A Strategic Synthesis of Orange Waste-Derived Porous Carbon via a Freeze-Drying Method: Morphological Characterization and Cytocompatibility Evaluation
Abstract
1. Introduction
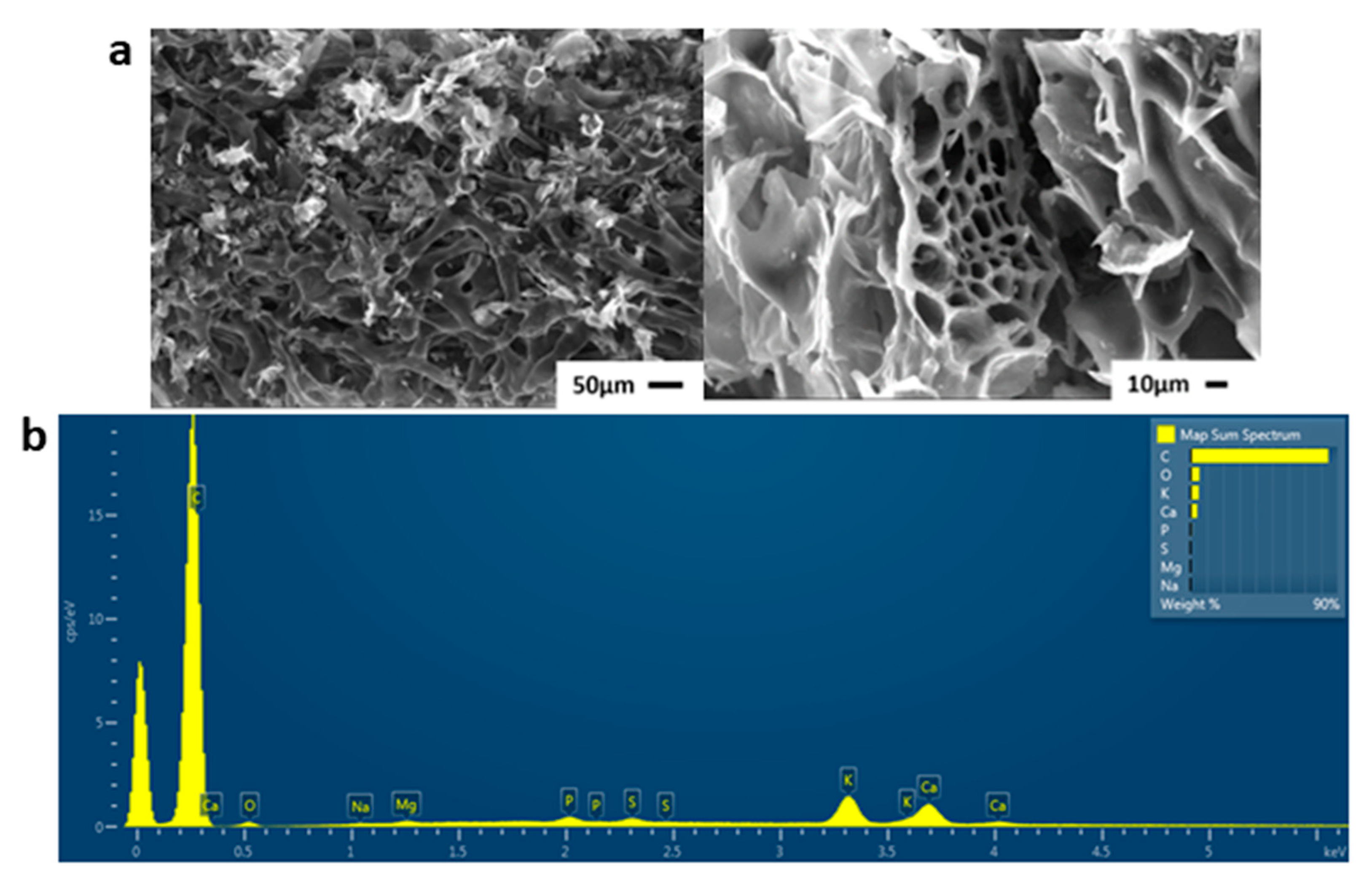
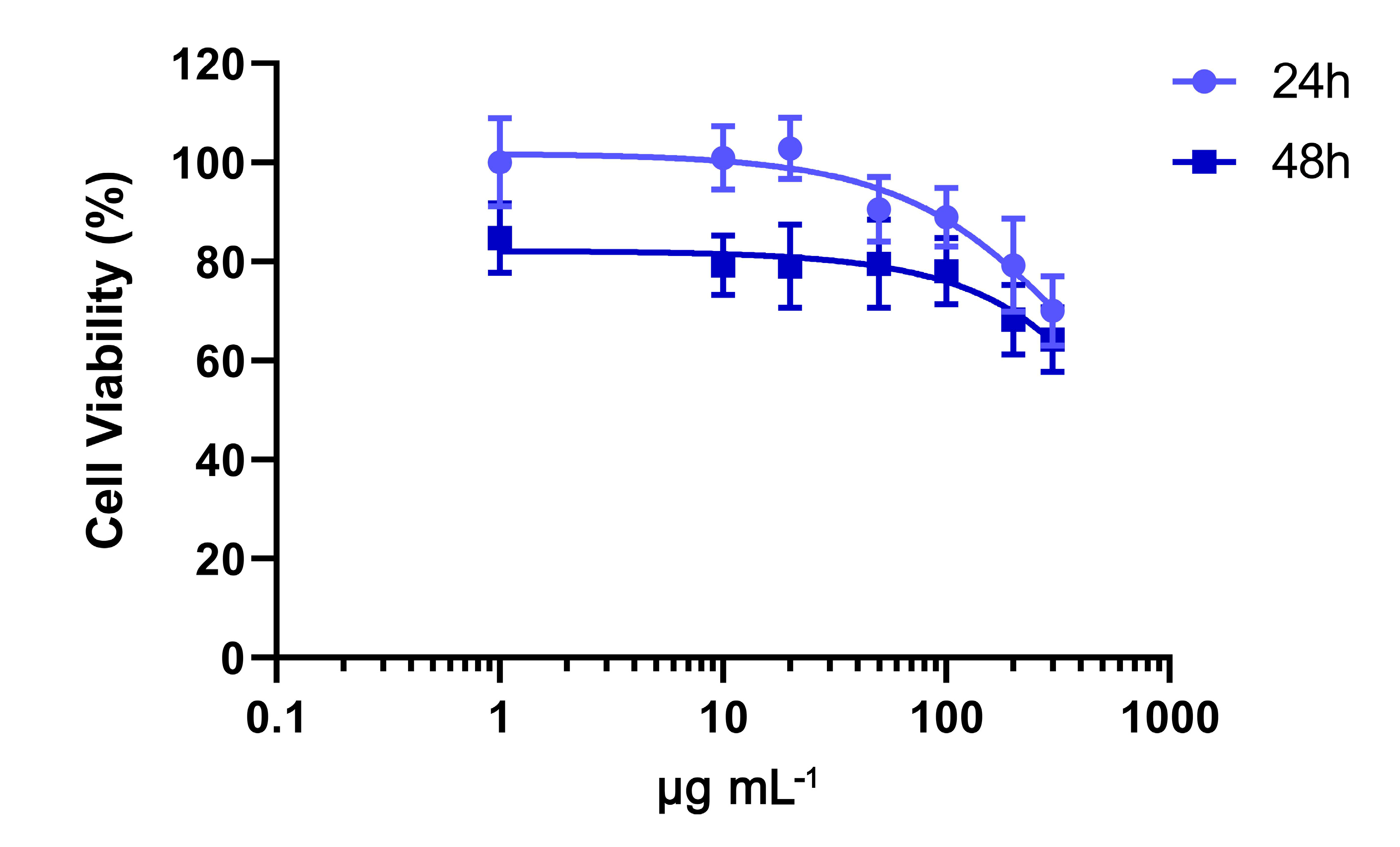
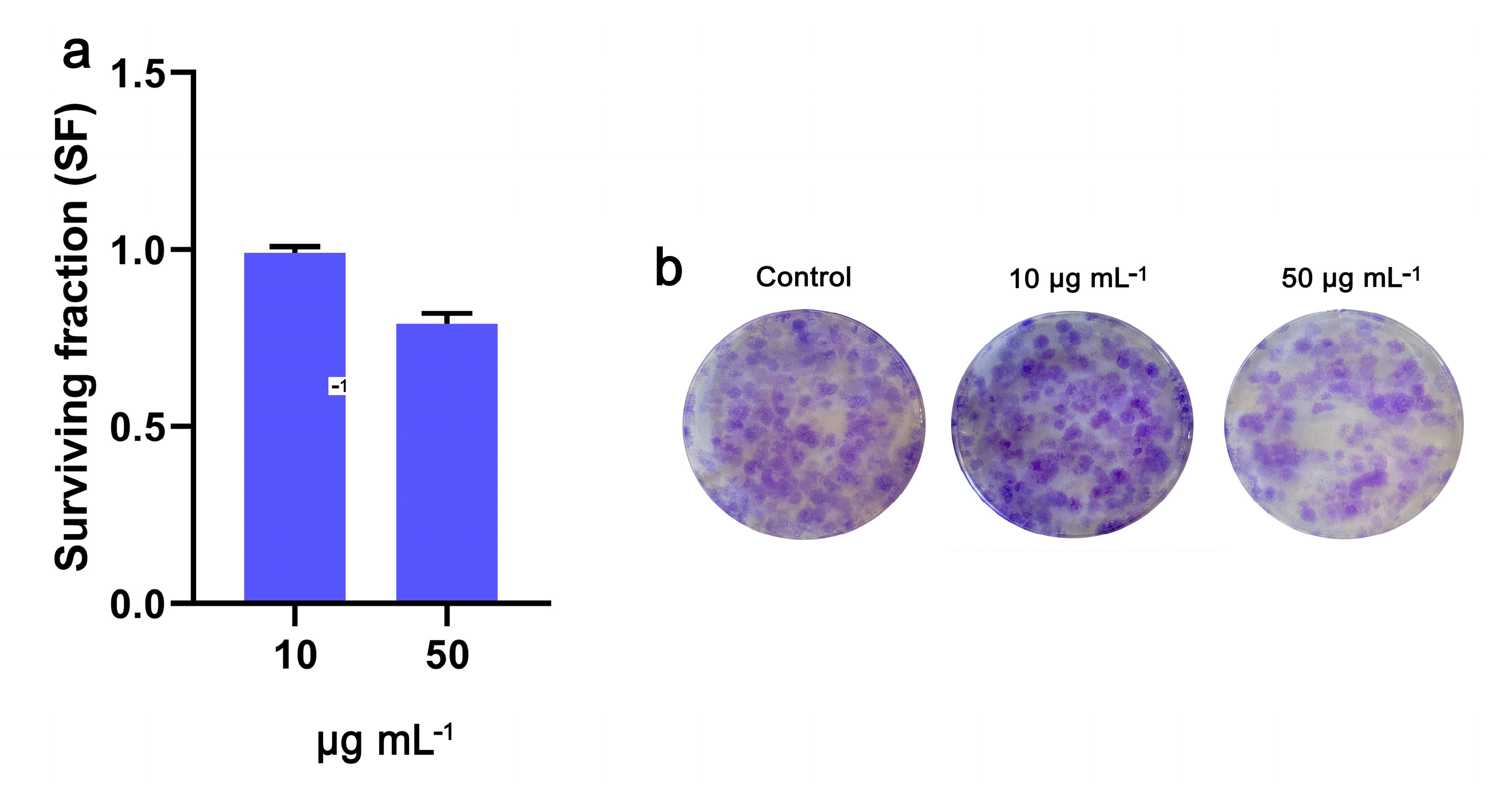
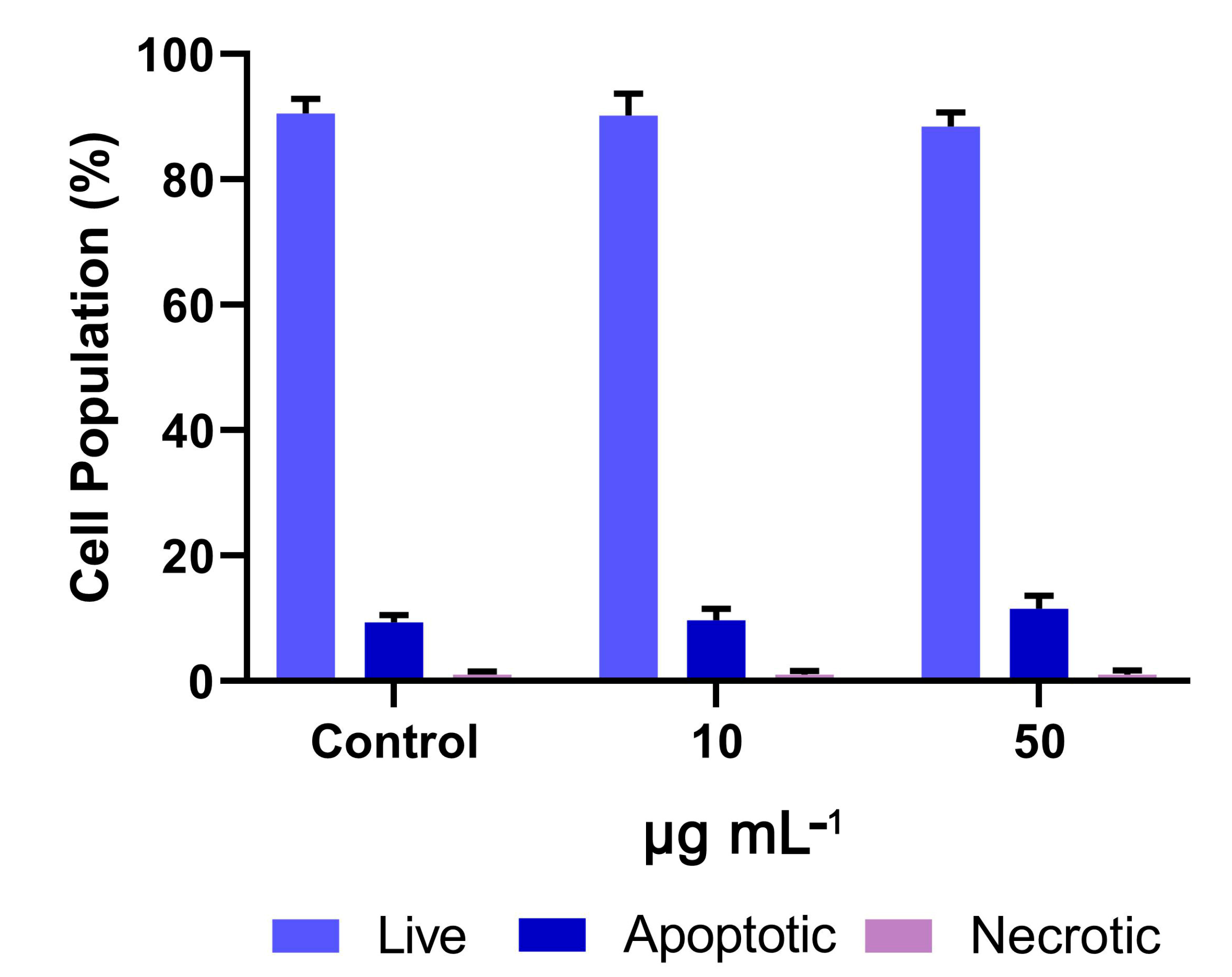
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Details
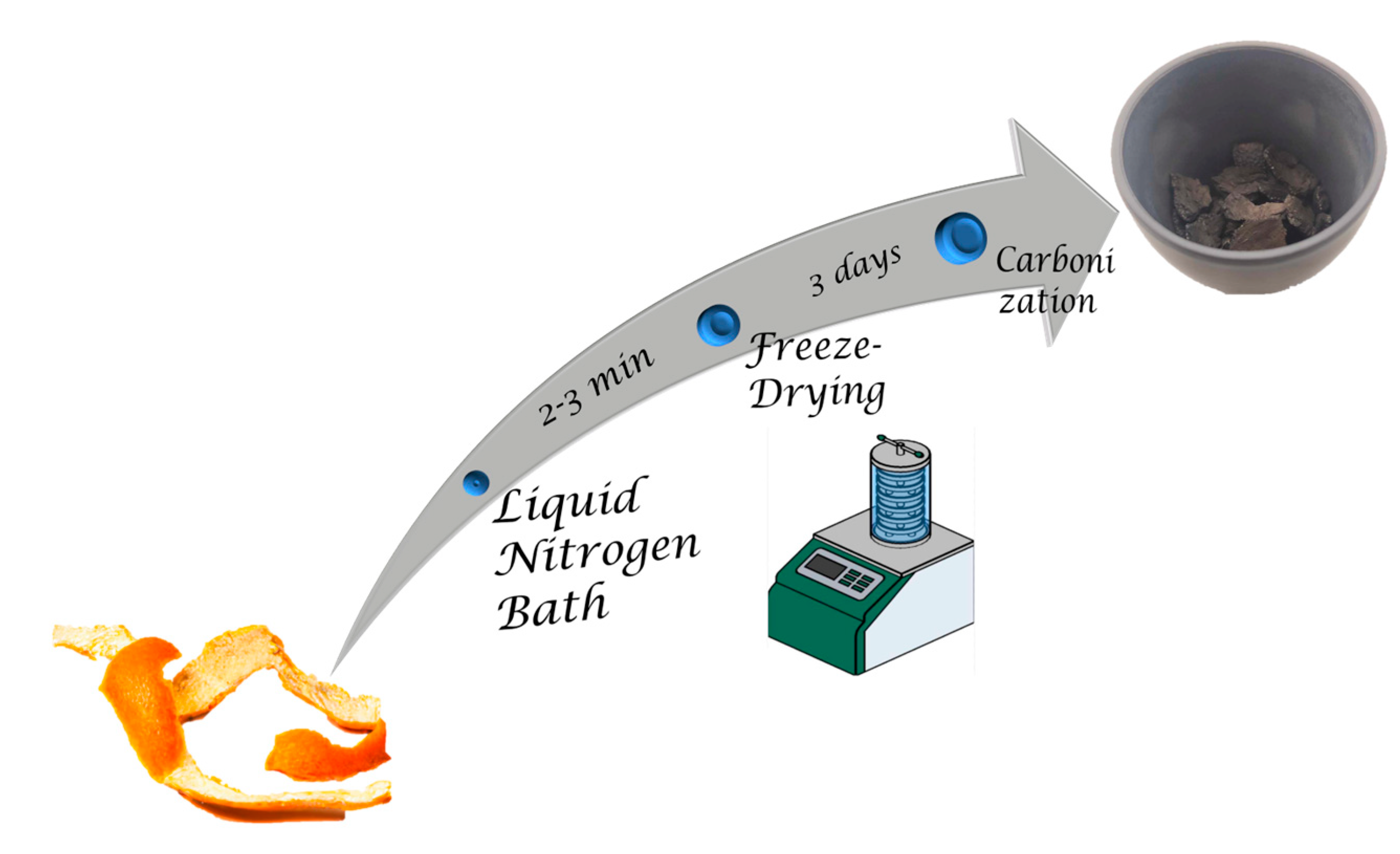
4.2. Preparation of the Porous Carbon from Orange Peels
4.3. Material Characterization
4.4. Cell Line
4.5. Cell Viability Assay
4.6. Ability of Cells to Form Colonies (Clonogenic Assay)
4.7. Flow Cytometry
4.7.1. Reactive Oxygen Species Formation
4.7.2. Detection of Apoptosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.-L.; Zhang, H.; Shao, L.-M.; Lü, F.; He, P.-J. Preparation and Application of Hierarchical Porous Carbon Materials from Waste and Biomass: A Review. Waste Biomass Valorization 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Ultrathin graphene-like 2D porous carbon nanosheets and its excellent capacitance retention for supercapacitor. J. Ind. Eng. Chem. 2018, 68, 257–266. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.; Wu, J.; Huang, J. Celery-derived porous carbon materials for superior performance supercapacitors. Nanoscale Adv. 2021, 3, 5363–5372. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Q.; Wang, J.; Zhao, Y. Recent insights in synthesis and energy storage applications of porous carbon derived from biomass waste: A review. Int. J. Hydrogen Energy 2022, 47, 39338–39363. [Google Scholar] [CrossRef]
