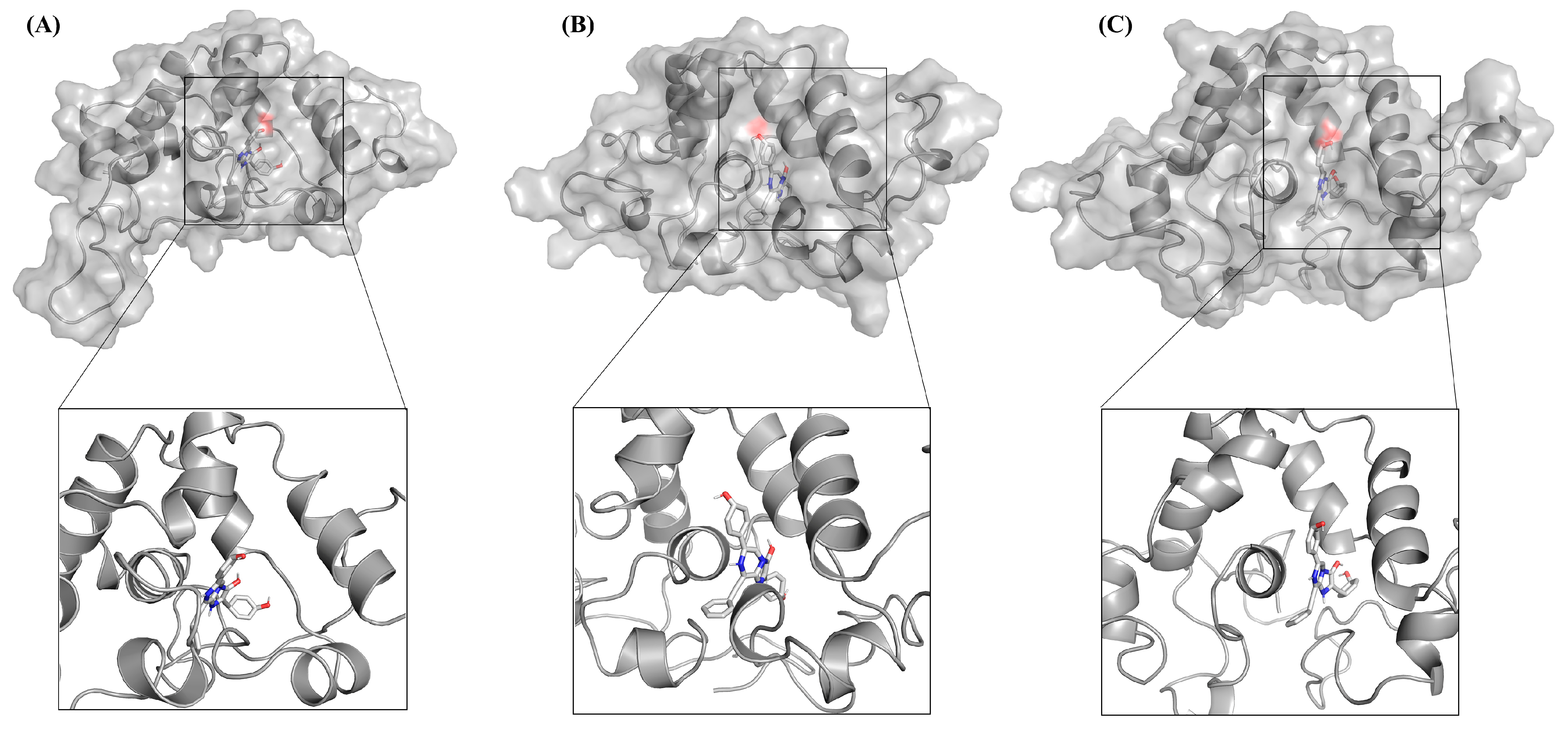
2.3. Molecular Mechanics/Poisson–Boltzmann Surface Area Calculation
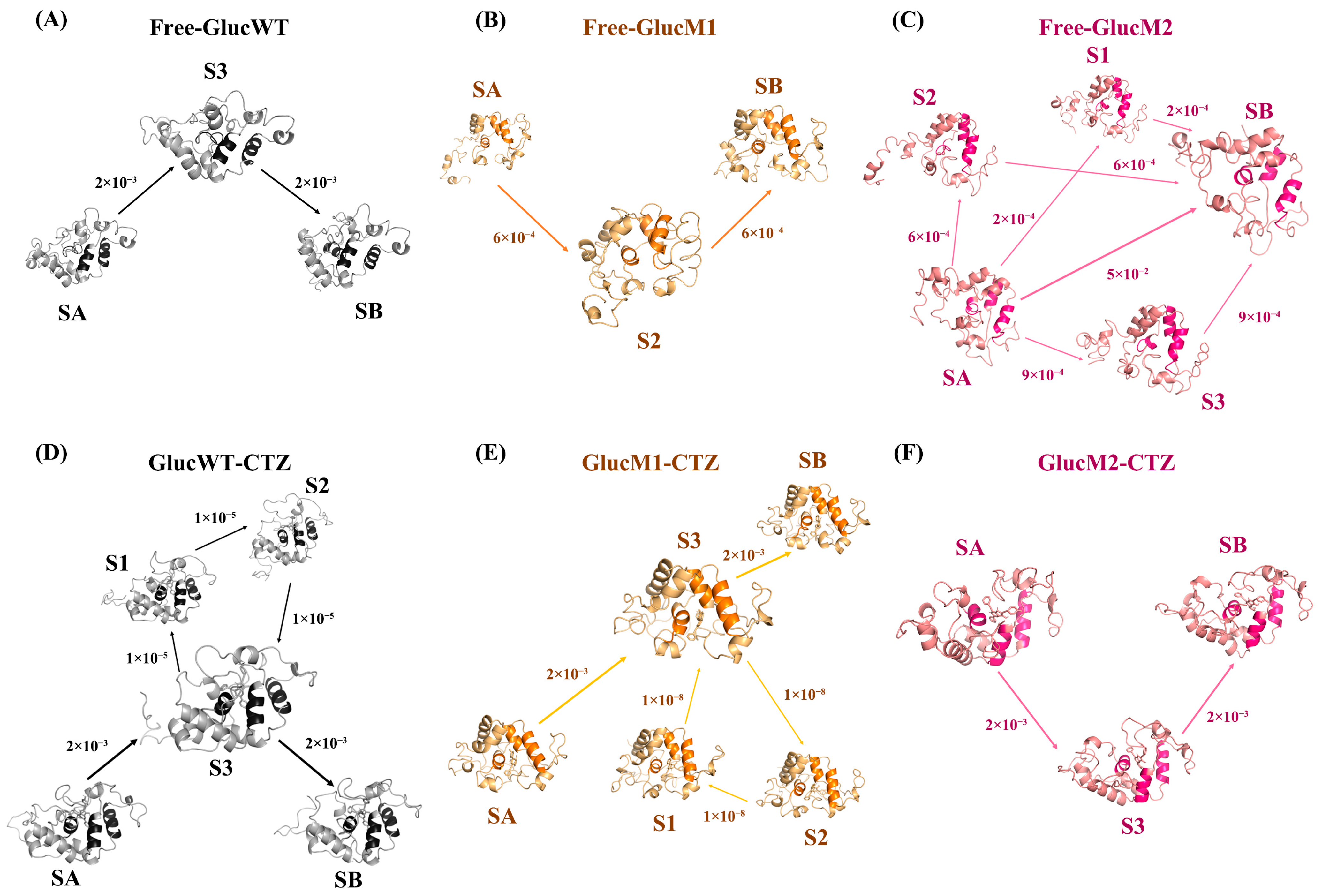
2.4. Conformational Changes during MD Simulations
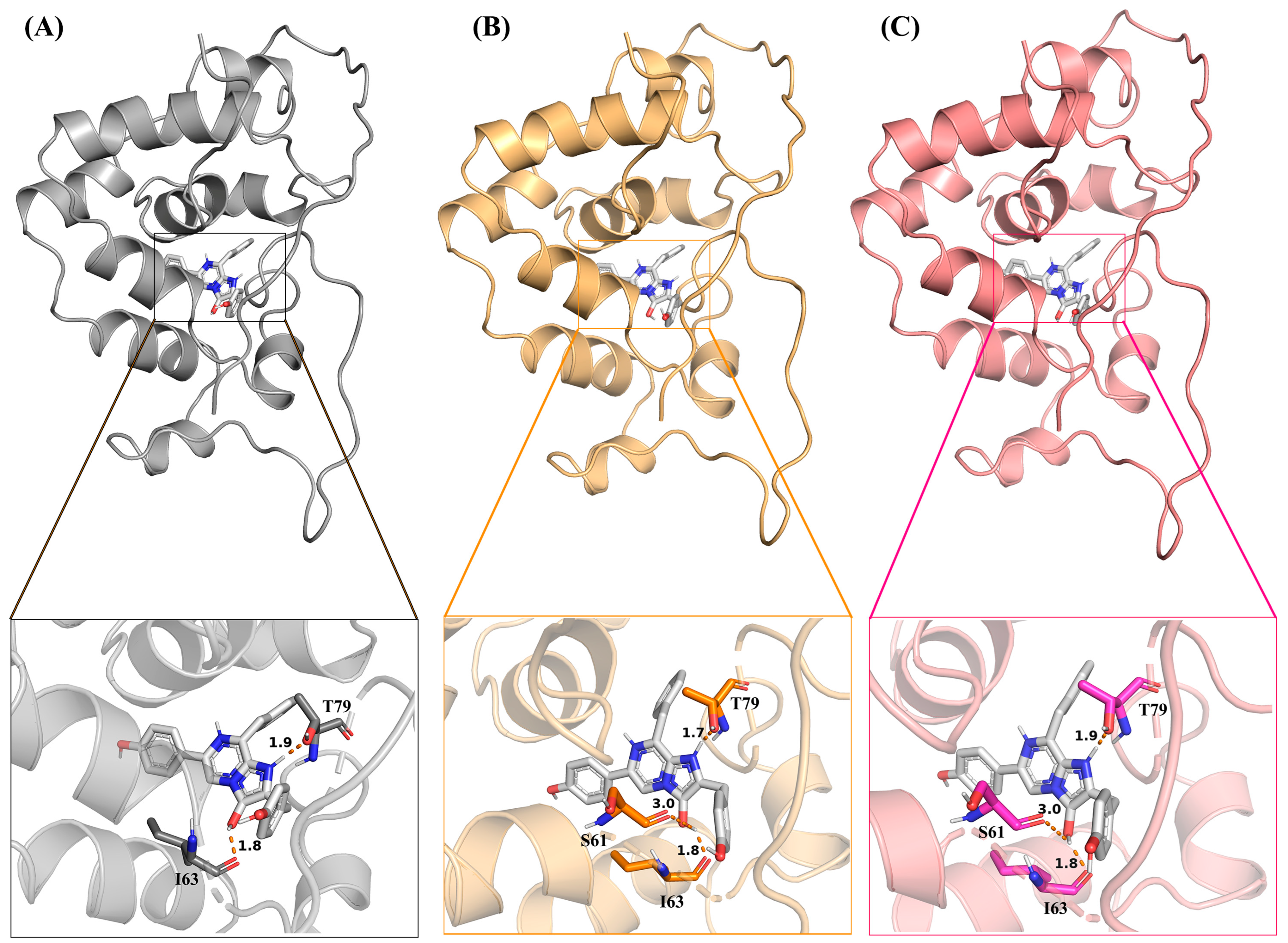
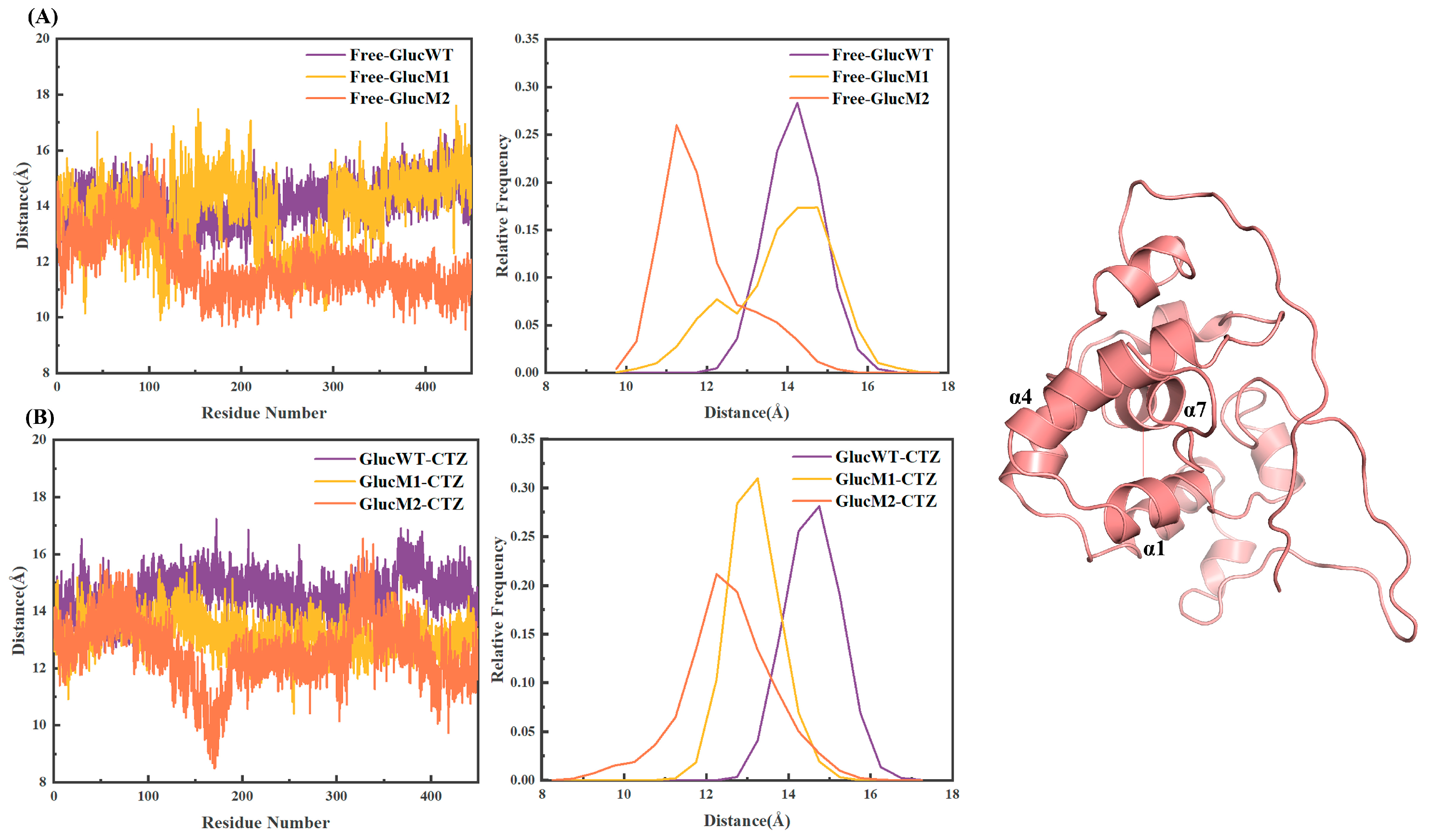
2.5. Distance Analysis
2.6. Neural Relationship Inference
3. Discussion
4. Materials and Methods
4.1. System Preparation
4.2. Molecular Dynamic Simulation
4.3. Trajectory Analysis
4.4. MM/PBSA Calculations
4.5. Markov Model
4.5.1. K-Means Clustering Algorithm
4.5.2. Determination of Time Lag Time
4.5.3. Flux Analysis
4.5.4. Neural Relationship Inference
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kimura, T.; Hiraoka, K.; Kasahara, N.; Logg, C.R. Optimization of enzyme-substrate pairing for bioluminescence imaging of gene transfer using Renilla and Gaussia luciferases. J. Gene Med. 2010, 12, 528–537. [Google Scholar] [CrossRef]
- Notka, F.; Wagner, R. Reprogramming a GFP reporter gene subjects it to complex lentiviral gene regulation. Methods Mol. Biol. 2012, 813, 85–106. [Google Scholar] [CrossRef]
- Laios, E.; Obeid, P.J.; Ioannou, P.C.; Christopoulos, T.K. Expression hybridization assays combining cDNAs from firefly and Renilla luciferases as labels for simultaneous determination of two target sequences. Anal. Chem. 2000, 72, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Yamashita, H.; Au, P.; Tannous, B.A.; Fukumura, D.; Jain, R.K. Secreted Gaussia luciferase as a biomarker for monitoring tumor progression and treatment response of systemic metastases. PLoS ONE 2009, 4, e8316. [Google Scholar] [CrossRef]
- Stuss, D.P.; Boyd, J.D.; Levin, D.B.; Delaney, K.R. MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice. PLoS ONE 2012, 7, e31896. [Google Scholar] [CrossRef] [PubMed]
