Hydrogen Production from Methanol Steam Reforming over Fe-Modified Cu/CeO2 Catalysts
Abstract
1. Introduction
2. Results and Discussion
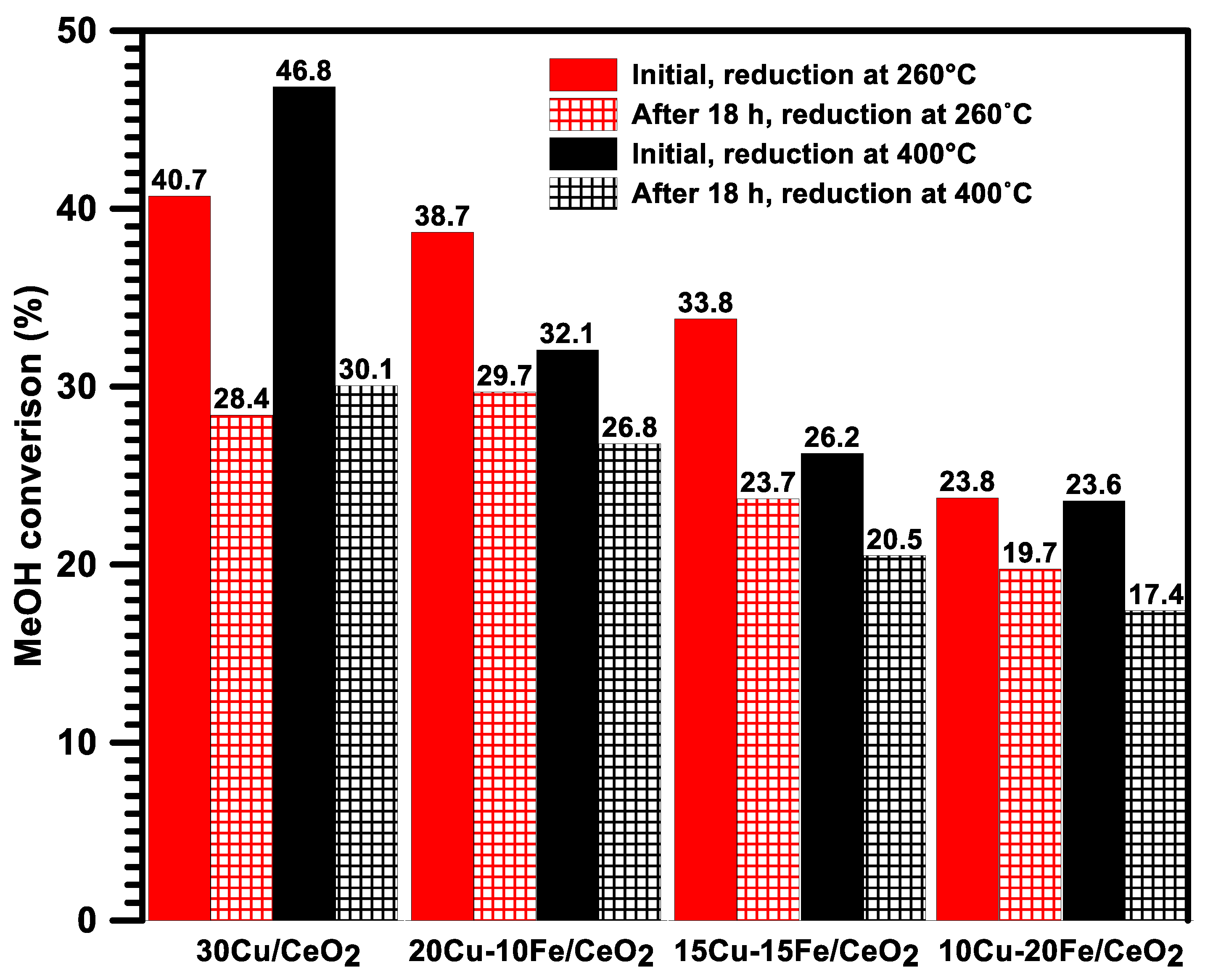
2.1. Optimisation of the Copper Catalyst Composition—Activity and Selectivity of Cu/CeO2 Catalysts in the SRM
2.2. Qualitative and Quantitative Composition of Elements in the Cu-Fe/CeO2 Catalysts
2.3. Specific Surface Area and Pore Size of Catalysts
