Micron-Sized Thiol-Functional Polysilsesquioxane Microspheres with Open and Interconnected Macropores: Effects of the System Composition on the Porous Structure and Particle Size of the Microspheres
Abstract
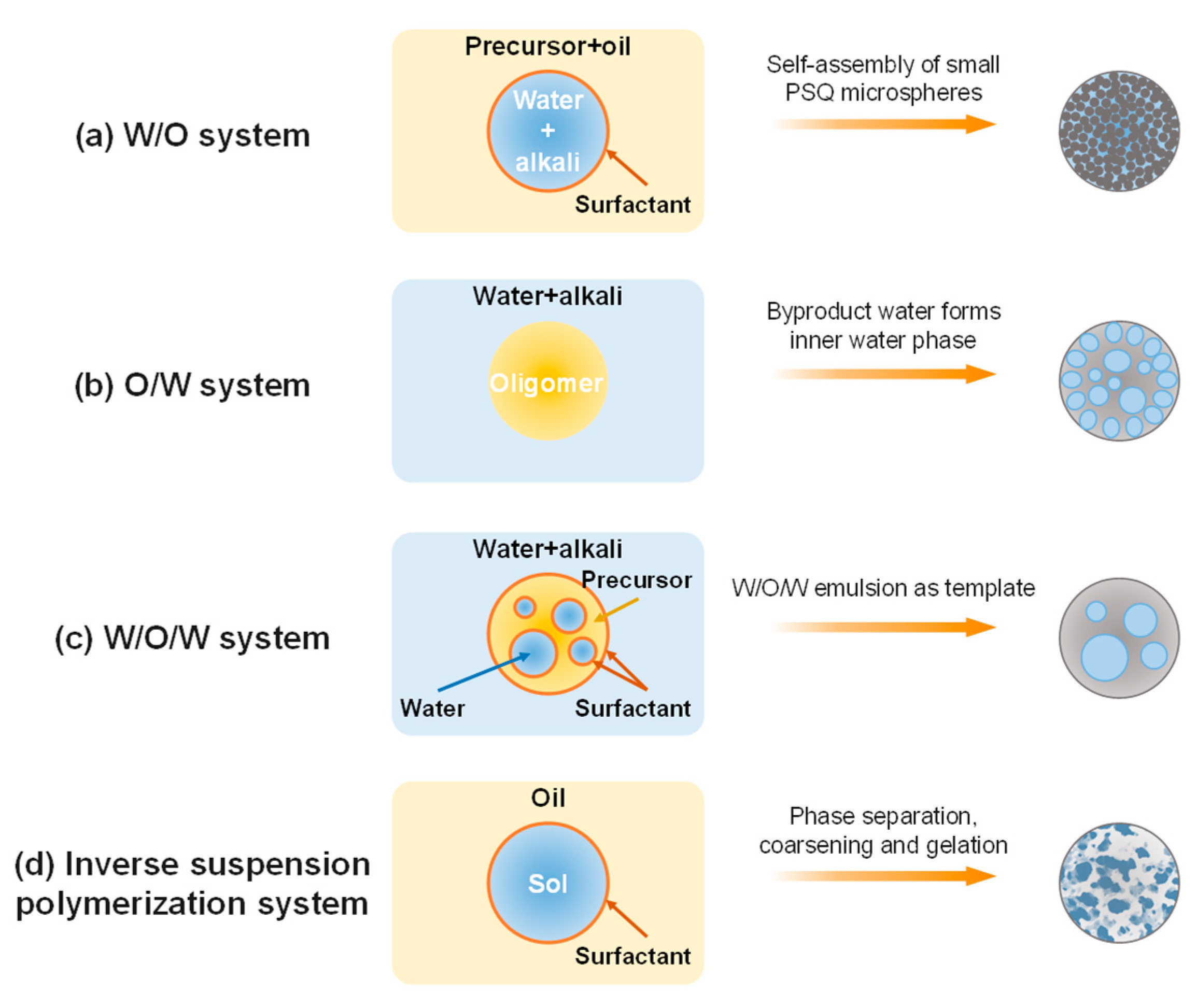
1. Introduction
2. Results and Discussion
2.1. Effects of the Composition of the Sol–Gel Disperse Phase
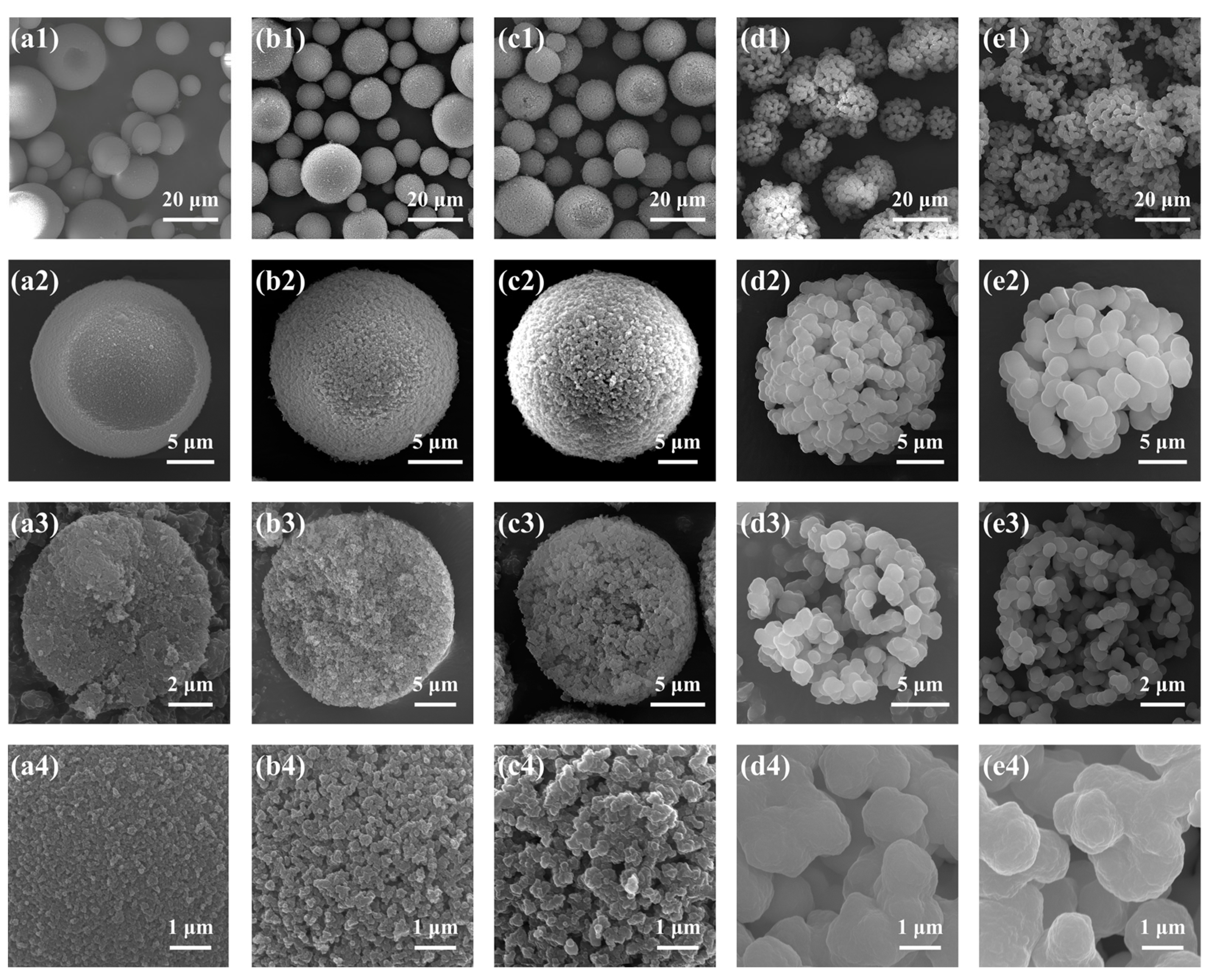
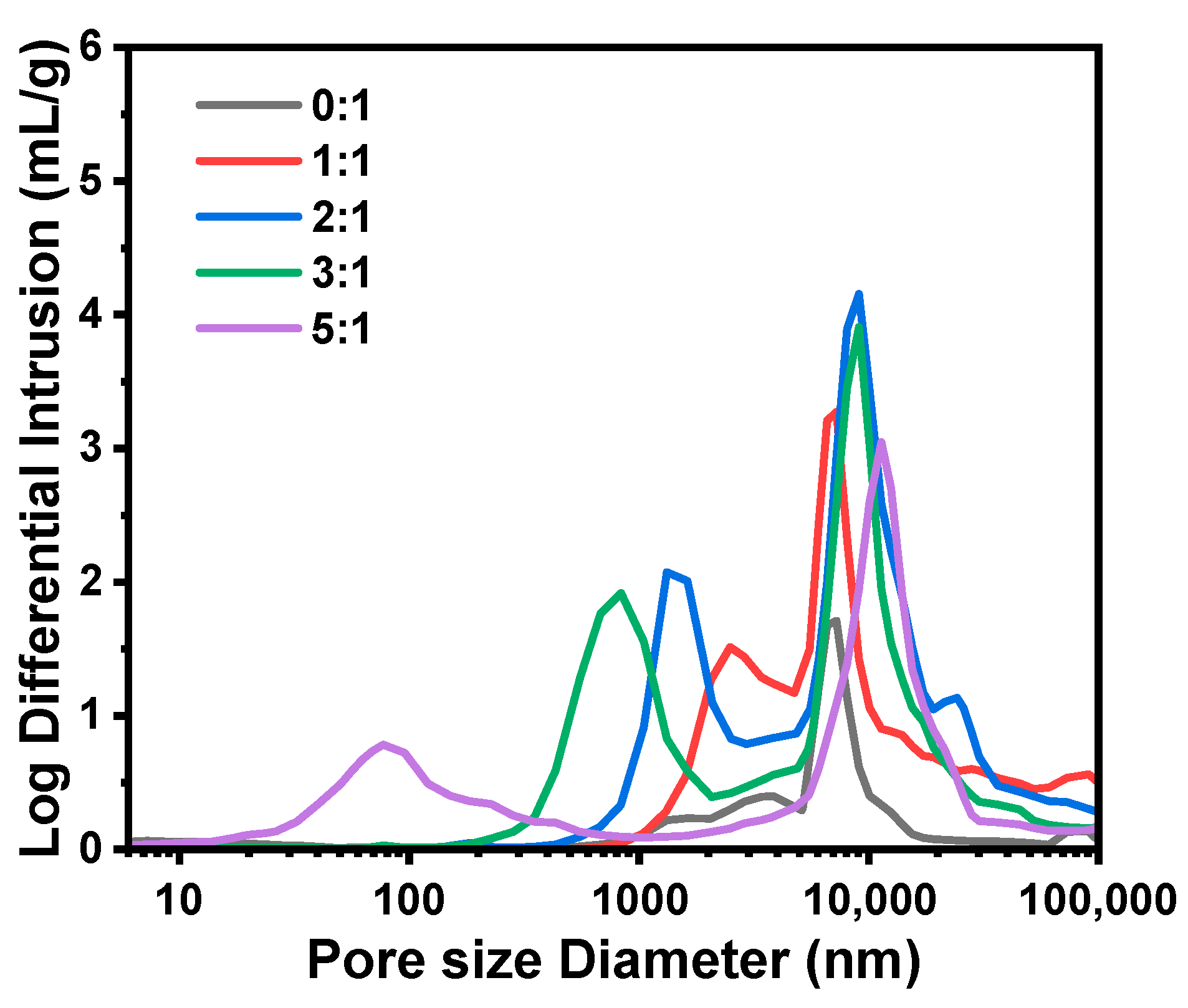
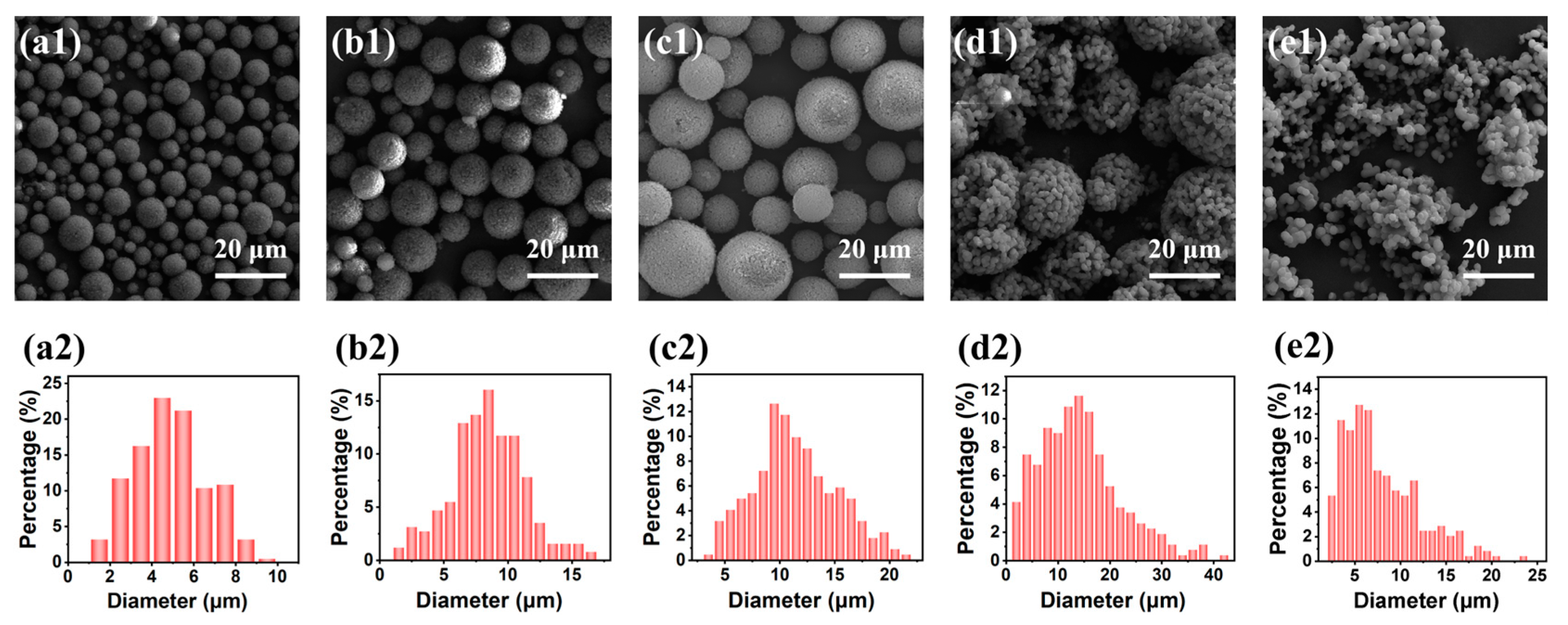
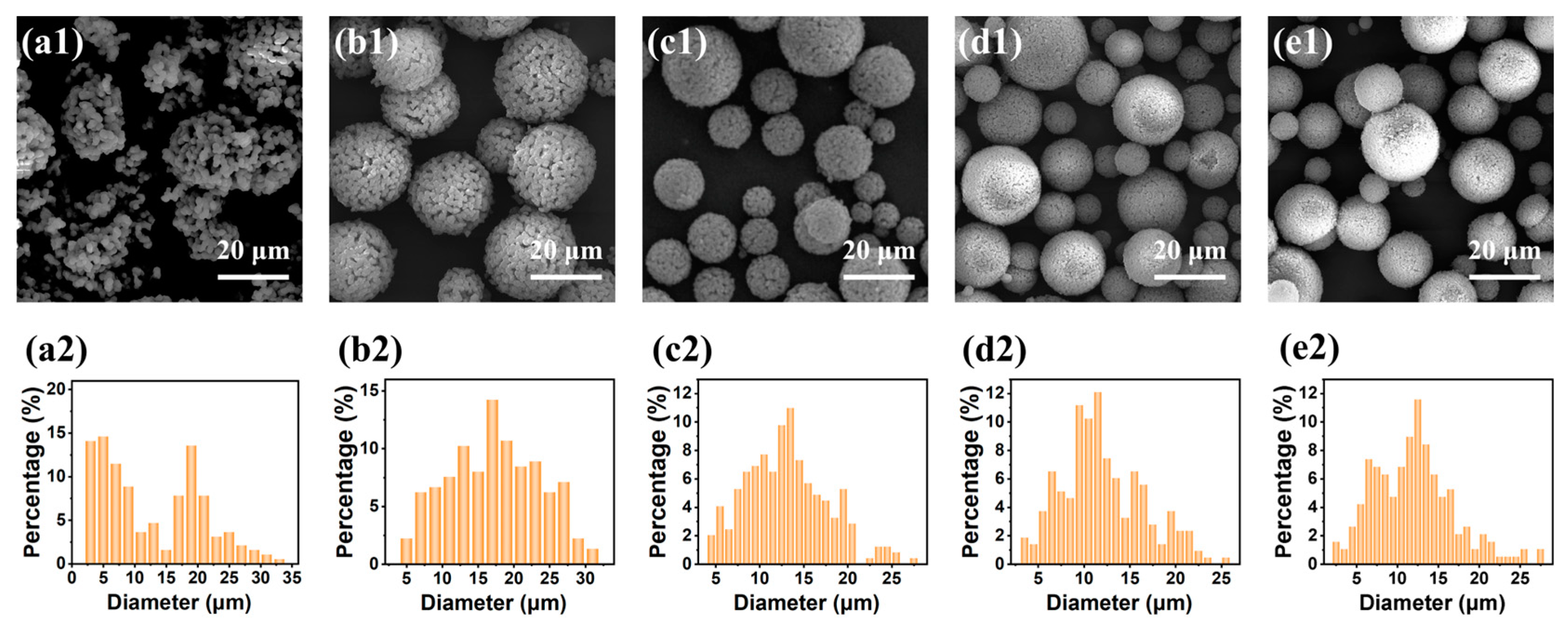
2.1.1. Effect of the Molar Ratio of H2O to Total Precursors (MRH2O/Si)
2.1.2. Effect of the Molar Ratio of MeOH to Total Precursors (MRMeOH/Si)
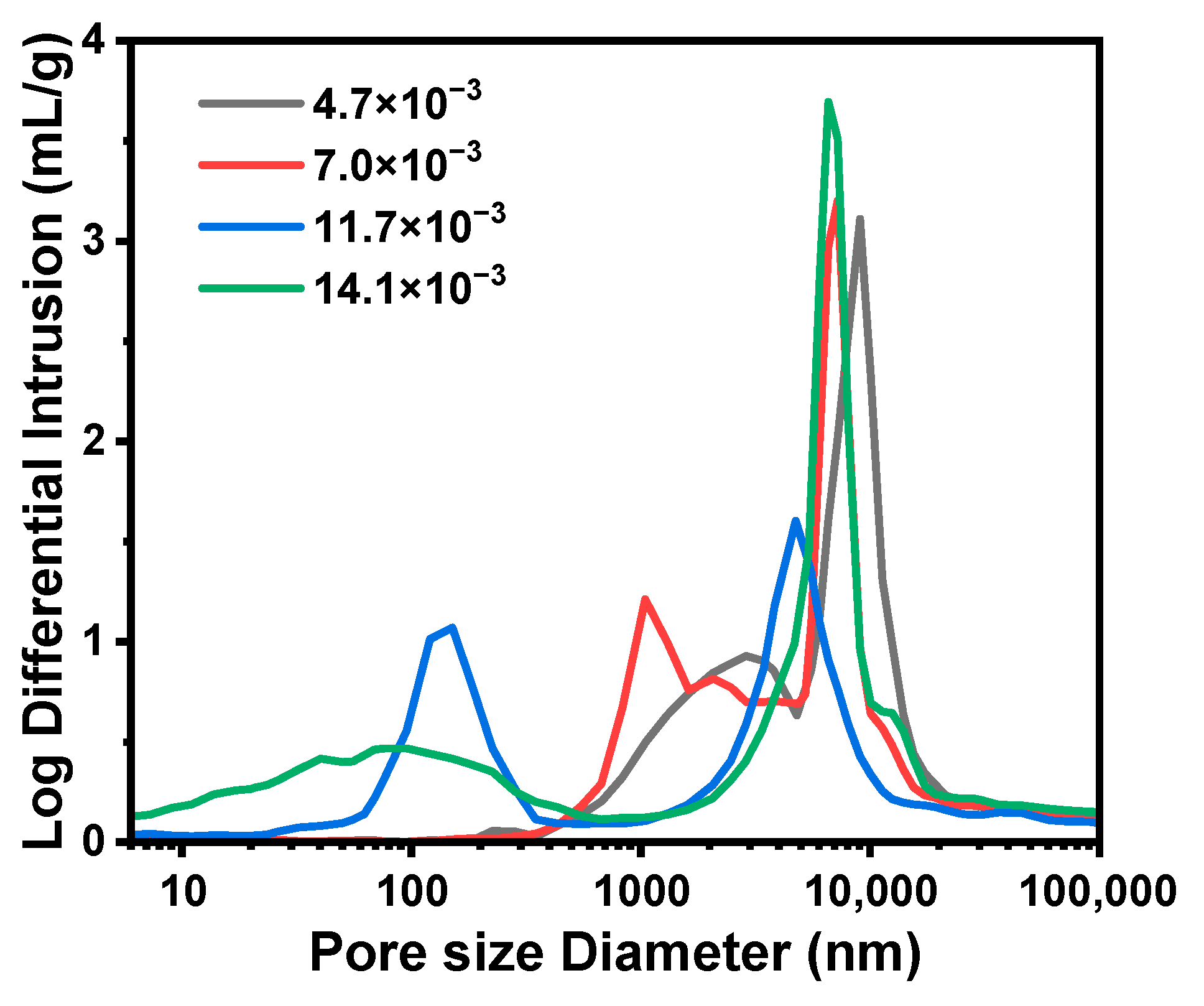
2.1.3. Effect of the Molar Ratio of NH4OH to Total Precursors (MRNH4OH/Si)
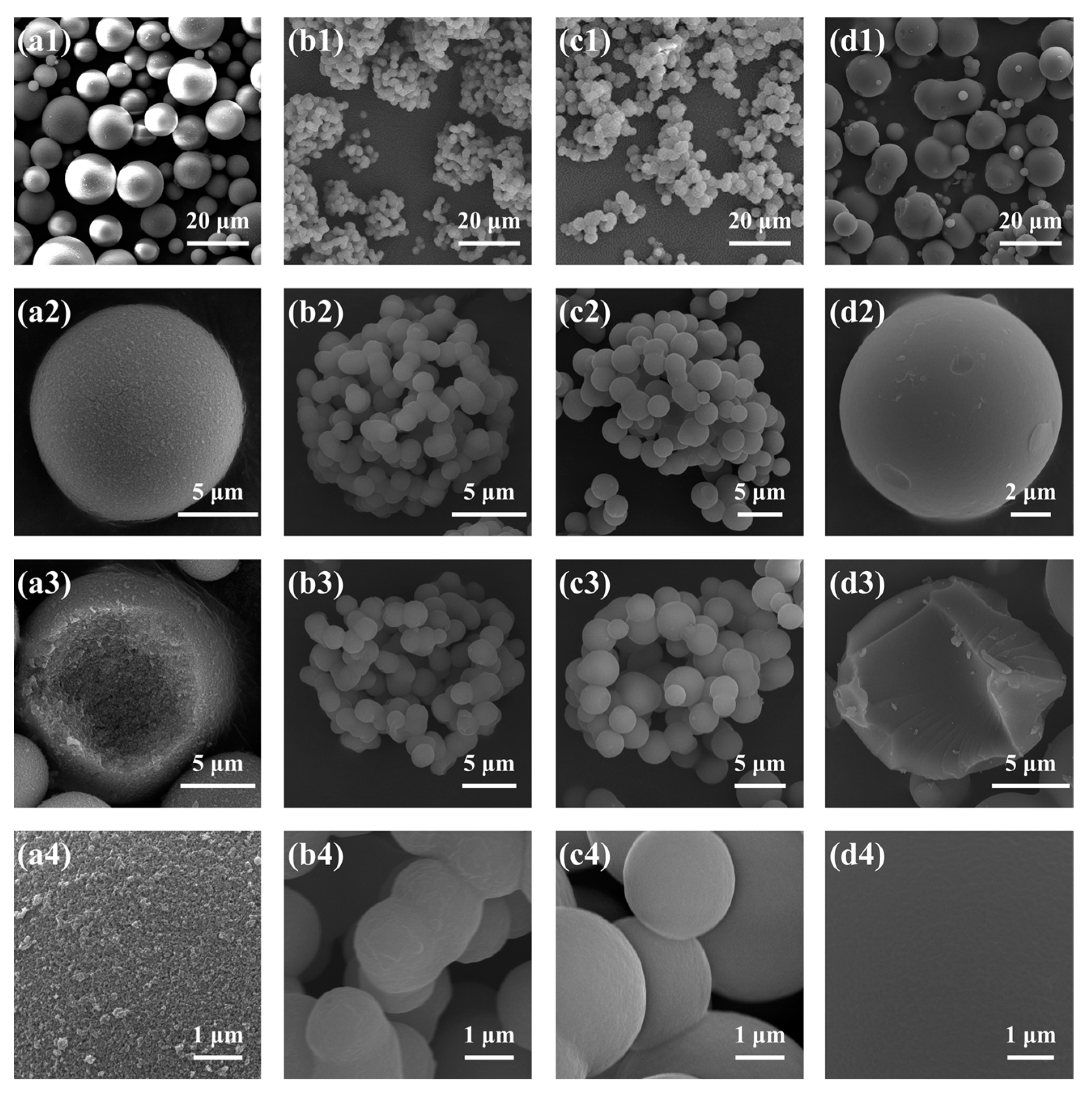
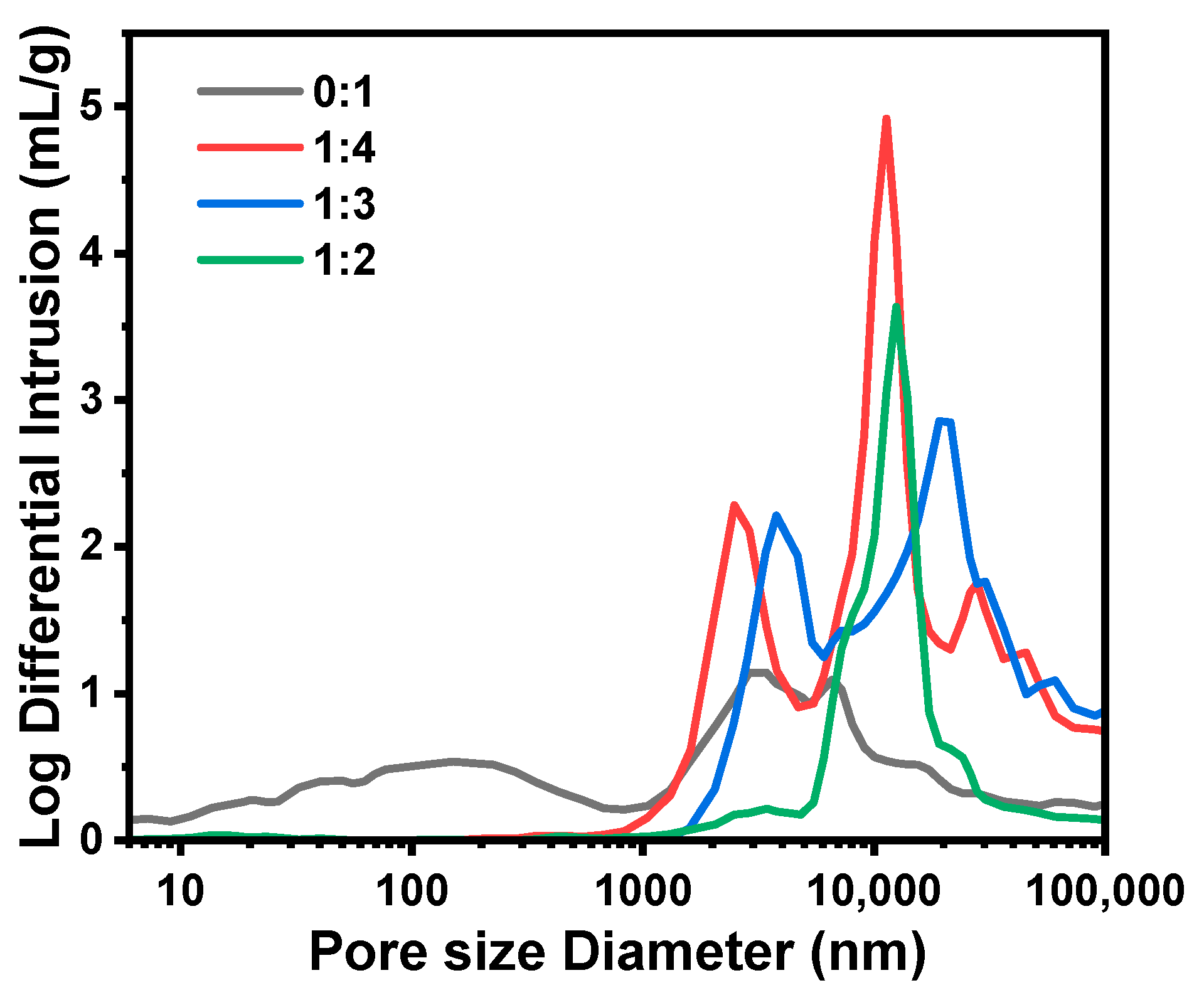
2.1.4. Effect of the MPTMS/MTMS Molar Ratio (MRMPTMS/MTMS)
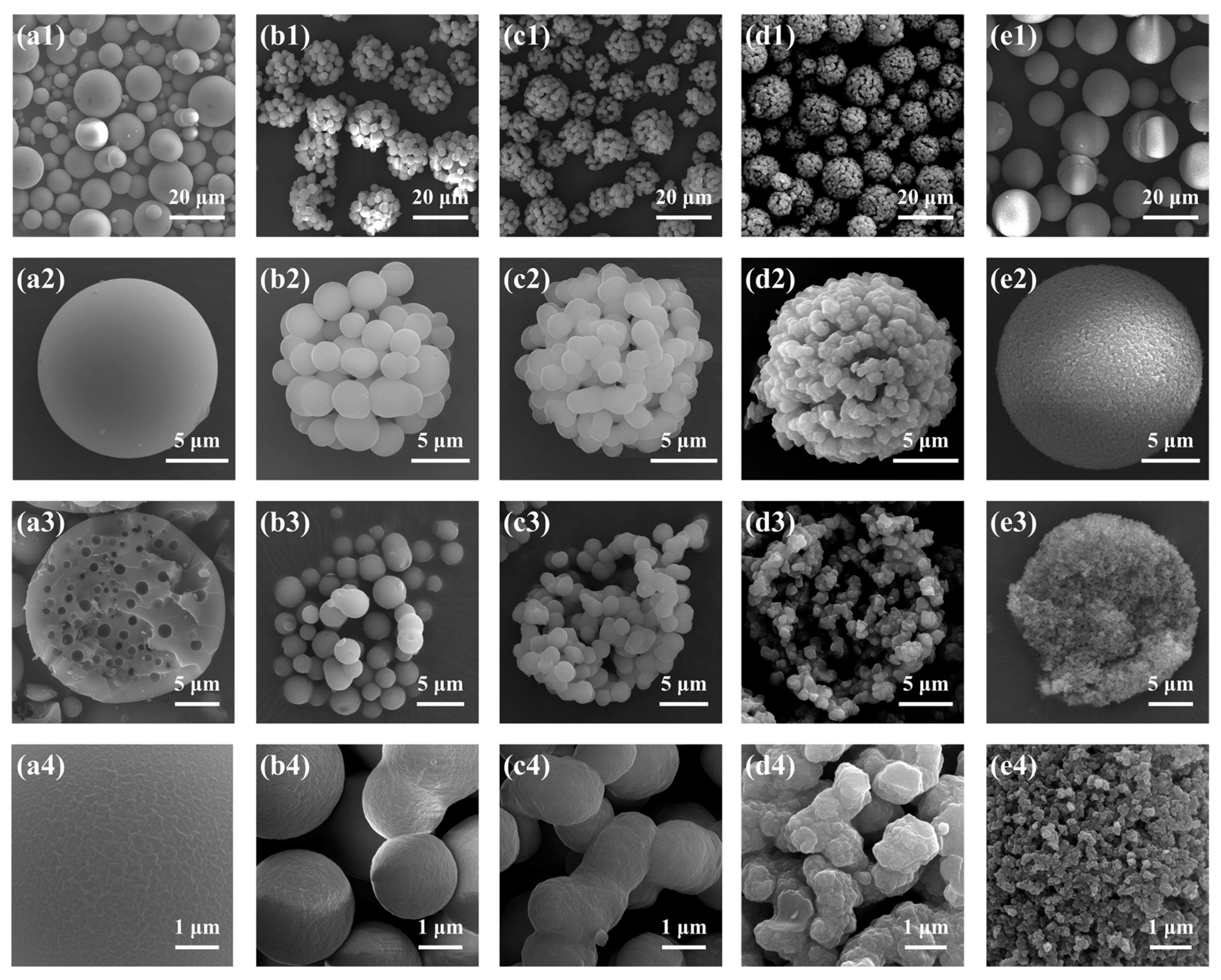
2.2. Effect of the Mass Ratio of Sol–Gel Disperse Phase to Oil Continuous Phase (WRW/O)
2.3. Effect of the Span 80 Mass Content in the Oil Continuous Phase
3. Materials and Methods
3.1. Materials
3.2. Preparation of TMPSQ Microspheres
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, G.Q.; Peng, J.X.; Zhang, Y.Y.; Zhang, H.X.; Lü, J.L.; Fang, Y. One-step synthesis of hydrophobic multicompartment organosilica microspheres with highly interconnected macro-mesopores for the stabilization of liquid marbles with excellent catalysis. Langmuir 2017, 33, 5223–5235. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Lei, S.; Liu, X.F.; Chen, D.Z.; Tang, J.N. Preparation of refractive-index-controlled silicone microspheres and their application in polycarbonate light diffusing materials. Polym.-Plast. Technol. Mater. 2019, 58, 1766–1780. [Google Scholar] [CrossRef]