- Soleja, N.; Manzoor, O.; Khan, I.; Ahmad, A.; Mohsin, M. Role of green fluorescent proteins and their variants in development of FRET-based sensors. J. Biosci. 2018, 43, 763–784. [Google Scholar] [CrossRef]
- Heim, R.; Tsien, R.Y. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr. Biol. 1996, 6, 178–182. [Google Scholar] [CrossRef]
- Rodriguez, E.A.; Campbell, R.E.; Lin, J.Y.; Lin, M.Z.; Miyawaki, A.; Palmer, A.E.; Shu, X.; Zhang, J.; Tsien, R.Y. The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins. Trends Biochem. Sci. 2017, 42, 111–129. [Google Scholar] [CrossRef]
- van Thor, J.J.; Champion, P.M. Photoacid Dynamics in the Green Fluorescent Protein. Annu. Rev. Phys. Chem. 2023, 74, 123–144. [Google Scholar] [CrossRef]
- Rizzuto, R.; Brini, M.; De Giorgi, F.; Rossi, R.; Heim, R.; Tsien, R.Y.; Pozzan, T. Double labelling of subcellular structures with organelle-targeted GFP mutants in vivo. Curr. Biol. 1996, 6, 183–188. [Google Scholar] [CrossRef]
- Fraga, H. Firefly luminescence: A historical perspective and recent developments. Photochem. Photobiol. Sci. 2008, 7, 146–158. [Google Scholar] [CrossRef]
- Tiffen, J.C.; Bailey, C.G.; Ng, C.; Rasko, J.E.; Holst, J. Luciferase expression and bioluminescence does not affect tumor cell growth in vitro or in vivo. Mol. Cancer 2010, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Yokobori, T.; Osone, K.; Tatsuki, H.; Takada, T.; Suto, T.; Yajima, R.; Kato, T.; Fujii, T.; Tsutsumi, S.; et al. Establishment of a novel method to evaluate peritoneal microdissemination and therapeutic effect using luciferase assay. Cancer Sci. 2016, 107, 341–346. [Google Scholar] [CrossRef]
