Rational Construction of Honeycomb-like Carbon Network-Encapsulated MoSe2 Nanocrystals as Bifunctional Catalysts for Highly Efficient Water Splitting
Abstract
1. Introduction
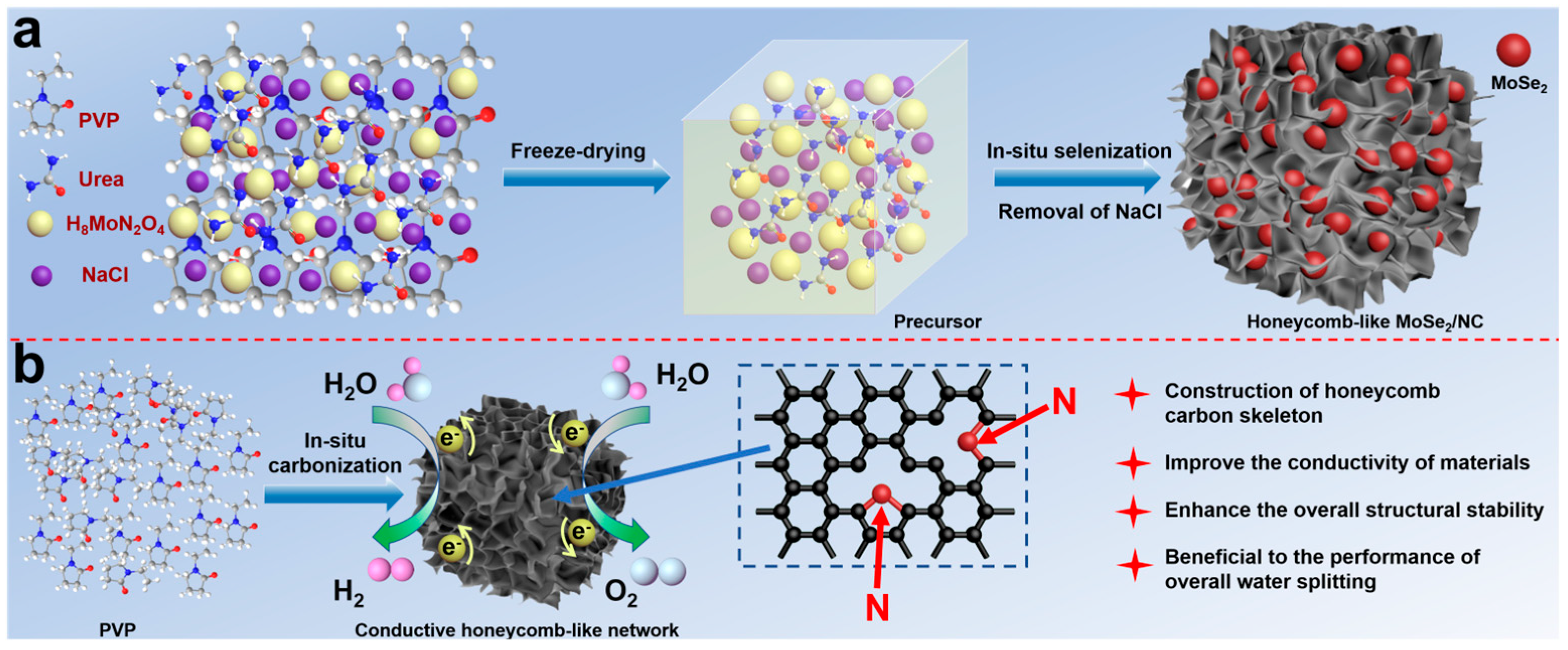
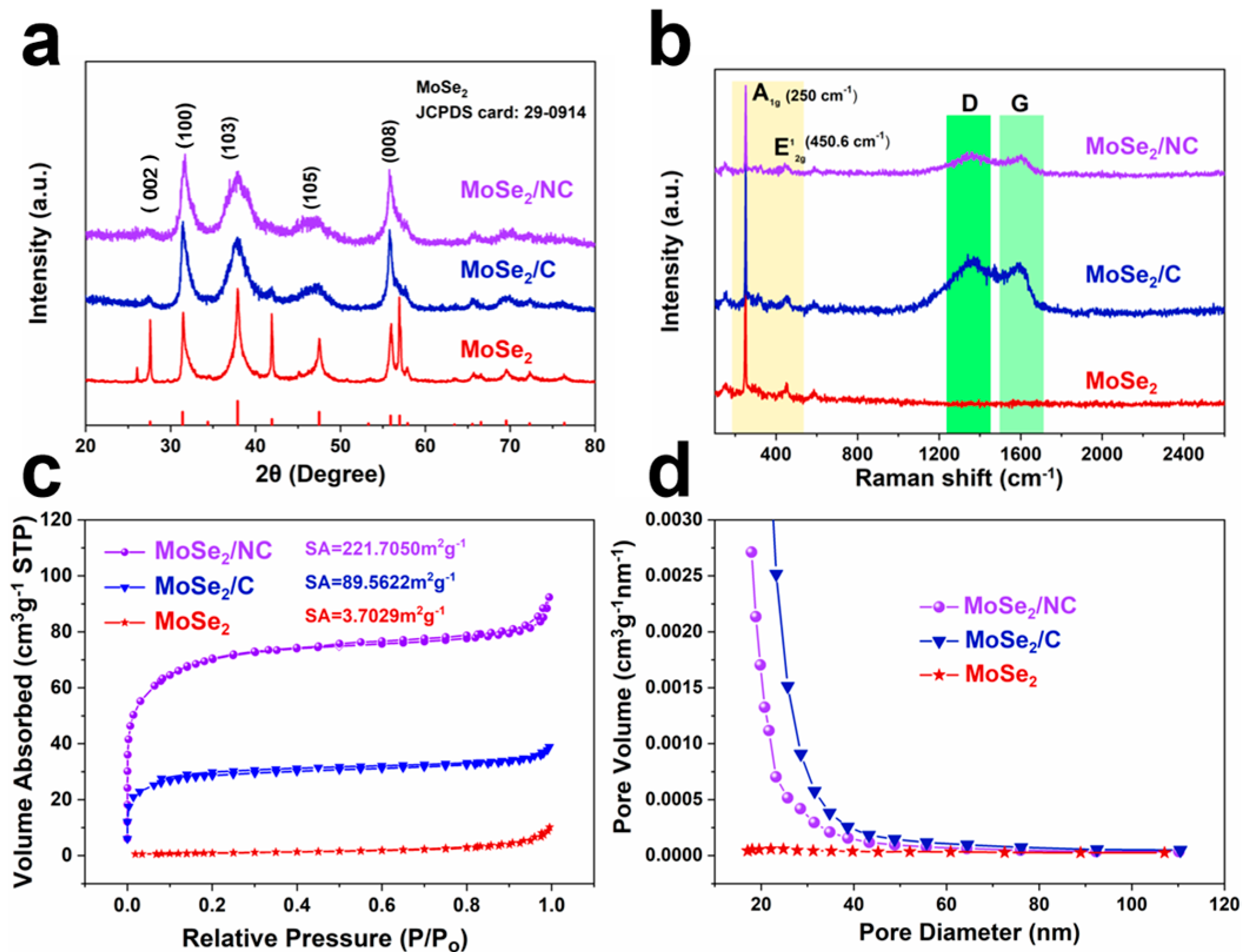
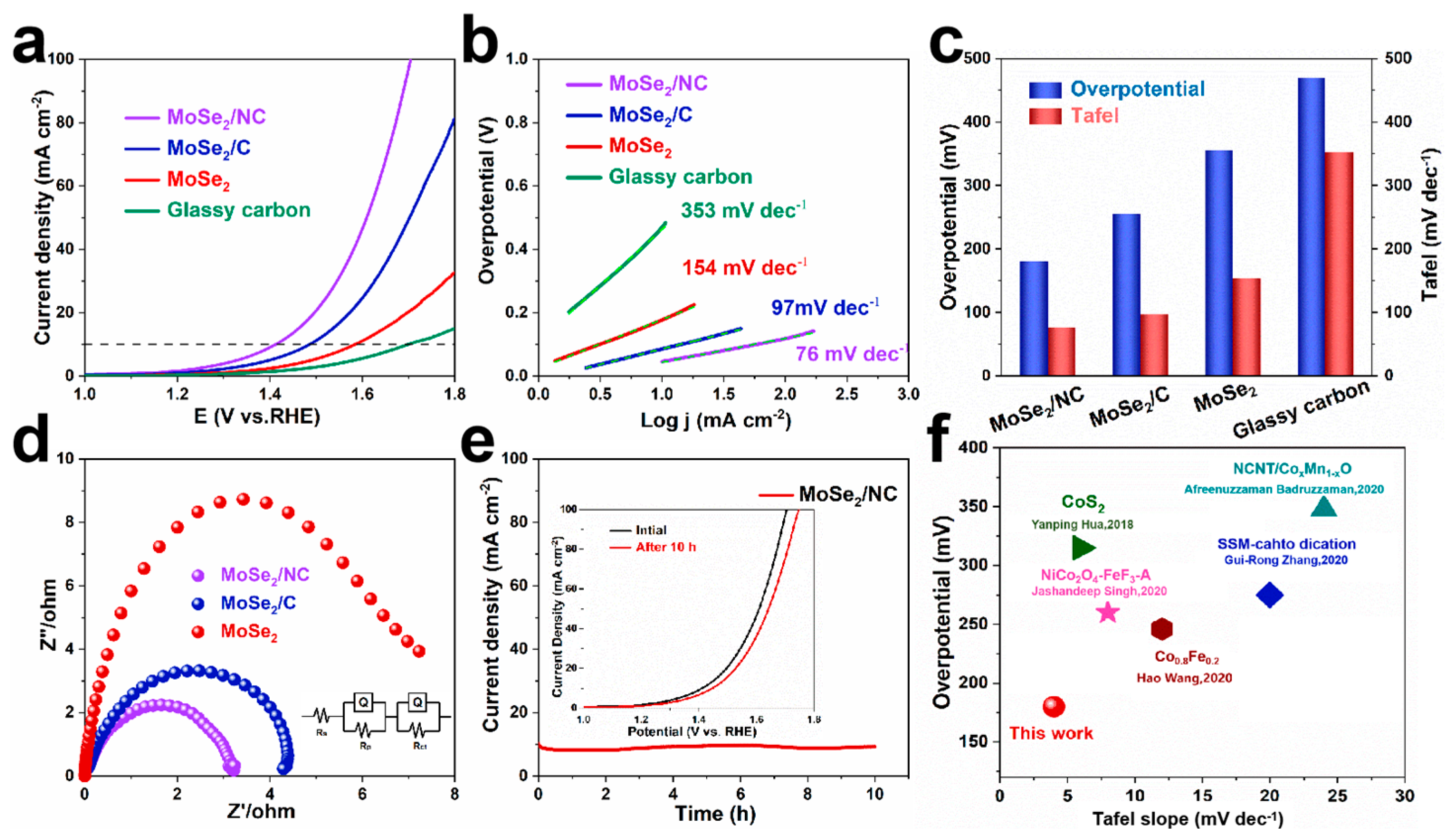
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of the MoSe2/NC Composite
3.2. Structural Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Z.; Jiao, W.; Huang, Z.; Chen, G.; Zhang, B.; Han, Y.; Huang, W. Design and synthesis of noble metal-based alloy electrocatalysts and their application in hydrogen evolution reaction. Small 2023, 19, 2301465. [Google Scholar] [CrossRef]
- Du, H.; Wang, T.; He, S.; Li, B.; Wang, K.; Chen, Q.; Du, Z.; Ai, W.; Huang, W. Mountain-shaped nickel nanostripes enabled by facet engineering of nickel foam: A new platform for high-current-density water splitting. Adv. Funct. Mater. 2023, 34, 2311854. [Google Scholar] [CrossRef]
- Guo, M.; Deng, R.; Wang, C.; Zhang, Q. Recent progress of advanced manganese oxide-based materials for acidic oxygen evolution reaction: Fundamentals, performance optimization, and prospects. J. Energy Chem. 2023, 78, 537–553. [Google Scholar] [CrossRef]
- Hyun Oh, J.; Ho Han, G.; Kim, J.; Eun Lee, J.; Kim, H.; Kyung Kang, S.; Kim, H.; Wooh, S.; Soo Lee, P.; Won Jang, H.; et al. Self-supported electrodes to enhance mass transfer for high-performance anion exchange membrane water electrolyzer. Chem. Eng. J. 2023, 460, 141727. [Google Scholar] [CrossRef]
- Jia, C.; Zhen, C.; Yin, L.; Zhu, H.; Du, P.; Han, A.; Liu, G.; Cheng, H.-M. Topologic transition-induced abundant undercoordinated Fe active sites in NiFeOOH for superior oxygen evolution. Nano Energy 2023, 106, 108044. [Google Scholar] [CrossRef]
