Low-Cost Ni-W Catalysts Supported on Glucose/Carbon Nanotube Hybrid Carbons for Sustainable Ethylene Glycol Synthesis
Abstract
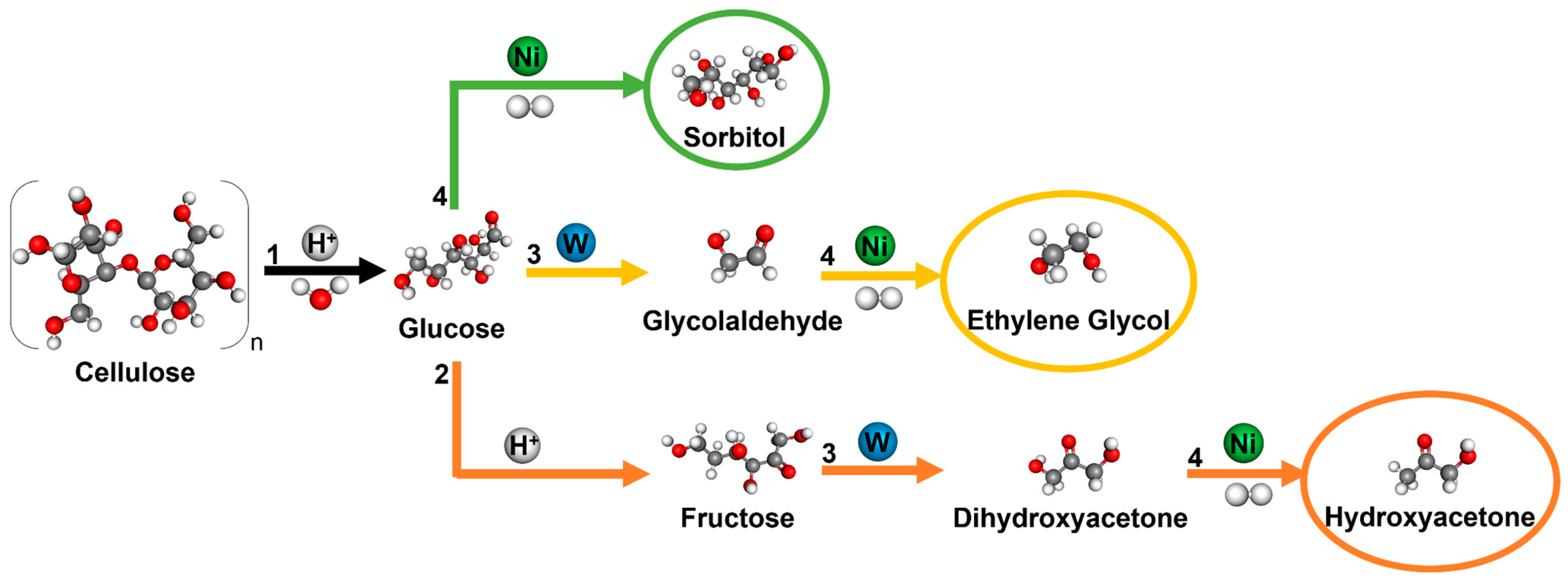
1. Introduction
2. Results and Discussion
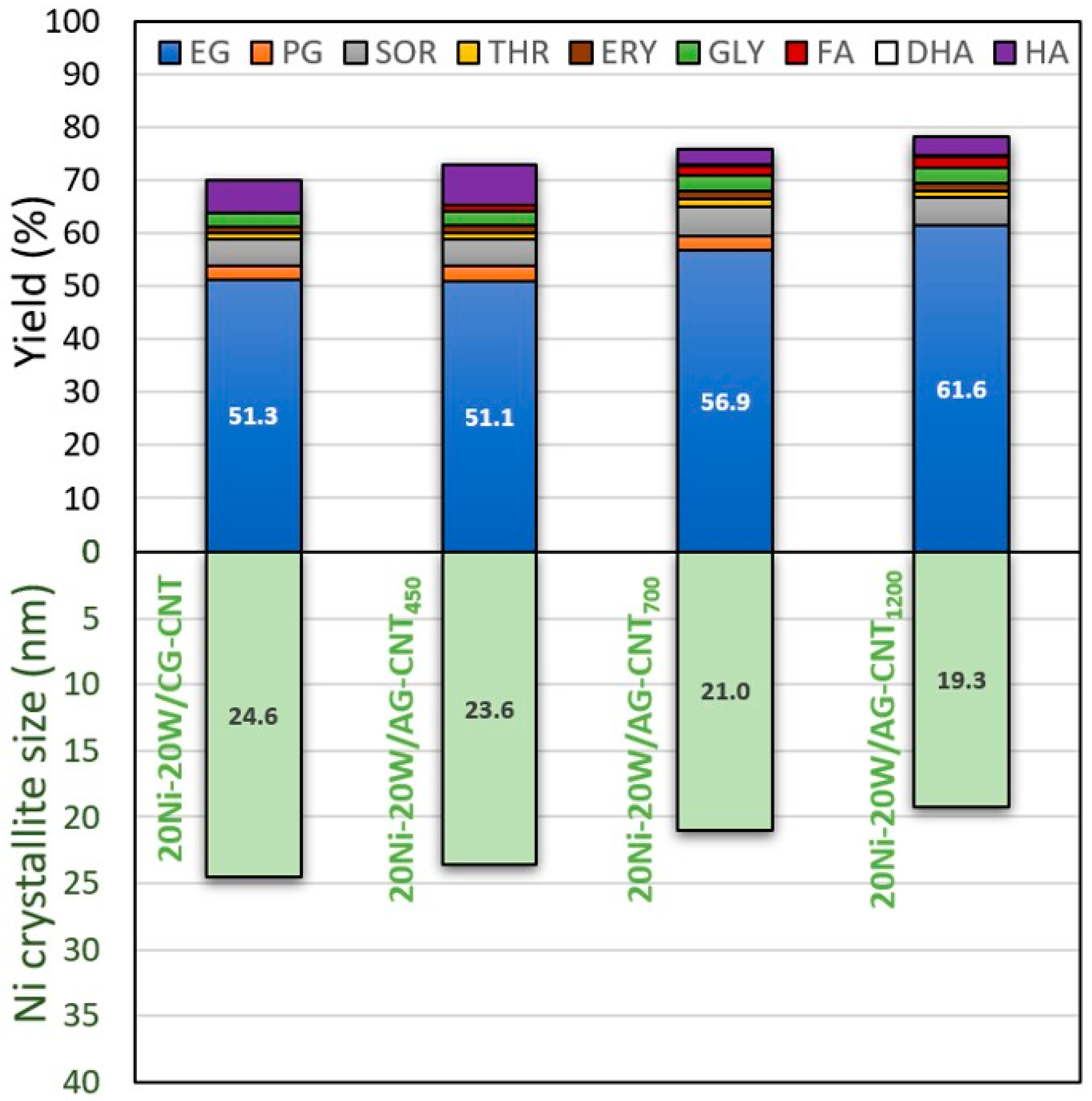
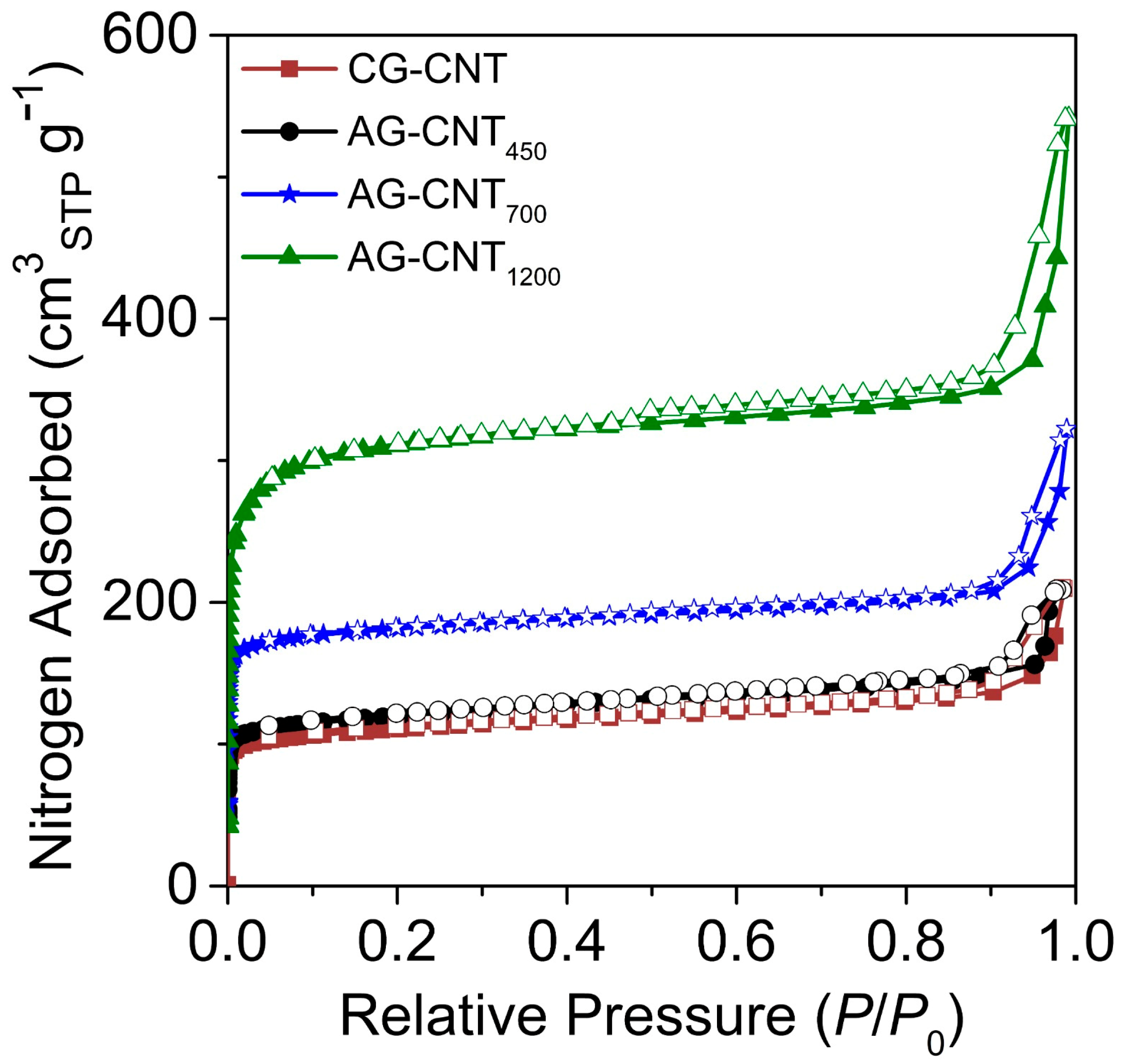
2.1. Material Characterisation
2.2. Catalytic Properties for Cellulose Conversion
3. Experimental Section
3.1. Chemicals
3.2. Preparation of Materials
3.3. Characterisation of the Supports and Catalysts
3.4. Catalysts’ Evaluation and Products Analysis
4. Main Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, Z.; Zhao, B.; Yu, S.; Huang, L.; Wu, Q. Nickel-Tungsten co-doped carbon-based catalyst for high selective production of ethylene glycol from cellulose hydrogenolysis. Fuel Process. Technol. 2023, 247, 107816. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, Z.; Zhang, H.; Fu, P. Reaction pathways and selectivity in the chemo-catalytic conversion of cellulose and its derivatives to ethylene glycol: A review. Chin. J. Chem. Eng. 2024, 66, 310–331. [Google Scholar] [CrossRef]
- Yan, W.; Guan, Q.; Jin, F. Catalytic conversion of cellulosic biomass to harvest high-valued organic acids. iScience 2023, 26, 107933. [Google Scholar] [CrossRef]
- Baniamerian, H.; Høj, M.; Beier, M.J.; Jensen, A.D. Catalytic conversion of sugars and polysaccharides to glycols: A review. Appl. Catal. B Environ. 2023, 330, 122650. [Google Scholar] [CrossRef]
- Ischia, G.; Cutillo, M.; Guella, G.; Bazzanella, N.; Cazzanelli, M.; Orlandi, M.; Miotello, A.; Fiori, L. Hydrothermal carbonization of glucose: Secondary char properties, reaction pathways, and kinetics. Chem. Eng. J. 2022, 449, 137827. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Fukuoka, A.; Dhepe, P. Catalytic conversion of cellulose into sugar alcohols. Angew. Chem. Int. Ed. 2006, 45, 5161–5163. [Google Scholar] [CrossRef]