- Quan, H.; Tao, W.; Wang, Y.; Chen, D. Enhanced supercapacitor performance of Camellia oleifera shell derived hierarchical porous carbon by carbon quantum dots. J. Energy Storage 2022, 55, 105573. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X. Recent Advances in Porous Carbon Materials for Electrochemical Energy Storage. Chem. Asian J. 2018, 13, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.K.R.; Jagadeesan, D.; Kulkarni, C.; Kamath, A.; Datta, R.; Eswaramoorthy, M. Observation of Pore-Switching Behavior in Porous Layered Carbon through a Mesoscale Order–Disorder Transformation. Angew. Chem. Int. Ed. 2011, 50, 3929–3933. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Zhong, K.; Li, M.; Yang, Y.; Zhang, H.; Zhang, B.; Tang, J.; Yan, J.; Su, M.; Yang, Z. Nitrogen-doped biochar derived from watermelon rind as oxygen reduction catalyst in air cathode microbial fuel cells. Appl. Energy 2019, 242, 516–525. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.J.; Mirihana, S.; Gunathilaka, H. Porous Carbon Materials in Biomedical Applications. Biomed. J. Sci. Tech. Res. 2019, 22, 16905–16907. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Ma, Y.; Tian, J.; Li, L.; Kong, L.; Liu, S.; Guo, K.; Chen, X. Interconnected hierarchical porous carbon synthesized from freeze-dried celery for supercapacitor with high performance. Int. J. Energy Res. 2021, 45, 9058–9068. [Google Scholar] [CrossRef]
- UNEP. Food Waste Index Report 2021; UNEP: Nairobi, Kenya, 2021. [Google Scholar]