- Lu, X.; Yin, Q.F.; Xin, Z.; Zhang, Z.Q. Powerful adsorption of silver(I) onto thiol-functionalized polysilsesquioxane microspheres. Chem. Eng. Sci. 2010, 65, 6471–6477. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhang, L.X.; Huo, Z.X.; Chen, L. High stability amino-derived reversed-phase/anion-exchange mixed-mode phase based on polysilsesquioxane microspheres for simultaneous separation of compound drugs. J. Pharm. Biomed. Anal. 2021, 203, 114187. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Vogel, R.; Surawski, P.P.T.; Corrie, S.R.; Rühmann, A.; Trau, M. Biomolecular screening with novel organosilica microspheres. Chem. Commun. 2005, 41, 4783–4785. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Nie, Z.Y.; Gao, R.S.; Kan, C.Y. Facile synthesis of micron-sized thiol-functional polysilsesquioxane microspheres through a one-step sol-gel method. J. Sol-Gel Sci. Technol. 2023, 109, 330–345. [Google Scholar] [CrossRef]
- Matsumoto, T.; Takayama, Y.; Wada, N.; Onoda, H.; Kojima, K.; Yamada, H.; Wakabayashi, H. Acid-free synthesis of poly-organo-siloxane spherical particles using a W/O emulsion. J. Mater. Chem. 2003, 13, 1764–1770. [Google Scholar] [CrossRef]
- Lu, X.; Hou, Y.H.; Zha, J.; Xin, Z. Size-controlled synthesis of monodispersed poly(3-mercaptopropylsilsesquioxane) microspheres by a two-step sol–gel method. Ind. Eng. Chem. Res. 2014, 53, 14659–14663. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Shi, Z.G. Waxberry-like hierarchically porous ethyl-bridged hybrid silica microsphere: A substrate for enzyme catalysis and high-performance liquid chromatography. J. Chromatogr. A 2019, 1587, 79–87. [Google Scholar] [CrossRef]