- You, S.J.; Baek, I.G.; Kim, Y.T.; Jeong, K.; Chae, H.; Kim, T.; Kim, C.; Jeong, S.; Kim, T.J.; Chung, Y.; et al. Direct conversion of cellulose into polyols or H2 over Pt/Na(H)-ZSM-5. Korean J. Chem. Eng. 2011, 28, 744–750. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, Y.; Xia, Q.; Liu, X.; Ren, J.; Lu, G.; Wang, Y. Direct conversion of cellulose into sorbitol with high yield by a novel mesoporous niobium phosphate supported Ruthenium bifunctional catalyst. Appl. Catal. A Gen. 2013, 459, 52–58. [Google Scholar] [CrossRef]
- Mankar, A.R.; Modak, A.; Pant, K.K. High yield synthesis of hexitols and ethylene glycol through one-pot hydrolytic hydrogenation of cellulose. Fuel Process. Technol. 2021, 218, 106847. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H. Kinetic insight into the effect of the catalytic functions on selective conversion of cellulose to polyols on carbon-supported WO3 and Ru catalysts. Catal. Today 2016, 269, 74–81. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, H. Direct conversion of cellulose into sorbitol using dual-functionalized catalysts in neutral aqueous solution. Catal. Commun. 2012, 19, 115–118. [Google Scholar] [CrossRef]
- Deng, W.; Tan, X.; Fang, W.; Zhang, Q.; Wang, Y. Conversion of Cellulose into Sorbitol over Carbon Nanotube-Supported Ruthenium Catalyst. Catal. Lett. 2009, 133, 167–174. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ito, Y.; Komanoya, T.; Hosaka, Y.; Dhepe, P.L.; Kasai, K.; Hara, K.; Fukuoka, A. Synthesis of sugar alcohols by hydrolytic hydrogenation of cellulose over supported metal catalysts. Green Chem. 2011, 13, 326–333. [Google Scholar] [CrossRef]
- Luo, C.; Wang, S.; Liu, H. Cellulose conversion into polyols catalysed by reversible formed acids and supported ruthenium clusters in hot water. Angew. Chem. Int. Ed. 2007, 46, 7636–7639. [Google Scholar] [CrossRef]