- Kamińska, A.; Miądlicki, P.; Kiełbasa, K.; Kujbida, M.; Sreńscek-Nazzal, J.; Wróbel, R.J.; Wróblewska, A. Activated Carbons Obtained from Orange Peels, Coffee Grounds, and Sunflower Husks—Comparison of Physicochemical Properties and Activity in the Alpha-Pinene Isomerization Process. Materials 2021, 14, 7448. [Google Scholar] [CrossRef]
- Saha, D.; Taylor, B.; Alexander, N.; Joyce, D.F.; Faux, G.I.; Lin, Y.; Shteyn, V.; Orkoulas, G. One-step conversion of agro-wastes to nanoporous carbons: Role in separation of greenhouse gases. Bioresour. Technol. 2018, 256, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O.; Eyo, E.U. Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants. Materials 2023, 16, 1092. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Sudhan, N.; Karnan, M.; Sathish, M. Orange Peel Derived Activated Carbon for Fabrication of High-Energy and High-Rate Supercapacitors. ChemistrySelect 2017, 2, 11384–11392. [Google Scholar] [CrossRef]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, Y. One-pot synthesis of biomass-derived porous carbons for multipurpose energy applications. J. Mater. Chem. A 2024, 12, 6211–6242. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Hao, R.; Wang, Y. Synthesis and applications of biomass-derived porous carbon materials in energy utilization and environmental remediation. Chemosphere 2023, 339, 139635. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.C.; Brewer, B.J. AMORPH: A statistical program for characterizing amorphous materials by X-ray diffraction. Comput. Geosci. 2018, 120, 21–31. [Google Scholar] [CrossRef]