- Baggett, B.; Roy, R.; Momen, S.; Morgan, S.; Tisi, L.; Morse, D.; Gillies, R.J. Thermostability of firefly luciferases affects efficiency of detection by in vivo bioluminescence. Mol. Imaging 2004, 3, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Koksharov, M.I.; Ugarova, N.N. Approaches to engineer stability of beetle luciferases. Comput. Struct. Biotechnol. J. 2012, 2, e201209004. [Google Scholar] [CrossRef]
- Shifera, A.S.; Hardin, J.A. Factors modulating expression of Renilla luciferase from control plasmids used in luciferase reporter gene assays. Anal. Biochem. 2010, 396, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Loening, A.M.; Fenn, T.D.; Wu, A.M.; Gambhir, S.S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng. Des. Sel. 2006, 19, 391–400. [Google Scholar] [CrossRef]
- Woo, J.; von Arnim, A.G. Mutational optimization of the coelenterazine-dependent luciferase from Renilla. Plant Methods 2008, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- de Wet, J.R.; Wood, K.V.; Helinski, D.R.; DeLuca, M. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proc. Natl. Acad. Sci. USA 1985, 82, 7870–7873. [Google Scholar] [CrossRef] [PubMed]
- Lembert, N.; Idahl, L.A. Regulatory effects of ATP and luciferin on firefly luciferase activity. Biochem. J. 1995, 305, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Tang, Y.; Breakefield, X.; Weissleder, R. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene 2003, 22, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
- Tannous, B.A.; Kim, D.E.; Fernandez, J.L.; Weissleder, R.; Breakefield, X.O. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 2005, 11, 435–443. [Google Scholar] [CrossRef]
- Tannous, B.A. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat. Protoc. 2009, 4, 582–591. [Google Scholar] [CrossRef]
- Wiles, S.; Ferguson, K.; Stefanidou, M.; Young, D.B.; Robertson, B.D. Alternative luciferase for monitoring bacterial cells under adverse conditions. Appl. Environ. Microbiol. 2005, 71, 3427–3432. [Google Scholar] [CrossRef]