- Qu, Q.S.; Si, Y.; Xuan, H.; Zhang, K.H.; Chen, X.M.; Ding, Y.; Feng, S.J.; Yu, H.Q.; Abdullah, M.A.; Alamry, K.A. Dendritic core-shell silica spheres with large pore size for separation of biomolecules. J. Chromatogr. A 2018, 1540, 31–37. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.W.; Gao, J.Q.; Wang, R.Q.; Gao, G.F.; Ren, G.M.; Na, S.N.; Hong, M.; Yang, S.H. Spherical binderless 4A/5A zeolite assemblies: Synthesis, characterization, and adsorbent applications. Molecules 2024, 29, 1432. [Google Scholar] [CrossRef] [PubMed]
- Strangfeld, C.; Wiehle, P.; Munsch, S.M. About the dominance of mesopores in physisorption in amorphous materials. Molecules 2021, 26, 7190. [Google Scholar] [CrossRef] [PubMed]
- Vale, M.; Loureiro, M.V.; Ferreira, M.J.; Marques, A.C. Silica-based microspheres with interconnected macroporosity by phase separation. J. Sol-Gel Sci. Technol. 2020, 95, 746–759. [Google Scholar] [CrossRef]
- Steinbach, J.C.; Fait, F.; Mayer, H.A.; Kandelbauer, A. Sol–gel-controlled size and morphology of mesoporous silica microspheres using hard templates. ACS Omega 2023, 8, 30273–30284. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.Q.; Zhu, Q.Y.; Tang, C.W.; Liu, J.W.; Yi, Y.K.; Bai, Q. Synthesis and application of 5 μm monodisperse porous silica microspheres with controllable pore size using polymeric microspheres as templates for the separation of small solutes and proteins by high-performance liquid chromatography. J. Chromatogr. A 2022, 1675, 463165. [Google Scholar] [CrossRef]