- Abdulsalam, J.; Mulopo, J.; Oboirien, B.; Bada, S.; Falcon, R. Experimental evaluation of activated carbon derived from South Africa discard coal for natural gas storage. Int. J. Coal Sci. Technol. 2019, 6, 459–477. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Xu, C.; Sun, J.; Gao, L. Facile one-step hydrazine-assisted solvothermal synthesis of nitrogen-doped reduced graphene oxide: Reduction effect and mechanisms. RSC Adv. 2013, 3, 1194–1200. [Google Scholar] [CrossRef]
- Milev, A.; Wilson, M.; Kannangara, G.S.K.; Tran, N. X-ray diffraction line profile analysis of nanocrystalline graphite. Mater. Chem. Phys. 2008, 111, 346–350. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- do Amaral Junior, M.A.; Matsushima, J.T.; Rezende, M.C.; Gonçalves, E.S.; Marcuzzo, J.S.; Baldan, M.R. Production and Characterization of Activated Carbon Fiber from Textile PAN Fiber. J. Aerosp. Technol. Manag. 2017, 9, 423–430. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]
- Zafar, Z.; Ni, Z.H.; Wu, X.; Shi, Z.X.; Nan, H.Y.; Bai, J.; Sun, L.T. Evolution of Raman spectra in nitrogen doped graphene. Carbon 2013, 61, 57–62. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Madito, M.J. Correlation of the Graphene Fermi-Level Shift and the Enhanced Electrochemical Performance of Graphene-Manganese Phosphate for Hybrid Supercapacitors: Raman Spectroscopy Analysis. ACS Appl. Mater. Interfaces 2021, 13, 37014–37026. [Google Scholar] [CrossRef]