- Shrestha, T.B.; Seo, G.M.; Basel, M.T.; Kalita, M.; Wang, H.; Villanueva, D.; Pyle, M.; Balivada, S.; Rachakatla, R.S.; Shinogle, H.; et al. Stem cell-based photodynamic therapy. Photochem. Photobiol. Sci. 2012, 11, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Wille, T.; Blank, K.; Schmidt, C.; Vogt, V.; Gerlach, R.G. Gaussia princeps luciferase as a reporter for transcriptional activity, protein secretion, and protein-protein interactions in Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2012, 78, 250–257. [Google Scholar] [CrossRef]
- Maguire, C.A.; Deliolanis, N.C.; Pike, L.; Niers, J.M.; Tjon-Kon-Fat, L.A.; Sena-Esteves, M.; Tannous, B.A. Gaussia luciferase variant for high-throughput functional screening applications. Anal. Chem. 2009, 81, 7102–7106. [Google Scholar] [CrossRef]
- Welsh, J.P.; Patel, K.G.; Manthiram, K.; Swartz, J.R. Multiply mutated Gaussia luciferases provide prolonged and intense bioluminescence. Biochem. Biophys. Res. Commun. 2009, 389, 563–568. [Google Scholar] [CrossRef]
- Degeling, M.H.; Bovenberg, M.S.; Lewandrowski, G.K.; de Gooijer, M.C.; Vleggeert-Lankamp, C.L.; Tannous, M.; Maguire, C.A.; Tannous, B.A. Directed molecular evolution reveals Gaussia luciferase variants with enhanced light output stability. Anal. Chem. 2013, 85, 3006–3012. [Google Scholar] [CrossRef]
- Kim, S.B.; Suzuki, H.; Sato, M.; Tao, H. Superluminescent variants of marine luciferases for bioassays. Anal. Chem. 2011, 83, 8732–8740. [Google Scholar] [CrossRef]
- Wu, N.; Kamioka, T.; Kuroda, Y. A novel screening system based on VanX-mediated autolysis-Application to Gaussia luciferase. Biotechnol. Bioeng. 