- Xia, H.J.; Wan, G.P.; Zhao, J.L.; Liu, J.W.; Bai, Q. Preparation and characterization of monodisperse large-porous silica microspheres as the matrix for protein separation. J. Chromatogr. A 2016, 1471, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M.; Wang, Y.J.; Sun, Y.W.; Luo, G.S.; Dai, Y.Y. Synthesis of micrometer-sized hard silica spheres with uniform mesopore size and textural pores. J. Colloid Interface Sci. 2006, 299, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, Q.; Zhou, H.; Sun, P.; Yuan, Z.; Li, B.; Ding, D.; Shi, A.C.; Chen, T. Budded, mesoporous silica hollow spheres: Hierarchical structure controlled by kinetic self-assembly. Adv. Mater. 2006, 18, 3284–3288. [Google Scholar] [CrossRef]
- Abdullah, M.; Iskandar, F.; Shibamoto, S.; Ogi, T.; Okuyama, K. Preparation of oxide particles with ordered macropores by colloidal templating and spray pyrolysis. Acta Mater. 2004, 52, 5151–5156. [Google Scholar] [CrossRef]
- Min, K.; Choi, C.H.; Kim, M.Y.; Choi, M. Aerosol-assisted controlled packing of silica nanocolloids: Templateless synthesis of mesoporous silicates with structural tunability and complexity. Langmuir 2015, 31, 542–550. [Google Scholar] [CrossRef]
- Shi, Z.G.; Feng, Y.Q. Synthesis and characterization of hierarchically porous silica microspheres with penetrable macropores and tunable mesopores. Microporous Mesoporous Mater. 2008, 116, 701–704. [Google Scholar] [CrossRef]
- Han, C.L.; Ma, L.; Tang, T.Y.; Deng, J.; Luo, G.S. Microdroplet-based synthesis of polymethylsilsesquioxane microspheres with controllable size, surface morphology, and internal structure. Chem. Eng. Sci. 2022, 262, 118054. [Google Scholar] [CrossRef]
- Johnston, A.P.R.; Battersby, B.J.; Lawrie, G.A.; Trau, M. Porous functionalised silica particles: A potential platform for biomolecular screening. Chem. Commun. 2005, 7, 848–850. [Google Scholar] [CrossRef]