- Li, Z.; Cao, S.; Chen, J.; Wu, L.; Chen, M.; Ding, H.; Wang, R.; Guo, W.; Bai, Y.; Liu, M.; et al. Modulating surface architecture and electronic conductivity of Li-rich manganese-based cathode. Small 2024, 2400641. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, D.; Xu, J.; Sun, H.; Shi, Z. Constructing surface concave defects on NiFe layered double hydroxides by electrochemical reduction for efficient oxygen evolution reaction. Chem. Eng. J. 2024, 482, 148858. [Google Scholar] [CrossRef]
- Liu, C.; Feng, J.; Zhou, P.; Liu, D.; Qiao, L.; Liu, D.; Cao, Y.; Su, S.-C.; Liu, H.; Pan, H. Multi-metal interaction boosts reconstructed FeCoCrCuOx@CF toward efficient alkaline water electrolysis under large current density. Chem. Eng. J. 2023, 476, 146710. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Z.; Tang, W.; Chen, Y.; Lan, C.; Zhu, L.; Jiang, W.; Wu, Y.; Wang, Y.; Yang, Z.; et al. In-situ direct seawater electrolysis using floating platform in ocean with uncontrollable wave motion. Nat. Commun. 2024, 15, 5305. [Google Scholar] [CrossRef]
- Malek, A.; Xue, Y.; Lu, X. Dynamically restructuring NixCryO electrocatalyst for stable oxygen evolution reaction in real seawater. Angew. Chem. Int. Ed. 2023, 62, 202309854. [Google Scholar] [CrossRef]
- Pan, S.; Li, C.; Xiong, T.; Xie, Y.; Luo, F.; Yang, Z. Hydrogen spillover in MoOxRh hierarchical nanosheets boosts alkaline HER catalytic activity. Appl. Catal. B Environ. 2024, 341, 123275. [Google Scholar] [CrossRef]
- Sasmal, S.; Chen, L.; Sarma, P.V.; Vulpin, O.T.; Simons, C.R.; Wells, K.M.; Spontak, R.J.; Boettcher, S.W. Materials descriptors for advanced water dissociation catalysts in bipolar membranes. Nat. Mater. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Sun, H.; Chen, H.C.; Humayun, M.; Qiu, Y.; Ju, J.; Zhang, Y.; Bououdina, M.; Xue, X.; Liu, Q.; Pang, Y.; et al. Unlocking the catalytic potential of platinum single atoms for industry-level current density chlorine tolerance hydrogen generation. Adv. Funct. Mater. 2024, 2408872. [Google Scholar] [CrossRef]
- Sun, J.; Ren, G.; Qin, S.; Zhao, Z.; Li, Z.; Zhang, Z.; Li, C.; Meng, X. Reconstruction Co-O-Mo in amorphous-crystalline MoOx/Co(OH)2 interface for industry-level active and stable electrocatalytic seawater hydrogen evolution. Nano Energy 2024, 121, 109246. [Google Scholar] [CrossRef]