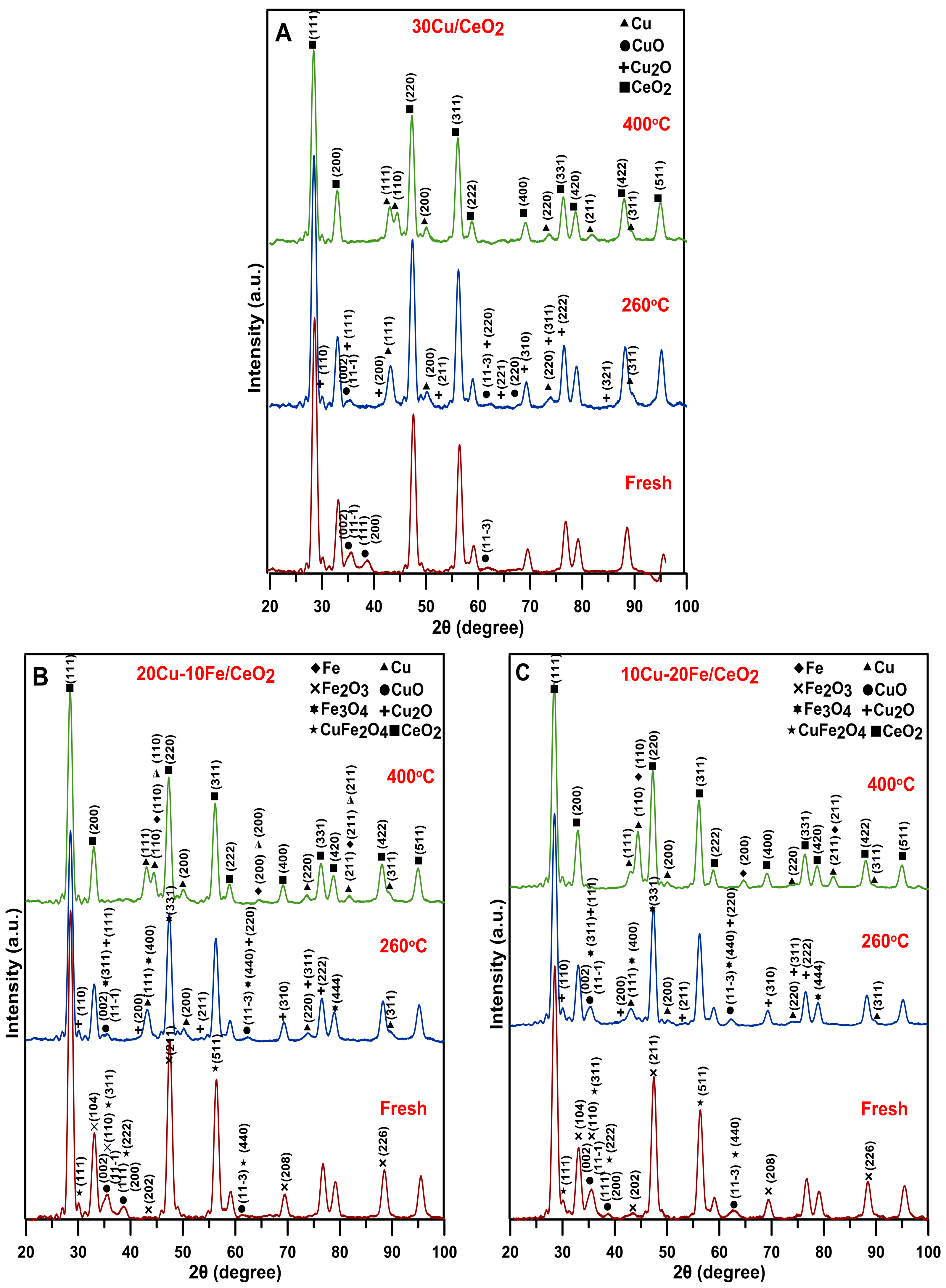
2.4. Phase Composition and Average Crystallite Size
2.4.1. XRD
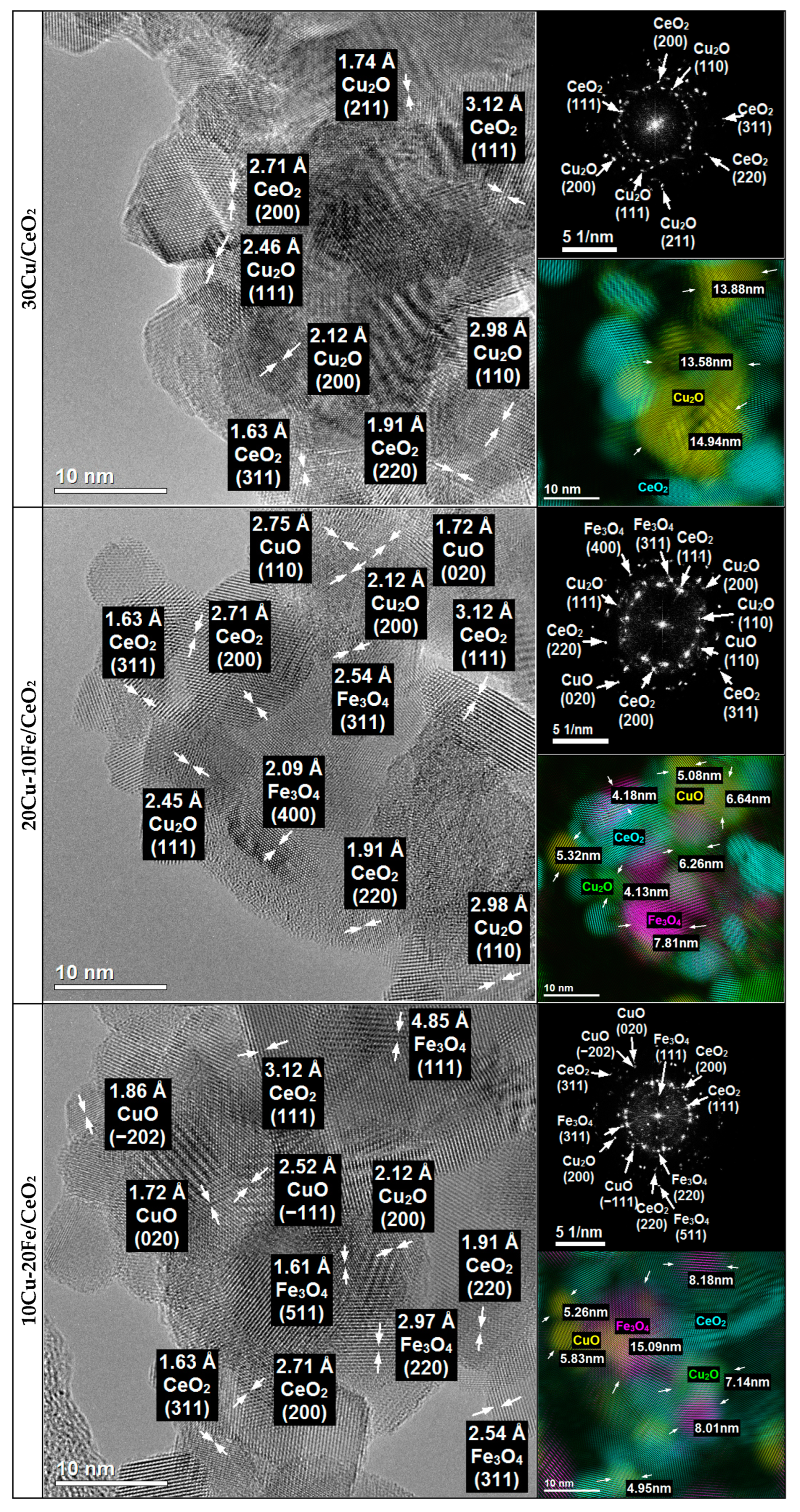
2.4.2. TEM
TEM Studies of Fresh Cu-Fe Catalysts
TEM and STEM-EDS Studies of Reduced Catalysts
2.5. H2-TPR Studies
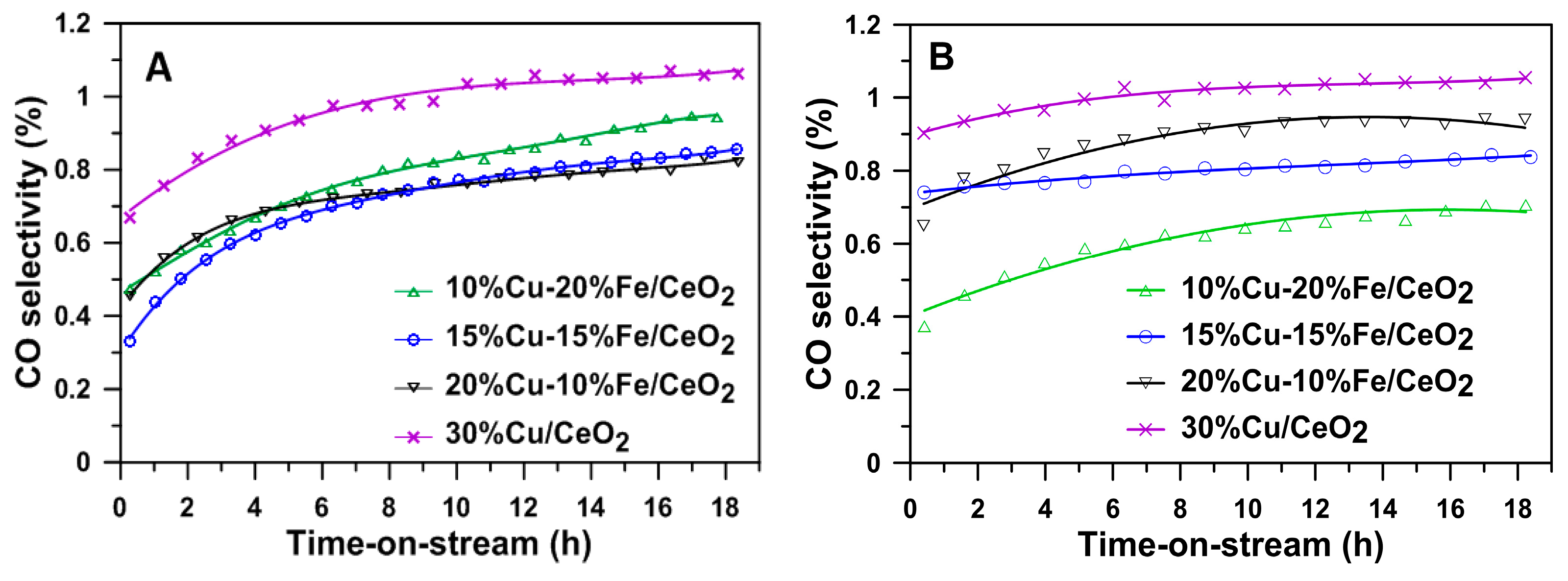
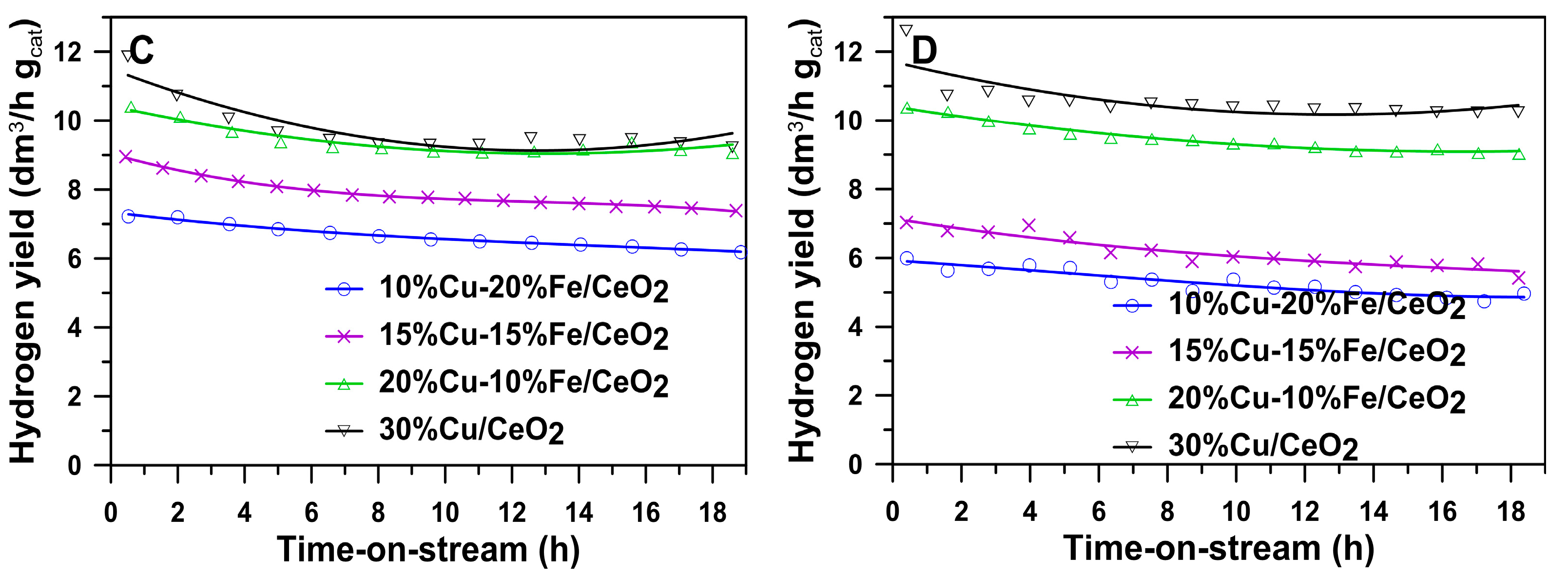
2.6. Activity, Selectivity and Stability of Cu-Fe/CeO2 Catalysts Reduced at 260/400 °C during an Isothermal Test at 260 °C in the SRM
2.7. TEM Studies of Cu-Fe Catalysts after Reaction
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterisation
3.2.1. Low-Temperature Adsorption of Nitrogen
3.2.2. XRF Measurements
3.2.3. XRD Measurements
3.2.4. H2-TPR Measurements
3.2.5. TEM Measurements
3.2.6. Catalytic Activity Measurement
- —the molar concentration of methanol in the reaction mixture, mol%;
- —the molar concentration of methanol in the post-reaction mixture, mol%.
- —the molar concentration of carbon-containing products in the post-reaction mixture, mol%;
- —the number of carbon atoms in the molecules of the post-reaction carbon products.
- —the molar concentration of the hydrogen in the post-reaction mixture, mol%.