- Johnston, A.P.R.; Battersby, B.J.; Lawrie, G.A.; Lambert, L.K.; Trau, M. A mechanism for forming large fluorescent organo-silica particles: potential supports for combinatorial synthesis. Chem. Mater. 2006, 18, 6163–6169. [Google Scholar] [CrossRef]
- Han, L.; Nie, Z.Y.; Gao, R.S.; Jiang, Z.Y.; Kan, C.Y. Micron-sized thiol-functional polysilsesquioxane microspheres with open and interconnected macropores: Preparation, characterization and formation mechanism. Molecules 2024, 29, 1204. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Kanamori, K. Organic-inorganic hybrid poly(silsesquioxane) monoliths with controlled macro- and mesopores. J. Mater. Chem. 2005, 15, 3776–3786. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase-separation in gelling silica organic polymer-solution—Systems containing poly(sodium styrenesulfonate). J. Am. Ceram. Soc. 1991, 74, 2518–2530. [Google Scholar] [CrossRef]
- Cai, W.W.; Yang, H.; Guo, X.Z. A facile one-step route to synthesize titania hollow microspheres with incontinuous multicavities. Chin. Chem. Lett. 2014, 25, 441–446. [Google Scholar] [CrossRef]
- Nakanishi, K. Pore structure control of silica gels based on phase separation. J. Porous Mater. 1997, 4, 67–112. [Google Scholar] [CrossRef]
- Marques, A.C.; Vale, M. Macroporosity control by phase separation in sol-gel derived monoliths and microspheres. Materials 2021, 14, 4247. [Google Scholar] [CrossRef]
- Lu, X.; Hou, Y.; Zha, J.; Xin, Z. Facile synthesis of Rhodamine B-doped poly(3-mercaptopropylsilsesquioxane) fluorescent microspheres with controllable size. Ind. Eng. Chem. Res. 2013, 52, 5880–5886. [Google Scholar] [CrossRef]