- Tan, F.; Zhou, Y.; Zhang, H.; Sun, P.; Li, H.; Liu, X.; Wågberg, T.; Hu, G. Improving the hydrogen evolution reaction activity of molybdenum-based heterojunction nanocluster capsules via electronic modulation by erbium–nitrogen–phosphorus ternary doping. Chem. Eng. J. 2023, 454, 140079. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, L.; Lv, R. Rational design of efficient electrocatalysts for hydrogen production by water electrolysis at high current density. Mater. Chem. Front. 2023, 7, 6035–6060. [Google Scholar] [CrossRef]
- Wang, M.; Ma, W.; Tan, C.; Qiu, Z.; Hu, L.; Lv, X.; Li, Q.; Dang, J. Designing efficient non-precious metal electrocatalysts for high-performance hydrogen production: A comprehensive evaluation strategy. Small 2024, 20, 2306631. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. Progress in hydrogen production coupled with electrochemical oxidation of small molecules. Angew. Chem. Int. Ed. 2022, 61, 202213328. [Google Scholar] [CrossRef]
- Elayappan, V.; Shanmugam, R.; Chinnusamy, S.; Yoo, D.J.; Mayakrishnan, G.; Kim, K.; Noh, H.S.; Kim, M.K.; Lee, H. Three-dimensional bimetal TMO supported carbon based electrocatalyst developed via dry synthesis for hydrogen and oxygen evolution. Appl. Surf. Sci. 2020, 505, 144642. [Google Scholar] [CrossRef]
- Prasanna, M.; Kwak, H.B.; Oh, M.J.; Yoo, D.J. Architecting a 1T-phase material with metal NPs enriching HER kinetics in alkaline and seawater electrolytes. Inorg. Chem. Front. 2024; advance article. [Google Scholar] [CrossRef]
- Setayeshgar, S.; Karimipour, M.; Molaei, M.; Moghadam, M.R.; Khazraei, S. Synthesis of scalable 1T/2H–MoSe2 nanosheets with a new source of Se in basic media and study of their HER activity. Int. J. Hydrogen Energy 2020, 45, 6090–6101. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Huang, B.; Cai, W.; Sui, J.; Yang, Z.; Wang, H.-E. MoSe2 nanoplatelets with enriched active edge sites for superior sodium-ion storage and enhanced alkaline hydrogen evolution activity. Chem. Eng. J. 2020, 382, 123047. [Google Scholar] [CrossRef]