- GMe—the initialmolar flow rateof methanol (mol/h);
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl. Catal. A 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Dhar, H.P.; Christner, L.G.; Kush, A.K. Nature of CO Adsorption during H2 oxidation in relation to modeling for CO poisoning of a fuel cell anode. J. Electrochem. Soc. 1987, 134, 3021–3026. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam reforming of methanol for hydrogen production: A critical analysis of catalysis, processes, and scope. Ind. Eng. Chem. Res. 2021, 60, 89–113. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Dagle, V.L.; Halevi, B.; Datye, A.K.; Wang, Y. Influence of ZnO facets on Pd/ZnO catalysts for methanol steam reforming. ACS Catal. 2014, 4, 2379–2386. [Google Scholar] [CrossRef]
- Liu, D.; Men, Y.; Wang, J.; Kolb, G.; Liu, X.; Wang, Y.; Sun, Q. Highly active and durable Pt/In2O3/Al2O3 catalysts in methanol steam reforming. Int. J. Hydrogen Energy 2016, 41, 21990–21999. [Google Scholar] [CrossRef]
- Heggen, M.; Penner, S.; Friedrich, M.; Dunin-Borkowski, R.E.; Armbrüster, M. Formation of ZnO patches on ZnPd/ZnO during methanol steam reforming: A strong metal-support interaction effect? J. Phys. Chem. C 2016, 120, 10460–10465. [Google Scholar] [CrossRef]
- Barbosa, R.L.; Papaefthimiou, V.; Law, Y.T.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Zapf, R.; Kolb, G.; Schlögl, R.; Zafeiratos, S. Methanol steam reforming over indium-promoted Pt/Al2O3 catalyst: Nature of the active surface. J. Phys. Chem. C 2013, 117, 6143–6150. [Google Scholar] [CrossRef]
- Ilinich, O.M.; Liu, Y.; Waterman, E.M.; Farrauto, R.J. Kinetics of methanol steam reforming with a Pd–Zn–Y/CeO2 catalyst under realistic operating conditions of a portable reformer in fuel cell applications. Ind. Eng. Chem. Res. 2012, 52, 638–644. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandăo, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Park, J.E.; Yim, S.-D.; Kim, C.S.; Park, E.D. Steam reforming of methanol over Cu/ZnO/ZrO2/Al2O3 catalyst. Int. J. Hydrogen. Energy 2014, 39, 11517–11527. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Mateos-Pedrero, C.; Boaventura, M.; Sousa, J.; Mendes, A. CuO/ZnO/Ga2O3 catalyst for low temperature MSR reaction: Synthesis, characterization and kinetic model. Appl. Catal. B 2018, 221, 371–379. [Google Scholar] [CrossRef]
- Wang, S.-S.; Su, H.-Y.; Gu, X.-K.; Li, W.-X. Differentiating intrinsic reactivity of copper, copperzinc alloy, and copper/zinc oxide interface for methanol steam reforming by first-principles theory. J. Phys. Chem. C 2017, 121, 21553–21559. [Google Scholar] [CrossRef]
- Lei, Y.; Luo, Y.; Li, X.; Lu, J.; Mei, Z.; Peng, W.; Chen, R.; Chen, K.; Chen, D.; He, D. The role of samarium on Cu/Al2O3 catalyst in the methanol steam reforming for hydrogen production. Catal. Today 2018, 307, 162–168. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Ning, P.; Song, Z.; Liu, X.; Duan, Y. In situ DRIFTS studies on CuO–Fe2O3 catalysts for low temperature selective catalytic oxidation of ammonia to nitrogen. Appl. Surf. Sci. 2017, 419, 733–743. [Google Scholar] [CrossRef]
- Thattarathody, R.; Sheintuch, M. Kinetics and dynamics of methanol steam reforming on CuO/ZnO/alumina catalyst. Appl. Catal. A 2017, 540, 47–56. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Zhang, L.; Li, Y.; Pan, L. Cu supported on ZnAl-LDHs precursor prepared by in situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming. Int. J. Hydrogen Energy 2017, 42, 9930–9937. [Google Scholar] [CrossRef]
- Litt, G.; Almquist, C. An investigation of CuO/Fe2O3 catalysts for the gas-phase oxidation of ethanol. Appl. Catal. B 2009, 90, 10–17. [Google Scholar] [CrossRef]
- Sanches, S.G.; Huertas Flores, J.; da Silva, M.I.P. Influence of aging time on the microstructural characteristics of a Cu/ZnO-based catalyst prepared by homogeneous precipitation for use inmethanol steam reforming. React. Kinet. Mech. Catal. 2017, 121, 473–485. [Google Scholar] [CrossRef]