| Sample | MRH2O/Si | Water-A b | Water-B b | Dmean | CV | SMIP | Vpore | Ptotal | Dintra | Vintra | Pintra |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (g) | (μm) | (%) | (m2/g) | (mL/g) | (%) | (nm) | (mL/g) | (%) | ||
| A1 | 7:1 | 2.59 | 2.35 | 11.4 | 36.5 | 52.8 | 1.49 | 61.0 | 40 | 0.39 | 29.1 |
| A2 | 8:1 | 3.13 | 2.89 | 11.5 | 37.9 | 35.3 | 1.85 | 63.9 | 56 | 0.54 | 33.9 |
| A3 | 8.5:1 | 3.40 | 3.16 | 11.4 | 33.2 | 19.4 | 2.00 | 66.7 | 183 | 0.55 | 35.2 |
| A4 | 9:1 | 3.67 | 3.43 | 12.6 | 36.9 | 3.6 | 3.07 | 72.3 | 1315 | 0.96 | 45.0 |
| A5 | 10:1 | 4.22 | 3.98 | 13.6 | 42.5 | 2.3 | 3.37 | 74.3 | 2509 | 0.94 | 44.8 |
| Sample | MRMeOH/Si | MeOH | Dmean | CV | SMIP | Vpore | Ptotal | Dintra | Vintra | Pintra |
|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (μm) | (%) | (m2/g) | (mL/g) | (%) | (nm) | (mL/g) | (%) | ||
| B1 | 0:1 | 0.00 | 8.1 | 44.4 | 13.6 | 0.70 | 47.9 | – | – | – |
| B2 | 1:1 | 1.92 | 11.3 | 36.3 | 1.6 | 2.15 | 67.4 | 2490 | 0.73 | 41.2 |
| B3 | 2:1 | 3.85 | 10.3 | 32.7 | 4.4 | 2.81 | 73.9 | 1321 | 0.80 | 44.6 |
| B4 | 3:1 | 5.77 | 10.6 | 35.6 | 13.2 | 2.23 | 70.5 | 833 | 0.74 | 44.3 |
| B5/A3 | 4:1 | 7.69 | 11.4 | 33.2 | 19.4 | 2.00 | 66.7 | 183 | 0.55 | 35.2 |
| B6 | 5:1 | 9.61 | 12.2 | 39.1 | 39.6 | 1.88 | 64.6 | 77 | 0.61 | 37.3 |
| Sample | MRNH4OH/Si | NH4OH (aq, 10 wt.%) | Water-B | Dmean | CV | SMIP | Vpore | Ptotal | Dintra | Vintra | Pintra |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (g) | (μm) | (%) | (m2/g) | (mL/g) | (%) | (nm) | (mL/g) | (%) | ||
| C1 | 4.7 × 10−3:1 | 0.80 | 3.88 | 8.1 | 59.4 | 3.8 | 2.39 | 73.0 | 2882 | 0.93 | 51.3 |
| C2 | 7.0 × 10−3:1 | 1.20 | 3.52 | 9.6 | 39.5 | 8.6 | 1.57 | 60.3 | 1044 | 0.64 | 38.2 |
| C3/B5/A3 | 9.4 × 10−3:1 | 1.60 | 3.16 | 11.4 | 33.2 | 19.4 | 2.00 | 66.7 | 183 | 0.55 | 35.2 |