- Palkovits, R.; Tajvidi, K.; Procelewska, J.; Rinaldi, R.; Ruppert, A. Hydrogenolysis of cellulose combining mineral acids and hydrogenation catalysts. Green Chem. 2010, 12, 972–978. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Geboers, J.; Dusselier, M.; Schepers, H.; Vosch, T.; Zhang, L.; Van Tendeloo, G.; Jacobs, P.A.; Sels, B.F. Selective Bifunctional Catalytic Conversion of Cellulose over Reshaped Ni Particles at the Tip of Carbon Nanofibers. ChemSusChem 2010, 3, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, Y.; Huang, Y.; Chen, X.; Zhang, T. Catalytic conversion of cellulose to hexitols with mesoporous carbon supported Ni-based bimetallic catalysts. Green Chem. 2012, 14, 614–617. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamakoshi, Y.; Hosaka, Y.; Yabushita, M.; Fukuoka, A. Production of sugar alcohols from real biomass by supported platinum catalyst. Catal. Today 2014, 226, 204–209. [Google Scholar] [CrossRef]
- Komanoya, T.; Kobayashi, H.; Hara, K.; Chun, W.-J.; Fukuoka, A. Kinetic Study of Catalytic Conversion of Cellulose to Sugar Alcohols under Low-Pressure Hydrogen. ChemCatChem 2014, 6, 230–236. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, T.; Zheng, M.; Wang, A.; Wang, H.; Wang, X.; Shu, Y.; Stottlemyer, A.L.; Chen, J.G. Catalytic conversion of cellulose into ethylene glycol over supported carbide catalysts. Catal. Today 2009, 147, 77–85. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, T.; Zheng, M.; Wang, A.; Wang, H.; Wang, X.; Chen, J.G. Direct catalytic conversion of cellulose into ethylene glycol using nickel-promoted tungsten carbide catalysts. Angew. Chem. Int. Ed. 2008, 47, 8510–8513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.Y.; Wang, A.Q.; Ji, N.; Pang, J.F.; Wang, X.D.; Zhang, T. Transition metal-tungsten bimetallic catalysts for the conversion of cellulose into ethylene glycol. ChemSusChem 2010, 3, 63–66. [Google Scholar] [CrossRef]
- Zhao, G.; Zheng, M.; Wang, A.; Zhang, T. Catalytic Conversion of Cellulose to Ethylene Glycol over Tungsten Phosphide Catalysts. Chin. J. Catal. 2010, 31, 928–932. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfão, J.J.M.; Pereira, M.F.R. Insights into the effect of the catalytic functions on selective production of ethylene glycol from lignocellulosic biomass over carbon supported ruthenium and tungsten catalysts. Bioresour. Technol. 