2016, 113, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Gheysens, O.; Mottaghy, F.M. Method of bioluminescence imaging for molecular imaging of physiological and pathological processes. Methods 2009, 48, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.M.; Herring, P.J.; Campbell, A.K. The widespread occurrence and tissue distribution of the imidazolopyrazine luciferins. J. Biolumin. Chemilumin. 1997, 12, 87–91. [Google Scholar] [CrossRef]
- Salehian, M.; Emamzadeh, R.; Nazari, M. Exploring the Potential of Arginine to Increase Coelenterazine-Renilla Luciferase Affinity and Enzyme Stability: Kinetic and Molecular Dynamics Studies. Protein J. 2024, 1–12. [Google Scholar] [CrossRef]
- Mortazavi, M.; Torkzadeh-Mahani, M.; Rahimi, M.; Maleki, M.; Lotfi, S.; Riahi-Madvar, A. Effects of synonymous mutations on kinetic properties and structure of firefly luciferase: Molecular dynamics simulation, molecular docking, RNA folding, and experimental study. Int. J. Biol. Macromol. 2023, 235, 123835. [Google Scholar] [CrossRef]
- Wu, N.; Kobayashi, N.; Tsuda, K.; Unzai, S.; Saotome, T.; Kuroda, Y.; Yamazaki, T. Solution structure of Gaussia Luciferase with five disulfide bonds and identification of a putative coelenterazine binding cavity by heteronuclear NMR. Sci. Rep. 2020, 10, 20069. [Google Scholar] [CrossRef]
- Haghshenas, H.; Tavakol, H.; Kaviani, B.; Mohammadnezhad, G. AMBER Force Field Parameters for Cobalt-Containing Biological Systems: A Systematic Derivation Study. J. Phys. Chem. B 2020, 124, 777–787. [Google Scholar] [CrossRef]
- Scherer, M.K.; Trendelkamp-Schroer, B.; Paul, F.; Perez-Hernandez, G.; Hoffmann, M.; Plattner, N.; Wehmeyer, C.; Prinz, J.H.; Noe, F. PyEMMA 2: A Software Package for Estimation, Validation, and Analysis of Markov Models. J. Chem. Theory Comput. 2015, 11, 5525–5542. [Google Scholar] [CrossRef]