| C4 | 11.7 × 10−3:1 | 2.00 | 2.80 | 11.5 | 39.5 | 28.6 | 1.38 | 55.2 | 151 | 0.52 | 31.9 |
| C5 | 14.1 × 10−3:1 | 2.40 | 2.44 | 11.9 | 38.3 | 71.3 | 1.80 | 62.6 | 95 | 0.50 | 31.6 |
| Sample | MRMPTMS/MTMS | MTMS | MPTMS | Dmean | CV | SMIP | Vpore | Ptotal | Dintra | Vintra | Pintra |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (g) | (μm) | (%) | (m2/g) | (mL/g) | (%) | (nm) | (mL/g) | (%) | ||
| D1 | 0:1 | 8.17 | 0 | 9.1 | 37.7 | 70.8 | 2.13 | 62.6 | 151 | 0.75 | 37.0 |
| D2/C3/B5/A3 | 1:5 | 6.81 | 1.96 | 11.4 | 33.2 | 19.4 | 2.00 | 66.7 | 183 | 0.55 | 35.2 |
| D3 | 1:4 | 6.54 | 2.36 | 9.8 | 62.3 | 2.1 | 3.28 | 74.7 | 2485 | 0.79 | 41.5 |
| D4 | 1:3 | 6.13 | 2.95 | 12.1 | 48.7 | 1.3 | 2.95 | 74.7 | 3774 | 0.53 | 34.5 |
| D5 | 1:2 | 5.45 | 3.93 | 8.3 | 48.2 | 4.7 | 1.35 | 59.4 | – | – | – |
| Sample | WRW/O | Precursors | Dmean | CV | SMIP | Vpore | Ptotal | Dintra | Vintra | Pintra |
|---|---|---|---|---|---|---|---|---|---|---|
| (mol) | (μm) | (%) | (m2/g) | (mL/g) | (%) | (nm) | (mL/g) | (%) | ||
| E1 | 0.11:1 | 0.02 | 4.9 | 35.7 | 15.9 | 1.24 | 61.3 | 151 | 0.23 | 23.1 |
| E2 | 0.22:1 | 0.04 | 8.4 | 33.7 | 13.3 | 1.44 | 58.6 | 227 | 0.46 | 31.2 |
| E3/D2/C3/B5/A3 | 0.32:1 | 0.06 | 11.4 | 33.2 | 19.4 | 2.00 | 66.7 | 183 | 0.55 | 35.2 |
| E4 | 0.43:1 | 0.08 | 14.4 | 56.0 | 2.8 | 2.92 | 78.0 | 1606 | 0.82 | 49.9 |
| E5 | 0.54:1 | 0.10 | 7.9 | 52.5 | 2.2 | 2.45 | 71.7 | 2883 | 0.60 | 38.1 |
| Sample | Span 80 b | Span 80 | Dmean | CV | SMIP | Vpore | Ptotal | Dintra | Vintra | Pintra |
|---|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | (g) | (μm) | (%) | (m2/g) | (mL/g) | (%) | (nm) | (mL/g) | (%) | |
| F1 | 1 | 0.80 | 12.6 | 61.5 | 2.5 | 3.13 | 79.3 | 2076 | 0.93 | 53.1 |