- Zygouri, P.; Spyrou, K.; Mitsari, E.; Barrio, M.; Macovez, R.; Patila, M.; Stamatis, H.; Verginadis, I.I.; Velalopoulou, A.P.; Evangelou, A.M.; et al. A facile approach to hydrophilic oxidized fullerenes and their derivatives as cytotoxic agents and supports for nanobiocatalytic systems. Sci. Rep. 2020, 10, 8244. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1,3-Diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. CrystEngComm 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Saraya, M.E.; Rokbaa, H.H. Preparation of Vaterite Calcium Carbonate in the Form of Spherical Nano-size Particles with the Aid of Polycarboxylate Superplasticizer as a Capping Agent. Am. J. Nanomater. 2016, 4, 44–51. [Google Scholar]
- Kumar, M.P.; Kesavan, T.; Kalita, G.; Ragupathy, P.; Narayanan, T.N.; Pattanayak, D.K. On the large capacitance of nitrogen doped graphene derived by a facile route. RSC Adv. 2014, 4, 38689–38697. [Google Scholar] [CrossRef]
- Zygouri, P.; Athinodorou, A.M.; Spyrou, K.; Simos, Y.V.; Subrati, M.; Asimakopoulos, G.; Vasilopoulos, K.C.; Vezyraki, P.; Peschos, D.; Tsamis, K.; et al. Oxidized-Multiwalled Carbon Nanotubes as Non-Toxic Nanocarriers for Hydroxytyrosol Delivery in Cells. Nanomaterials 2023, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Zygouri, P.; Spyrou, K.; Papayannis, D.K.; Asimakopoulos, G.; Dounousi, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Comparative Study of Various Graphene Oxide Structures as Efficient Drug Release Systems for Ibuprofen. AppliedChem 2022, 2, 93–105. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Thommes, M. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Chem. Int. 2016, 38, 25. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X.; Miao, Y.; Gao, B.; Shi, Y.; Zhao, J.; Tan, S.H. Eggplant biomass based porous carbon for fast and efficient dye adsorption from wastewater. Ind. Crops Prod. 2022, 187, 115510. [Google Scholar] [CrossRef]
- Li, J.; Zan, G.; Wu, Q. Nitrogen and sulfur self-doped porous carbon from brussel sprouts as electrode materials for high stable supercapacitors. RSC Adv. 2016, 6, 57464–57472. [Google Scholar] [CrossRef]
- Liu, J.; Min, S.; Wang, F.; Zhang, Z. Biomass-derived three-dimensional porous carbon membrane electrode for high-performance aqueous supercapacitors: An alternative of powdery carbon materials. J. Power Sources 2020, 466, 228347. [Google Scholar] [CrossRef]
- Lu, S.; Yang, W.; Zhou, M.; Qiu, L.; Tao, B.; Zhao, Q.; Wang, X.; Zhang, L.; Xie, Q.; Ruan, Y. Nitrogen- and oxygen-doped carbon with abundant micropores derived from biomass waste for all-solid-state flexible supercapacitors. J. Colloid Interface Sci. 2022, 610, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Alothaid, H. Evaluation of cytotoxicity, oxidative stress and organ-specific effects of activated carbon from Al-Baha date palm kernels. Saudi J. Biol. Sci. 2022, 29, 103387. [Google Scholar] [CrossRef]
- Magno, L.M.; Hinds, D.T.; Duffy, P.; Yadav, R.B.; Ward, A.D.; Botchway, S.W.; Colavita, P.E.; Quinn, S.J. Porous Carbon Microparticles as Vehicles for the Intracellular Delivery of Molecules. Front. Chem. 2020, 8, 576175. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Heldt, C.L.; Gencoglu, M.F.; Vijayaragavan, K.S.; Chen, J.; Saksule, A. A study on the cytotoxicity of carbon-based materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Biniecka, P.; Bugajska, Ż.; Daniluk, K.; Dyjak, S.; Strojny, B.; Kutwin, M.; Wierzbicki, M.; Grodzik, M.; Chwalibog, A. Analysis of the cytotoxicity of hierarchical nanoporous graphenic carbon against human glioblastoma grade IV cells. Int. J. Nanomed. 2017, 12, 3839–3849. [Google Scholar] [CrossRef][Green Version]
- Barnes, L.-M.; Phillips, G.J.; Davies, J.G.; Lloyd, A.W.; Cheek, E.; Tennison, S.R.; Rawlinson, A.P.; Kozynchenko, O.P.; Mikhalovsky, S.V. The cytotoxicity of highly porous medical carbon adsorbents. Carbon 2009, 47, 1887–1895. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Geissmann, Q. OpenCFU, a New Free and Open-Source Software to Count Cell Colonies and Other Circular Objects. PLoS ONE 2013, 8, e54072. [Google Scholar] [CrossRef]
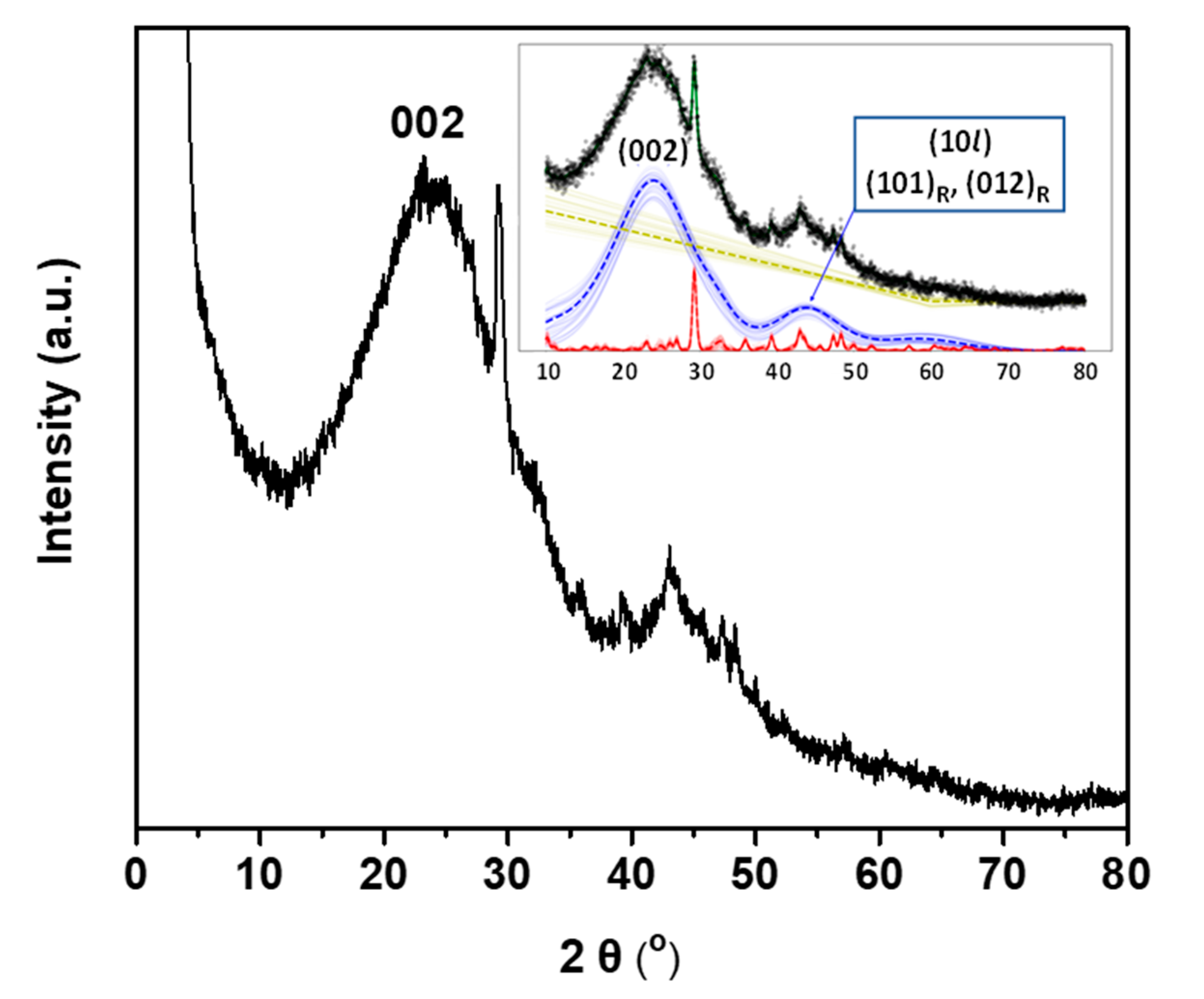
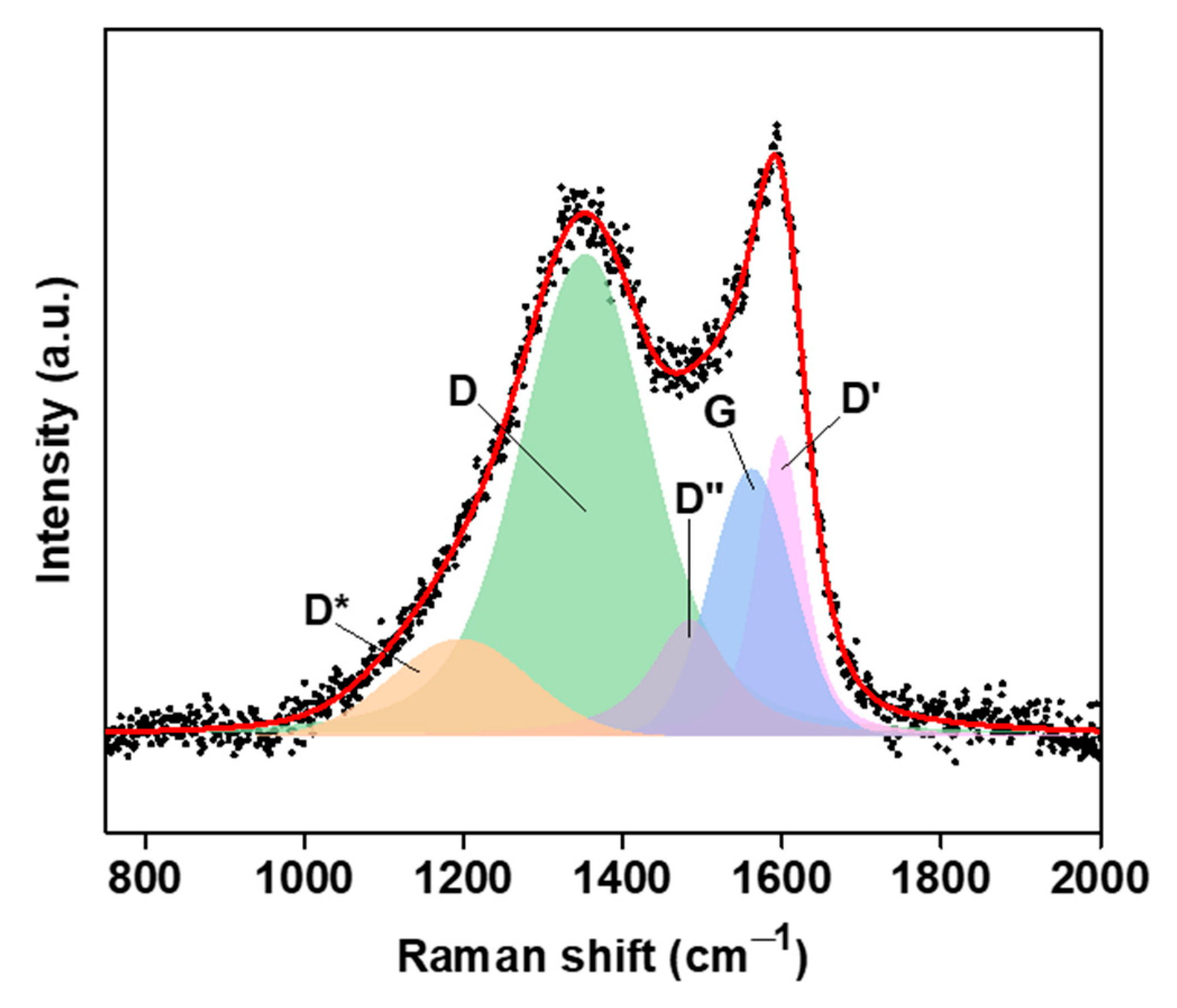
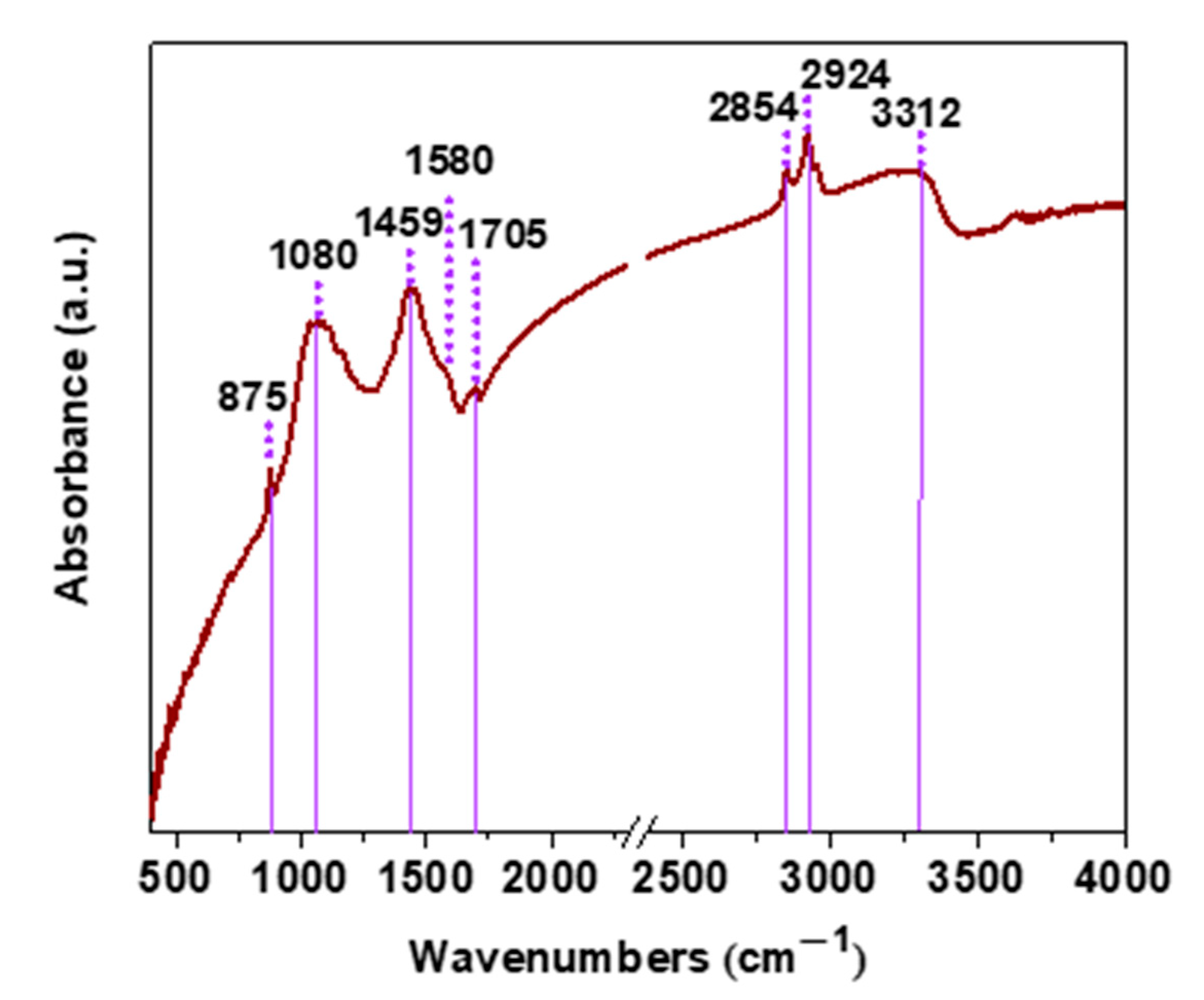
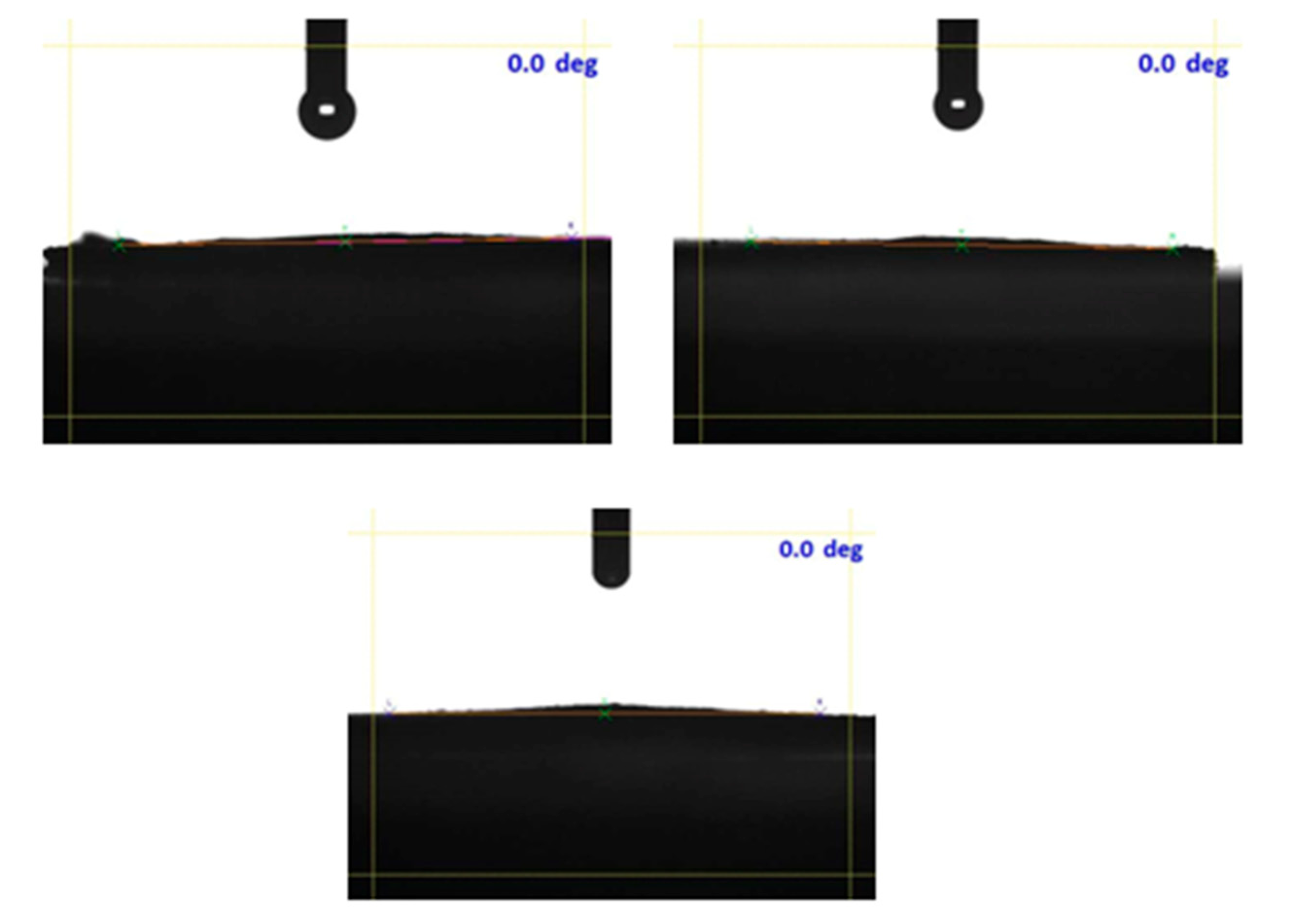


| Parameters | Raman Bands | ||||
|---|---|---|---|---|---|
| D* | D | D″ | G | D′ | |
| 1197 cm−1 | 1354 cm−1 | 1486 cm−1 | 1564 cm−1 | 1600 cm−1 | |
| 26,796 cm−1 | 147,808 cm−1 | 26,026 cm−1 | 44,473 cm−1 | 35,818 cm−1 | |
| BET SSA (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) |
|---|---|---|---|