- da Silva, F.A.; Dancini-Pontes, I.; DeSouza, M.; Fernandes, N.R.C. Kinetics of ethanol steam reforming over Cu–Ni/NbxOy catalyst. React. Kinet. Mech. Catal. 2017, 122, 557–574. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Maiti, S.; Llorca, J.; Dominguez, M.; Colussi, S.; Trovarelli, A.; Priolkar, K.R.; Aquilanti, G.; Gayen, A. Combustion synthesized copper-ion substituted FeAl2O4 (Cu0.1Fe0.9Al2O4): A superior catalyst for methanol steam reforming compared to its impregnated analogue. J. Power Sources 2016, 304, 319–331. [Google Scholar] [CrossRef]
- Pohar, A.; Hočevar, S.; Likozar, B.; Levec, J. Synthesis and characterization of gallium-promoted copper–ceria catalyst and its application for methanol steam reforming in a packed bed reactor. Catal. Today 2015, 256, 358–364. [Google Scholar] [CrossRef]
- Chang, C.-C.; Wang, J.-W.; Chang, C.-T.; Liaw, B.-J.; Chen, Y.-Z. Effect of ZrO2 on steam reforming of methanol over CuO/ZnO/ZrO2/Al2O3 catalysts. Chem. Eng. J. 2012, 192, 350–356. [Google Scholar] [CrossRef]
- Kim, S.; Kang, M. Hydrogen production from methanol steam reforming over Cu–Ti–P oxide catalysts. J. Ind. Eng. Chem. 2012, 18, 969–978. [Google Scholar] [CrossRef]
- Zhang, X.R.; Shi, P.F. Production of hydrogen by steam reforming of methanol on CeO2 promoted Cu/Al2O3 catalysts. J. Mol. Catal. A 2003, 194, 99–105. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wang, S.-F.; Tsai, A.-P.; Kameoka, S. Reduction behaviors and catalytic properties for methanol steam reforming of Cu-based spinel compounds CuX2O4 (X = Fe, Mn, Al, La). Ceram. Int. 2014, 40, 4541–4551. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kikuchi, R.; Takeguchi, T.; Eguchi, K. Steam reforming of dimethyl ether over composite catalysts of γ-Al2O3 and Cu-based spinel. Appl. Catal. B 2005, 57, 211–222. [Google Scholar] [CrossRef]
- Yang, S.-C.; Su, W.-N.; Lin, S.D.; Rick, J.; Cheng, J.-H.; Liu, J.-Y.; Pan, C.-J.; Liu, D.-G.; Lee, J.-F.; Chan, T.-S.; et al. Preparation of nano-sized Cu from a rod-like CuFe2O4: Suitable for high performance catalytic applications. Appl. Catal. B 2011, 106, 650–656. [Google Scholar] [CrossRef]
- Kameoka, S.; Tanabe, T.; Tsai, A.P. Spinel CuFe2O4: A precursor for copper catalyst with high thermal stability and activity. Catal. Lett. 2005, 100, 89–93. [Google Scholar] [CrossRef]
- Baneshi, J.; Haghighi, M.; Jodeiri, N.; Abdollahifar, M.; Ajamein, H. Homogeneous precipitation synthesis of CuO–ZrO2–CeO2–Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming for fuel cell applications. Energy Con. Manag. 2014, 87, 928–937. [Google Scholar] [CrossRef]
- Nakajima, H.; Lee, D.; Lee, M.-T.; Grigoropoulos, C.P. Hydrogen production with CuO/ZnO nanowire catalyst for a nanocatalytic solar thermal steam-methanol reformer. Int. J. Hydrogen Energy 2016, 41, 16927–16931. [Google Scholar] [CrossRef]
- Yang, R.-X.; Chuang, K.-H.; Wey, M.-Y. Hydrogen production through methanol steam reforming: Effect of synthesis parameters on Ni–Cu/CaO–SiO2 catalysts activity. Int. J. Hydrogen Energy 2014, 39, 19494–19501. [Google Scholar] [CrossRef]
- Jin, S.; Li, D.; Wang, Z.; Wang, Y.; Sun, L.; Zhu, M. Dynamics of the Cu/CeO2catalyst during methanol steam reforming. Catal. Sci. Technol. 2022, 12, 7003–7009. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Li, S.; Chen, Z.; Xu, X.; Fang, X.; Wang, X. Using XRD extrapolation method to design Ce-Cu-O solid solution catalysts for methanol steam reforming to produce H2: The effect of CuO lattice capacity on the reaction performance. Catal. Today 2022, 402, 228–240. [Google Scholar] [CrossRef]
- Wang, X.; Rodriguez, J.A.; Hanson, J.C.; Gamarra, D.; Martínez-Arias, A.; Fernández-García, M. In situ studies of the active sites for the water gas shift reaction over Cu-CeO2 catalysts: Complex interaction between metallic copper and oxygen vacancies of ceria. J. Phys. Chem. B 2006, 110, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yao, D.; Yang, Y.; Yang, W.; Li, Z.; Lv, J.; Huang, S.; Wang, Y.; Ma, X. Active Cu0-Cuσ+ sites for the hydrogenation of carbon-oxygen bonds over Cu/CeO2 catalysts. ACS Catal. 2022, 12, 1315–1325. [Google Scholar] [CrossRef]