| F2 | 2 | 1.60 | 17.3 | 36.4 | 4.1 | 2.73 | 77.0 | 1049 | 0.86 | 51.4 |
| F3 | 4 | 3.20 | 13.1 | 35.5 | 9.3 | 2.08 | 69.8 | 434 | 0.70 | 44.0 |
| F4/E3/D2/C3/B5/A3 | 6 | 4.80 | 11.4 | 33.2 | 19.4 | 2.00 | 66.7 | 183 | 0.55 | 35.2 |
| F5 | 8 | 6.40 | 12.1 | 37.5 | 13.3 | 1.86 | 69.9 | 227 | 0.51 | 38.8 |
| F6 | 10 | 8.00 | 12.0 | 41.0 | 19.3 | 1.96 | 69.6 | 183 | 0.56 | 39.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Nie, Z.; Gao, R.; Kan, C. Micron-Sized Thiol-Functional Polysilsesquioxane Microspheres with Open and Interconnected Macropores: Effects of the System Composition on the Porous Structure and Particle Size of the Microspheres. Molecules 2024, 29, 2841. https://doi.org/10.3390/molecules29122841
Han L, Nie Z, Gao R, Kan C. Micron-Sized Thiol-Functional Polysilsesquioxane Microspheres with Open and Interconnected Macropores: Effects of the System Composition on the Porous Structure and Particle Size of the Microspheres. Molecules. 2024; 29(12):2841. https://doi.org/10.3390/molecules29122841
Chicago/Turabian StyleHan, Lu, Zhenyu Nie, Rongsheng Gao, and Chengyou Kan. 2024. "Micron-Sized Thiol-Functional Polysilsesquioxane Microspheres with Open and Interconnected Macropores: Effects of the System Composition on the Porous Structure and Particle Size of the Microspheres" Molecules 29, no. 12: 2841. https://doi.org/10.3390/molecules29122841
APA StyleHan, L., Nie, Z., Gao, R., & Kan, C. (2024). Micron-Sized Thiol-Functional Polysilsesquioxane Microspheres with Open and Interconnected Macropores: Effects of the System Composition on the Porous Structure and Particle Size of the Microspheres. Molecules, 29(12), 2841. https://doi.org/10.3390/molecules29122841