- Oyama, H.; Morita, I.; Kiguchi, Y.; Miyake, S.; Moriuchi, A.; Akisada, T.; Niwa, T.; Kobayashi, N. Gaussia Luciferase as a Genetic Fusion Partner with Antibody Fragments for Sensitive Immunoassay Monitoring of Clinical Biomarkers. Anal. Chem. 2015, 87, 12387–12395. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Liu, N.N.; Hu, L.; Wang, H.; Sahni, N. Base-resolution stratification of cancer mutations using functional variomics. Nat. Protoc. 2017, 12, 2323–2341. [Google Scholar] [CrossRef]
- Chopra, A. Gaussia Princeps Luciferase. In Molecular Imaging and Contrast Agent Database (MICAD); Bethesda (MD): Rockville, MD, USA, 2004. [Google Scholar]
- Inouye, S.; Sahara, Y. Identification of two catalytic domains in a luciferase secreted by the copepod Gaussia princeps. Biochem. Biophys. Res. Commun. 2008, 365, 96–101. [Google Scholar] [CrossRef]
- Kim, S.B. Labor-effective manipulation of marine and beetle luciferases for bioassays. Protein Eng. Des. Sel. 2012, 25, 261–269. [Google Scholar] [CrossRef]
- Wu, N.; Rathnayaka, T.; Kuroda, Y. Bacterial expression and re-engineering of Gaussia princeps luciferase and its use as a reporter protein. Biochim. Biophys. Acta 2015, 1854, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Richards, F.M. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 1971, 55, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Loening, A.M.; Fenn, T.D.; Gambhir, S.S. Crystal structures of the luciferase and green fluorescent protein from Renilla reniformis. J. Mol. Biol. 2007, 374, 1017–1028. [Google Scholar] [CrossRef]
- Pascarella, S.; Argos, P. Analysis of insertions/deletions in protein structures. J. Mol. Biol. 1992, 224, 461–471. [Google Scholar] [CrossRef]