2018, 263, 402–409. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Rey-Raap, N.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Glucose-Based carbon materials as supports for the efficient catalytic transformation of cellulose directly to ethylene glycol. Cellulose 2019, 26, 7337–7353. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Pires, A.L.F.; Órfão, J.J.M.; Pereira, M.F.R. Paving the way towards an eco- and budget-friendly one-pot catalytic conversion of cellulose and lignocellulosic residues into ethylene glycol over Ni–W/CNT catalysts. Renew. Energ. 2022, 200, 1008–1022. [Google Scholar] [CrossRef]
- Huang, H.; Chen, L.; Gu, C.; Zhang, X.; Liu, J.; Zhang, Q.; Wang, C.; Ma, L.; Liao, Y. In-Situ synthesis of Ru–WOX/biochar catalyst for conversion of cellulose toward ethylene glycol. Cellulose 2022, 29, 8195–8211. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Z.; Zhao, B.; Zhao, R.; Yu, S.; Huang, L. Nickel-Tungsten co-doped biochar catalyst boosting ethylene glycol production from cellulose hydrogenolysis. Ind. Crops Prod. 2024, 207, 117752. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Órfão, J.J.M.; Pereira, M.F.R. Direct catalytic conversion of agro-forestry biomass wastes into ethylene glycol over CNT supported Ru and W catalysts. Ind. Crops Prod. 2021, 166, 113461. [Google Scholar] [CrossRef]
- Taylor, A. Lattice Parameter of Binary Nickel-Cobalt Alloys. J. Inst. Met. 1950, 77, 585–594. [Google Scholar]
- Morris, M.C.; McMurdie, H.F.; Evans, E.H.; Paretzkin, B.; Parker, H.S.; Panagiotopoulos, N.C. Iron oxide (hematite), α-Fe2O3. In Standard X-ray Diffraction Powder Patterns; CODEN:NBSMA6; National Bureau of Standards: Gaithersburg, MD, USA, 1981; p. 37. [Google Scholar]
- Keeling, J.R.O. The structure of NiWO4. Acta Crystallogr. 1957, 10, 209–213. [Google Scholar] [CrossRef]