| 644 | 0.4 | 0.2 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaloudi, A.S.; Zygouri, P.; Spyrou, K.; Athinodorou, A.-M.; Papanikolaou, E.; Subrati, M.; Moschovas, D.; Datta, K.K.R.; Sideratou, Z.; Avgeropoulos, A.; et al. A Strategic Synthesis of Orange Waste-Derived Porous Carbon via a Freeze-Drying Method: Morphological Characterization and Cytocompatibility Evaluation. Molecules 2024, 29, 3967. https://doi.org/10.3390/molecules29163967
Kaloudi AS, Zygouri P, Spyrou K, Athinodorou A-M, Papanikolaou E, Subrati M, Moschovas D, Datta KKR, Sideratou Z, Avgeropoulos A, et al. A Strategic Synthesis of Orange Waste-Derived Porous Carbon via a Freeze-Drying Method: Morphological Characterization and Cytocompatibility Evaluation. Molecules. 2024; 29(16):3967. https://doi.org/10.3390/molecules29163967
Chicago/Turabian StyleKaloudi, Angela S., Panagiota Zygouri, Konstantinos Spyrou, Antrea-Maria Athinodorou, Eirini Papanikolaou, Mohammed Subrati, Dimitrios Moschovas, K. K. R. Datta, Zili Sideratou, Apostolos Avgeropoulos, and et al. 2024. "A Strategic Synthesis of Orange Waste-Derived Porous Carbon via a Freeze-Drying Method: Morphological Characterization and Cytocompatibility Evaluation" Molecules 29, no. 16: 3967. https://doi.org/10.3390/molecules29163967
APA StyleKaloudi, A. S., Zygouri, P., Spyrou, K., Athinodorou, A.-M., Papanikolaou, E., Subrati, M., Moschovas, D., Datta, K. K. R., Sideratou, Z., Avgeropoulos, A., Simos, Y. V., Tsamis, K. I., Peschos, D., Yentekakis, I. V., & Gournis, D. P. (2024). A Strategic Synthesis of Orange Waste-Derived Porous Carbon via a Freeze-Drying Method: Morphological Characterization and Cytocompatibility Evaluation. Molecules, 29(16), 3967. https://doi.org/10.3390/molecules29163967