- Xu, J.; Ruan, J.; Jian, Y.; Lao, J.; Li, Z.; Xie, F.; Jin, Y.; Yu, X.; Lee, M.H.; Wang, Z.; et al. Cobalt-doping induced formation of five-coordinated nickel selenide for enhanced ethanol assisted overall water splitting. Small 2024, 20, 2305905. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Y.; Shi, J.; Hao, X.; Ma, Z.; Yang, K.; Zhang, T.; Li, C.; Zhang, D.; Huang, X.; et al. Corrosion-resistant cobalt phosphide electrocatalysts for salinity tolerance hydrogen evolution. Nat. Commun. 2023, 14, 7708. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, X.; Wang, S.; Rui, K.; Chen, Y.; Yu, H.; Ma, J.; Dou, S.X.; Sun, W. Heteroatom-doped MoSe2 nanosheets with enhanced hydrogen evolution kinetics for alkaline water splitting. Chem. Asian J. 2019, 14, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, H.; Li, M.; Wang, Y.; Wang, D. Nanoflower-like 1T/2H mixed-phase MoSe2 as an efficient electrocatalyst for hydrogen evolution. J. Alloys Compd. 2021, 878, 160381. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Li, H.; Li, H.; Wu, Z.; Liang, C.; Zhu, X.; Sun, Y. Dual surfactants applied in synthesis of MoSe2 for high-efficiency hydrogen evolution reaction. J. Alloys Compd. 2021, 863, 158092. [Google Scholar] [CrossRef]
- Li, H.; Hao, X.; Gong, H.; Jin, Z.; Zhao, T. Efficient hydrogen production at a rationally designed MoSe2@Co3O4 p-n heterojunction. J. Colloid Interface Sci. 2021, 586, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, T.; Xia, B.; Xi, P.; Gao, D. Zn-doped MoSe2 nanosheets as high-performance electrocatalysts for hydrogen evolution reaction in acid media. Electrochim. Acta 2019, 296, 701–708. [Google Scholar] [CrossRef]