- Kawsar, M.; Hossain, M.S.; Bahadur, N.M.; Ahmed, S. Synthesis of nano-crystallite hydroxyapatites in different media and a comparative study for estimation of crystallite size using Scherrer method, Halder-Wagner method size-strain plot, and Williamson-Hall model. Heliyon 2024, 10, e25347. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Kang, M.; Zhu, Y. Catalytic conversion of glucose and cellobiose into ethylene glycol over various tungsten-based catalysts. J. Fuel Chem. Technol. 2016, 44, 845–852. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Morais, R.G.; Órfão, J.J.M.; Pereira, M.F.R. Unlocking the value of food waste: Sustainable production of ethylene glycol over low-cost Ni-W catalysts supported on glucose-derived carbons. Sustain. Energy Fuels, 2024; under review. [Google Scholar]
- Ribeiro, L.S.; Órfão, J.J.M.; Pereira, M.F.R. Enhanced direct production of sorbitol by cellulose ball-milling. Green Chem. 2015, 17, 2973–2980. [Google Scholar] [CrossRef]


| Sample | SBET (m2 g−1) | Sext (m2 g−1) | Vµpores (cm3 g−1) | Vp (cm3 g−1) | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| CG-CNT | 431 | 68 | 0.142 | 0.32 | 93.3 | 1.4 | 0.0 | 0.0 | 5.3 |
| AG-CNT450 | 465 | 82 | 0.150 | 0.32 | 94.4 | 1.2 | 0.0 | 0.0 | 4.4 |
| AG-CNT700 | 727 | 70 | 0.250 | 0.50 | 94.7 | 0.6 | 0.0 | 0.0 | 4.7 |
| AG-CNT1200 | 1220 | 111 | 0.432 | 0.83 | 95.7 | 0.4 | 0.0 | 0.0 | 3.9 |
| Catalyst | SBET (m2 g−1) | Sext (m2 g−1) | Vµpores (cm3 g−1) | Vp (cm3 g−1) | Ni (wt.%) [a] | Ni (wt.%) [b] | Ni Crystallite Size (nm) |
|---|---|---|---|---|---|---|---|
| 20Ni-20W/CG-CNT | 281 | 66 | 0.085 | 0.26 | 32.6 | 32.6 | 24.6 |
| 20Ni-20W/AG-CNT450 | 191 | 66 | 0.052 | 0.21 | 29.3 | 29.3 | 23.6 |
| 20Ni-20W/AG-CNT700 | 219 | 88 | 0.055 | 0.34 | 23.9 | 24.1 | 21.0 |
| 20Ni-20W/AG-CNT1200 | 549 | 120 | 0.176 | 0.46 | 23.6 | 22.9 | 19.3 |
| 5Ni-20W/AG-CNT1200 | 821 | 109 | 0.277 | 0.57 | 4.6 | 5.9 | n.d. |
| 10Ni-20W/AG-CNT1200 | 692 | 101 | 0.236 | 0.57 | 10.0 | 11.2 | n.d. |
| 30Ni-20W/AG-CNT1200 | 245 | 125 | 0.051 | 0.30 | 37.5 | 35.4 | n.d. |