- Sun, H.; Wang, H.; Qu, Z. Construction of CuO/CeO2 catalysts via the ceria shape effect for selective catalytic oxidation of ammonia. ACS Catal. 2023, 13, 1077–1088. [Google Scholar] [CrossRef]
- Chen, A.; Yu, X.; Zhou, Y.; Miao, S.; Li, Y.; Kuld, S.; Sehested, J.; Liu, J.; Aoki, T.; Hong, S.; et al. Structure of the catalytically active copper-ceria interfacial perimeter. Nat. Catal. 2019, 2, 334–341. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Tsunoda, T.; Suzuki, K.; Hamakawa, S.; Murata, K.; Shiozaki, R.; Ishii, T.; Kumagai, M. Steam reforming of methanol over Cu/CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts. Top. Catal. 2003, 22, 205–213. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Liu, Y.; Zhang, L.; Chen, Y.; Wang, H.; Tian, Y.; Zhang, C.; Liu, D. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 7252–7261. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Lv, S.; Huang, Y. A novel synthetic route for MOF-derived CuO-CeO2 catalyst with remarkable methanol steam reforming performance. Catal. Commun. 2021, 149, 106215. [Google Scholar] [CrossRef]
- Oguchi, H.; Nishiguchi, T.; Matsumoto, T.; Kanai, H.; Utani, K.; Matsumura, Y.; Imamura, S. Steam reforming of methanol over Cu/CeO2/ZrO2 catalysts. Appl. Catal. A Gen. 2005, 281, 69–73. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Li, Y.; Xu, Y.; Xin, Q.; Shen, W. Carbon monoxide oxidation over CuO/CeO2 catalysts. Catal Today 2004, 93–95, 191–198. [Google Scholar] [CrossRef]
- Yu, C.-L.; Sakthinathan, S.; Hwang, B.-Y.; Lin, S.-Y.; Chiu, T.-W.; Yu, B.-S.; Fan, Y.-J.; Chuang, C. CuFeO2–CeO2nanopowder catalyst prepared by self-combustion glycine nitrate process and applied for hydrogen production from methanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 15752–15762. [Google Scholar] [CrossRef]
- Cao, L.; Lu, M.; Li, G.; Zhang, S. Hydrogen production from methanol steam reforming catalyzed by Fe modified Cu supported on attapulgite clay. React. Kinet. Mech. Catal. 2019, 126, 137–152. [Google Scholar] [CrossRef]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N. Highly porous monolith/TiO2 supported Cu, Cu-Ni, Ru, and Pt catalysts in methanol steam reforming process for H2 generation. Appl. Catal. A Gen. 2018, 554, 44–53. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.; Li, D.; Yuan, J.; Bao, G.; Li, K. Enhanced low-temperature activity of CO2 methanation over Ni/CeO2 catalyst: Influence of preparation methods. Appl. Catal. Open 2024, 192, 206956. [Google Scholar] [CrossRef]
- Bordoloi, A.; Anton, J.; Ruland, H.; Muhler, M.; Kaluza, S. Metal–support interactions in surface-modified Cu–Co catalysts applied in higher alcohol synthesis. Catal. Sci. Technol. 2015, 5, 3603–3612. [Google Scholar] [CrossRef][Green Version]
- Shinoda, M.; Zhang, Y.; Yoneyama, Y.; Hasegawa, K.; Tsubaki, N. New bimodal pore catalysts for Fischer–Tropsch synthesis. Fuel Process Technol. 2004, 86, 73–85. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Z.; Wang, S.; Shan, Y.; Wang, L.; Xu, T.; He, P. Catalytic oxidation of ethyl acetate over Y (Y = Cu, Mn, Co)-modified CeO2 derived from Ce-MOF. Catal. Commun. 2024, 186, 106832. [Google Scholar] [CrossRef]
- Tan, J.; He, J.; Gao, K.; Zhu, S.; Cui, J.; Huang, L.; Zhu, Y.; Zhao, Y. Catalytic hydrogenation of furfural over Cu/CeO2 Catalyst: The effect of support morphology and exposed facet. Appl. Surf. Sci. 2022, 604, 154472. [Google Scholar] [CrossRef]
- Ge, C.; Yu, Y.; An, D.; Tong, Q.; Tang, C.; Gao, F.; Sun, J.; Dong, L. Surface configuration modulation for FeOx-CeO2/c-Al2O3 catalysts and its influence in CO oxidation. J. Catal. 2020, 386, 139–150. [Google Scholar] [CrossRef]
- Lukyanov, A.; Churakova, A.; Filatov, A.; Levin, E.; Valiev, R.; Gunderov, D.; Antipov, E. Microstructure refinement in Cu-Fe alloy using high pressure torsion. Mater. Sci. Eng. 2014, 63, 012102. [Google Scholar] [CrossRef]