- Xu, L.; Ma, L.; Zhou, X.; Liu, Z.; Luo, D.; Xu, X.; Zhang, L. Boosting electrocatalytic activity of ultrathin MoSe2/C composites for hydrogen evolution via a surfactant assisted hydrothermal method. Int. J. Hydrogen Energy 2018, 43, 15749–15761. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Chen, D. Sheet-like MoSe2/C composites with enhanced Li-ion storage properties. J. Mater. Chem. A 2015, 3, 11857–11862. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Dong, C.; Wu, L.; Liang, D.; Cao, H.; Lu, P. Hydrogen evolution reaction on in-plane platinum and palladium dichalcogenides via single-atom doping. Int. J. Hydrogen Energy 2021, 46, 18294–18304. [Google Scholar] [CrossRef]
- Zeb, Z.; Huang, Y.; Chen, L.; Zhou, W.; Liao, M.; Jiang, Y.; Li, H.; Wang, L.; Wang, L.; Wang, H.; et al. Comprehensive overview of polyoxometalates for electrocatalytic hydrogen evolution reaction. Coord. Chem. Rev. 2023, 482, 215058. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Chen, H.; Yang, Q.; Liu, H.; Bao, S.; Tian, Z.; Slavcheva, E.; Lu, Z. Progress in anode stability improvement for seawater electrolysis to produce hydrogen. Adv. Mater. 2024, 2311322. [Google Scholar] [CrossRef]
- Yao, R.; Sun, K.; Zhang, K.; Wu, Y.; Du, Y.; Zhao, Q.; Liu, G.; Chen, C.; Sun, Y.; Li, J. Stable hydrogen evolution reaction at high current densities via designing the Ni single atoms and Ru nanoparticles linked by carbon bridges. Nat. Commun. 2024, 15, 2218. [Google Scholar] [CrossRef] [PubMed]
- Badruzzaman, A.; Yuda, A.; Ashok, A.; Kumar, A. Recent advances in cobalt based heterogeneous catalysts for oxygen evolution reaction. Inorg. Chim. Acta 2020, 511, 119854. [Google Scholar] [CrossRef]
- Hua, Y.; Jiang, H.; Jiang, H.; Zhang, H.; Li, C. Hierarchical porous CoS2 microboxes for efficient oxygen evolution reaction. Electrochim. Acta 2018, 278, 219–225. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, A.; Goutam, U.K.; Kumar, A. Microstructure and electrochemical performance of La2ZnMnO6 nanoflakes synthesized by facile hydrothermal route. Appl. Phys. A 2020, 126, 11. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wan, H.; Wu, D.; Chen, G.; Zhang, N.; Cao, Y.; Liu, X.; Ma, R. Ultrathin nanosheet-assembled Co-Fe hydroxide nanotubes: Sacrificial template synthesis, topotactic transformation, and their application as electrocatalysts for efficient oxygen evolution reaction. ACS Appl. Mater. 2020, 12, 46578–46587. [Google Scholar] [CrossRef]
- Zhang, G.-R.; Shen, L.-L.; Schmatz, P.; Krois, K.; Etzold, B.J.M. Cathodic activated stainless steel mesh as a highly active electrocatalyst for the oxygen evolution reaction with self-healing possibility. J. Energy Chem. 2020, 49, 153–160. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, H.; Xiong, J.; Zhao, Z.; Zhang, D.; Chen, L. Anion-induced vacancy enhances Co3Se4/Fe3Se4 heterostructures for high-efficiency hydrogen production. Fuel 2024, 360, 130651. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.; Li, Z.; Xu, X.; Zhang, Z.; Li, C.; Wang, L.; Meng, X. Rapid synthesis of efficient Mo-based electrocatalyst for the hydrogen evolution reaction in alkaline seawater with 11.28% solar-to-hydrogen efficiency. J. Mater. Chem. A 2023, 11, 10346–10359. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, B.; Tang, K.; Qin, W.; Tan, C.; Yao, J.; Li, Y.; Jiang, L.; Wang, X.; Sun, Y. In-situ phase transition induced nanoheterostructure for overall water splitting. Chem. Eng. J. 2021, 409, 128156. [Google Scholar] [CrossRef]
- Yang, D.; Hou, W.; Lu, Y.; Zhang, W.; Chen, Y. Scalable synthesis of self-assembled bimetallic phosphide/N-doped graphene nanoflakes as an efficient electrocatalyst for overall water splitting. Nanoscale 2019, 11, 12837–12845. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Y.; Li, J.; Chen, Q.; Du, Y.; Rao, P.; Li, R.; Jia, C.; Kang, Z.; Deng, P.; et al. Engineering ruthenium-based electrocatalysts for effective hydrogen evolution reaction. Nanomicro Lett. 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; He, M.; Zou, H.; Yu, J.; Fan, K. 0D/3D MoS2-NiS2/N-doped graphene foam composite for efficient overall water splitting. Appl. Catal. B-Environ. 