| 10Ni-5W/AG-CNT1200 | n.d. | n.d. | n.d. | n.d. | 15.8 | n.d. | n.d. |
| 10Ni-10W/AG-CNT1200 | 933 | 164 | 0.309 | 0.84 | 14.3 | n.d. | n.d. |
| 10Ni-30W/AG-CNT1200 | 794 | 98 | 0.274 | 0.49 | 12.1 | n.d. | n.d. |
| Catalyst | T (°C) | (bar) | t (h) | X (%) | YEG (%) | Reference |
|---|---|---|---|---|---|---|
| 0.4Ru-30W/CNT | 205 | 50 | 3 | 100 | 42.4 | [26] |
| 0.4Ru-30W/CG | 205 | 50 | 5 | 100 | 42.1 | [27] |
| 5Ru-25W/AC | 245 | 60 [a] | 0.5 | 100 | 61.7 | [24] |
| 5Ru-40WOx/BC | 220 | 50 | 10 | 99.8 | 68.8 | [29] |
| 2Ni-30W2C/AC | 245 | 60 [a] | 0.5 | 100 | 61.0 | [23] |
| 2Ni-20WP/AC | 245 | 60 [a] | 0.5 | 100 | 46.0 | [25] |
| 20Ni-20W/CNT | 205 | 50 | 5 | 100 | 50.3 | [28] |
| 9Ni-13.5W/GC850 | 220 | 50 [a] | 2 | 100 | 69.2 | [2] |
| 9Ni-12WOx/GC700 | 220 | 50 [a] | 2 | 100 | 63.0 | [30] |
| 20Ni-10W/AG850 | 205 | 50 | 5 | 100 | 62.1 | [38] |
| 10Ni-5W/AG-CNT1200 | 205 | 50 | 5 | 100 | 61.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, R.G.; Ribeiro, L.S.; Órfão, J.J.M.; Pereira, M.F.R. Low-Cost Ni-W Catalysts Supported on Glucose/Carbon Nanotube Hybrid Carbons for Sustainable Ethylene Glycol Synthesis. Molecules 2024, 29, 3962. https://doi.org/10.3390/molecules29163962
Morais RG, Ribeiro LS, Órfão JJM, Pereira MFR. Low-Cost Ni-W Catalysts Supported on Glucose/Carbon Nanotube Hybrid Carbons for Sustainable Ethylene Glycol Synthesis. Molecules. 2024; 29(16):3962. https://doi.org/10.3390/molecules29163962
Chicago/Turabian StyleMorais, Rafael G., Lucília S. Ribeiro, José J. M. Órfão, and Manuel Fernando R. Pereira. 2024. "Low-Cost Ni-W Catalysts Supported on Glucose/Carbon Nanotube Hybrid Carbons for Sustainable Ethylene Glycol Synthesis" Molecules 29, no. 16: 3962. https://doi.org/10.3390/molecules29163962
APA StyleMorais, R. G., Ribeiro, L. S., Órfão, J. J. M., & Pereira, M. F. R. (2024). Low-Cost Ni-W Catalysts Supported on Glucose/Carbon Nanotube Hybrid Carbons for Sustainable Ethylene Glycol Synthesis. Molecules, 29(16), 3962. https://doi.org/10.3390/molecules29163962