- Schenkmayerova, A.; Pinto, G.P.; Toul, M.; Marek, M.; Hernychova, L.; Planas-Iglesias, J.; Daniel Liskova, V.; Pluskal, D.; Vasina, M.; Emond, S.; et al. Engineering the protein dynamics of an ancestral luciferase. Nat. Commun. 2021, 12, 3616. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Husic, B.E.; Pande, V.S. Markov State Models: From an Art to a Science. J. Am. Chem. Soc. 2018, 140, 2386–2396. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Han, W.; Xu, D. Neural relational inference to learn long-range allosteric interactions in proteins from molecular dynamics simulations. Nat. Commun. 2022, 13, 1661. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.; Zheng, P.; Zhang, T.; Ye, X.; Liu, M.; Huang, M.; Wan, Q.; Zhang, J. Based on UPLC-Q-TOF-MS/MS, Systematic Network Pharmacology, and Molecular Docking to Explore the Potential Mechanism of Fructus Aurantii for Major Depression Disorder. Evid. Based Complement. Alternat. Med. 2021, 2021, 6486287. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Pathak, A.K.; Bandyopadhyay, T. Temperature Induced Dynamical Transition of Biomolecules in Polarizable and Nonpolarizable TIP3P Water. J. Chem. Theory Comput. 2019, 15, 2706–2718. [Google Scholar] [CrossRef]
- Sattelle, B.M.; Almond, A. Less is more when simulating unsulfated glycosaminoglycan 3D-structure: Comparison of GLYCAM06/TIP3P, PM3-CARB1/TIP3P, and SCC-DFTB-D/TIP3P predictions with experiment. J. Comput. Chem. 2010, 31, 2932–2947. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., 3rd. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Fischer, S.; Crow, M.; Harris, B.D.; Gillis, J. Scaling up reproducible research for single-cell transcriptomics using MetaNeighbor. Nat. Protoc. 2021, 16, 4031–4067. [Google Scholar] [CrossRef]
- Chodera, J.D.; Noe, F. Markov state models of biomolecular conformational dynamics. Curr. Opin. Struct. Biol. 2014, 25, 135–144. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System, Version 2.0. Schrödinger, LLC. Available online: http://www.pymol.org/pymol (accessed on 14 August 2022).