- Guo, J.; Lu, D.; Zou, J. Investigation on solidification in Cu-20wt%Fe alloy through in situ observation. Metals 2023, 13, 581. [Google Scholar] [CrossRef]
- Delahay, G.; Coq, B.; Broussous, L. Selective catalytic reduction of nitrogen monoxide by decane on copper-exchanged beta zeolites. Appl. Catal. B Environ. 1997, 12, 49–59. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.R.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Lalla, N.P. Structural investigation of combustion synthesized Cu/CeO2 catalysts by EXAFS and other physical techniques: Formation of a Ce1-xCuxO2-δ solid solution. Chem. Mater. 2002, 14, 3591–3601. [Google Scholar] [CrossRef]
- Luo, M.; Zhong, Y.; Yuan, X.; Zheng, X. TPR and TPD studies of CuO/CeO2 catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 1997, 162, 121–131. [Google Scholar] [CrossRef]
- Tschöpe, A.; Ying, J.Y.; Chiang, Y.M. Processing and structural evolution of nanocrystalline Cu–CeO2-x catalysts. Mater. Sci. Eng. A 1995, 204, 267–271. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Xiao, M.; Han, D.; Lu, Y.; Meng, Y. Novel Cu–Fe bimetal catalyst for the formation of dimethyl carbonate from carbon dioxide and methanol. RSC Adv. 2012, 2, 6831–6837. [Google Scholar] [CrossRef]
- Koley, P.; Shit, S.C.; Joseph, B.; Pollastri, S.; Sabri, Y.M.; Mayes, E.L.H.; Nakka, L.; Tardio, J.; Mondal, J. Leveraging Cu/CuFe2O4-catalyzed biomass-derived furfural hydrodeoxygenation: A nanoscale metal–organic-framework template is the prime key. ACS Appl. Mater. Interfaces 2020, 12, 21682–21700. [Google Scholar] [CrossRef]
- Agarwal, V.; Patel, S.; Pant, K.K. H2 production by steam reforming of methanol over Cu/ZnO/Al2O3 catalysts: Transient deactivation kinetics modeling. Appl. Catal. A Gen. 2005, 279, 155–164. [Google Scholar] [CrossRef]
- Gac, W.; Słowik, G.; Zawadzki, W. Structural and surface changes of copper modified manganese oxides. Appl. Surf. Sci. 2016, 370, 536–544. [Google Scholar] [CrossRef]





| Catalyst | Metal Content (wt.%) 1 | Surface Area [m2/g] 2 | Pore Size [nm] 2 | |
|---|---|---|---|---|
| Cu | Fe | |||
| CeO2 | - | - | 63.9 | 15.4 |
| 30Cu/CeO2 | 31.0 | - | 44.3 | 14.0 |
| 20Cu-10Fe/CeO2 | 20.6 | 8.9 | 53.4 | 11.8 |
| 15Cu-15Fe/CeO2 | 16.5 | 14.3 | 51.5 | 13.1 |
| 10Cu-20Fe/CeO2 | 11.6 | 21.1 | 65.1 | 10.9 |
| Form | Phase | Catalyst | ||
|---|---|---|---|---|
| 30Cu/CeO2 | 20Cu-10Fe/CeO2 | 10Cu-20Fe/CeO2 | ||
| Crystallite Size [nm] | ||||
| Fresh | CuO | 6.16–9.73 | 5.52–8.31 | 7.86 |
| Fe2O3 | - | 6.85 | 8.52 | |
| Fe3O4/CuFe2O4 | - | 6.57 | 8.15 | |
| Reduced at 260 °C | Cu2O | 5.82–6.85 | 3.41–4.42 | 6.45 |
| CuO | - | 4.22–6.95 | 4.07–4.67 | |
| Fe3O4/CuFe2O4 | - | 5.75–6.56 | 6.26–14.12 | |
| Reduced at 400 °C | Cu2O | 13.86–14.94 | 4.13 | 7.14 |
| CuO | - | 5.08–6.64 | 4.95–5.83 | |
| Fe3O4/CuFe2O4 | - | 4.18–7.81 | 8.01–15.09 | |
| After reaction reduced at 260 °C | CuO | 5.00–8.02 | 7.03–8.78 | 7.01 |
| Fe3O4/CuFe2O4 | - | 5.26–7.45 | 7.28–11.23 | |
| After reaction reduced at 400 °C | CuO | 11.61 | 4.95–8.38 | 5.26–5.78 |
| Fe3O4/CuFe2O4 | - | 7.57–9.59 | 9.65–15.98 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słowik, G.; Rotko, M.; Ryczkowski, J.; Greluk, M. Hydrogen Production from Methanol Steam Reforming over Fe-Modified Cu/CeO2 Catalysts. Molecules 2024, 29, 3963. https://doi.org/10.3390/molecules29163963
Słowik G, Rotko M, Ryczkowski J, Greluk M. Hydrogen Production from Methanol Steam Reforming over Fe-Modified Cu/CeO2 Catalysts. Molecules. 2024; 29(16):3963. https://doi.org/10.3390/molecules29163963
Chicago/Turabian StyleSłowik, Grzegorz, Marek Rotko, Janusz Ryczkowski, and Magdalena Greluk. 2024. "Hydrogen Production from Methanol Steam Reforming over Fe-Modified Cu/CeO2 Catalysts" Molecules 29, no. 16: 3963. https://doi.org/10.3390/molecules29163963
APA StyleSłowik, G., Rotko, M., Ryczkowski, J., & Greluk, M. (2024). Hydrogen Production from Methanol Steam Reforming over Fe-Modified Cu/CeO2 Catalysts. Molecules, 29(16), 3963. https://doi.org/10.3390/molecules29163963