2019, 254, 15–25. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharyya, S. Porous NiFe-Oxide nanocubes as bifunctional electrocatalysts for efficient water-splitting. ACS Appl. Mater. 2017, 9, 41906–41915. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Huang, H.; Zhou, S.; Han, X.; Zhao, C.; Yang, J.; Li, S.; Guo, W.; An, B.; Zhao, J.; et al. An electrocatalyst with anti-oxidized capability for overall water splitting. Nano Res. 2018, 11, 3411–3418. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Q.; Long, S.; Huang, X. Cobalt-molybdenum nanosheet arrays as highly efficient and stable earth-abundant electrocatalysts for overall water splitting. Nano Energy 2018, 45, 448–455. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, C.; Huang, Z.; Yan, X.; Kong, X.; Chen, X.; Li, S.; Wang, L.; Wan, Z. Rational Construction of Honeycomb-like Carbon Network-Encapsulated MoSe2 Nanocrystals as Bifunctional Catalysts for Highly Efficient Water Splitting. Molecules 2024, 29, 3877. https://doi.org/10.3390/molecules29163877
Ou C, Huang Z, Yan X, Kong X, Chen X, Li S, Wang L, Wan Z. Rational Construction of Honeycomb-like Carbon Network-Encapsulated MoSe2 Nanocrystals as Bifunctional Catalysts for Highly Efficient Water Splitting. Molecules. 2024; 29(16):3877. https://doi.org/10.3390/molecules29163877
Chicago/Turabian StyleOu, Changjie, Zhongkai Huang, Xiaoyu Yan, Xiangzhong Kong, Xi Chen, Shi Li, Lihua Wang, and Zhongmin Wan. 2024. "Rational Construction of Honeycomb-like Carbon Network-Encapsulated MoSe2 Nanocrystals as Bifunctional Catalysts for Highly Efficient Water Splitting" Molecules 29, no. 16: 3877. https://doi.org/10.3390/molecules29163877
APA StyleOu, C., Huang, Z., Yan, X., Kong, X., Chen, X., Li, S., Wang, L., & Wan, Z. (2024). Rational Construction of Honeycomb-like Carbon Network-Encapsulated MoSe2 Nanocrystals as Bifunctional Catalysts for Highly Efficient Water Splitting. Molecules, 29(16), 3877. https://doi.org/10.3390/molecules29163877