- Chen, C.W.; Luo, J.; Parker, K.J. Image segmentation via adaptive K-mean clustering and knowledge-based morphological operations with biomedical applications. IEEE Trans. Image Process. 1998, 7, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, W.; Wang, R.; Wang, L. Brain MR image segmentation with spatial constrained K-mean algorithm and dual-tree complex wavelet transform. J. Med. Syst. 2014, 38, 93. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, L.; Bai, D.; Zhao, M.Y. K-mean cluster analysis for incisal jaw morphology of normal occlusion subjects among different vertical facial skeletal types. Hua Xi Kou Qiang Yi Xue Za Zhi 2005, 23, 299–302, 309. [Google Scholar] [PubMed]
- Prinz, J.H.; Wu, H.; Sarich, M.; Keller, B.; Senne, M.; Held, M.; Chodera, J.D.; Schutte, C.; Noe, F. Markov models of molecular kinetics: Generation and validation. J. Chem. Phys. 2011, 134, 174105. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.R. An overview and practical guide to building Markov state models. Adv. Exp. Med. Biol. 2014, 797, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Noe, F.; Schutte, C.; Vanden-Eijnden, E.; Reich, L.; Weikl, T.R. Constructing the equilibrium ensemble of folding pathways from short off-equilibrium simulations. Proc. Natl. Acad. Sci. USA 2009, 106, 19011–19016. [Google Scholar] [CrossRef]
- Weinan, E.; Vanden-Eijnden, E. Transition-path theory and path-finding algorithms for the study of rare events. Annu. Rev. Phys. Chem. 2010, 61, 391–420. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, S.; Chen, F.; Long, G.; Zhang, C.; Yu, P.S. A Comprehensive Survey on Graph Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2021, 32, 4–24. [Google Scholar] [CrossRef]
- Gianni, S.; Walma, T.; Arcovito, A.; Calosci, N.; Bellelli, A.; Engstrom, A.; Travaglini-Allocatelli, C.; Brunori, M.; Jemth, P.; Vuister, G.W. Demonstration of long-range interactions in a PDZ domain by NMR, kinetics, and protein engineering. Structure 2006, 14, 1801–1809. [Google Scholar] [CrossRef]
- Di Paola, L.; Giuliani, A. Protein contact network topology: A natural language for allostery. Curr. Opin. Struct. Biol. 2015, 31, 43–48. [Google Scholar] [CrossRef]




| Energy Component | Average | Std. Dev. | Std. Err. of Mean |
|---|---|---|---|
| ∆EvdW | −65.7423 | 4.2555 | 0.2006 |
| ∆Eele | −101.9711 | 31.8121 | 1.4996 |
| ∆Ggas | −167.7133 | 33.4725 | 1.5779 |
| ∆Gsolv | 126.3252 | 30.6287 | 1.4438 |
| ∆Gtotal | −41.3882 | 5.8702 | 0.2767 |
| Energy Component | Average | Std. Dev. | Std. Err. of Mean |
|---|---|---|---|
| ∆EvdW | −65.4621 | 4.5203 | 0.2131 |
| ∆Eele | −106.6130 | 36.7542 | 1.7326 |
| ∆Ggas | −172.0751 | 36.7367 | 1.7318 |
| ∆Gsolv | 125.5495 | 31.0207 | 1.4623 |
| ∆Gtotal | −46.5255 | 7.8161 | 0.3685 |
| Energy Component | Average | Std. Dev. | Std. Err. of Mean |
|---|---|---|---|
| ∆EvdW | −60.0448 | 5.8718 | 0.2768 |
| ∆Eele | −156.8636 | 30.0942 | 1.4187 |
| ∆Ggas | −216.9083 | 31.1263 | 1.4673 |
| ∆Gsolv | 179.5908 | 29.8570 | 1.4075 |
| ∆Gtotal | −37.3175 | 6.0562 | 0.2855 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, R.; Han, W.; Han, L. Molecular Dynamics Simulation Combined with Neural Relationship Inference and Markov Model to Reveal the Relationship between Conformational Regulation and Bioluminescence Properties of Gaussia Luciferase. Molecules 2024, 29, 4029. https://doi.org/10.3390/molecules29174029
Yang X, Zhang R, Han W, Han L. Molecular Dynamics Simulation Combined with Neural Relationship Inference and Markov Model to Reveal the Relationship between Conformational Regulation and Bioluminescence Properties of Gaussia Luciferase. Molecules. 2024; 29(17):4029. https://doi.org/10.3390/molecules29174029
Chicago/Turabian StyleYang, Xiaotang, Ruoyu Zhang, Weiwei Han, and Lu Han. 2024. "Molecular Dynamics Simulation Combined with Neural Relationship Inference and Markov Model to Reveal the Relationship between Conformational Regulation and Bioluminescence Properties of Gaussia Luciferase" Molecules 29, no. 17: 4029. https://doi.org/10.3390/molecules29174029
APA StyleYang, X., Zhang, R., Han, W., & Han, L. (2024). Molecular Dynamics Simulation Combined with Neural Relationship Inference and Markov Model to Reveal the Relationship between Conformational Regulation and Bioluminescence Properties of Gaussia Luciferase. Molecules, 29(17), 4029. https://doi.org/10.3390/molecules29174029








