Abstract
The tea tussock moth is a pest that damages tea leaves, affecting the quality and yield of tea and causing huge economic losses. The efficient asymmetric total synthesis of the sex pheromone of the tea tussock moth was achieved using commercially available starting materials with a 25% overall yield in 11 steps. Moreover, the chiral moiety was introduced by Evans’ template and the key C-C bond construction was accomplished through Julia–Kocienski olefination coupling. The synthetic sex pheromone of the tea tussock moth will facilitate the subsequent assessment and implementation of pheromones as environmentally friendly tools for pest management.
1. Introduction
Tea plays a significant role in the agricultural economy of China [1]. However, the infestation of pests on tea plants poses a more severe threat; in particular, the tea tussock moth, Euproctis pseudoconspersa (Strand) (Lepidoptera: Lymantridae), is a major pest of tea planation in China. Its larvae consume tea leaves, leading to complete defoliation in severe cases, significantly compromising the quality and yield of tea. Additionally, the venomous hairs of larvae also pose a risk of injury to farmers working in the tea plantations [2]. Currently, the primary means of pest control relies heavily on chemical pesticides, resulting in challenges such as exceeding pesticide residue standards, which has become a hindrance to the development of China’s tea industry. In response to the growing health consciousness among individuals, it is crucial to investigate pollution-free preventive and control measures in tea gardens. Insect pheromones offer numerous advantages, such as their non-toxic nature, sparing of natural predators, and environmental friendliness [3]. Consequently, the utilization of insect pheromones for pest control has attracted significant interest among scientists. The chemical structure of the sex pheromone emitted by the tea tussock moth was identified as 10, 14-dimethyl-l-pentadecyl isobutyrate 1 (Figure 1) [4,5]. The naturally occurring pheromone of the tea tussock moth was preliminarily assigned to the (R)-configuration at the C-10 methyl group [6]. However, field trials revealed that both the (R)-enantiomer and (S)-enantiomer exhibit attractive properties [7,8].
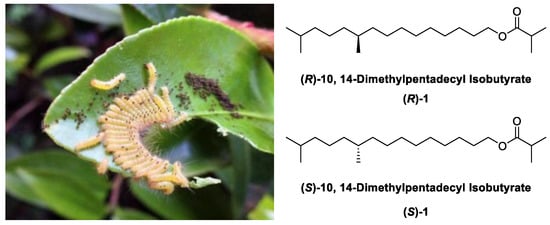
Figure 1.
Tea tussock moth and chemical structure of its sex pheromone.
Insect pheromones are commonly classified as either chiral or achiral compounds, characterized by their low molecular weight and simple functional groups, typically esters [9]. The synthesis of insect pheromones heavily relies on the construction of carbon chains, for which various synthetic strategies exist based on different raw materials [10]. The synthesis of the sex pheromone of the tea tussock moth in the early literature often relied primarily on alkylation methods [5]. There are two main problems including the construction of the C-C skeleton and the utilization of expensive chiral starting materials. For instance, Ichikawa et al. reported the first asymmetric total synthesis of the sex pheromone of the tea tussock moth by employing a Wittig reaction strategy using commercially available (R)- and (S)-citronellol (Scheme 1) [6]. Zhao and co-workers reported the second total synthesis employing an alkylation strategy by using commercially available (R)- and (S)-citronellyl bromide (Scheme 1a) [8]. Our group have reported the total synthesis of the sex pheromone of (R)-1 and (S)-1 through a Julia–Kocienski olefination coupling strategy (Scheme 1) [11,12].
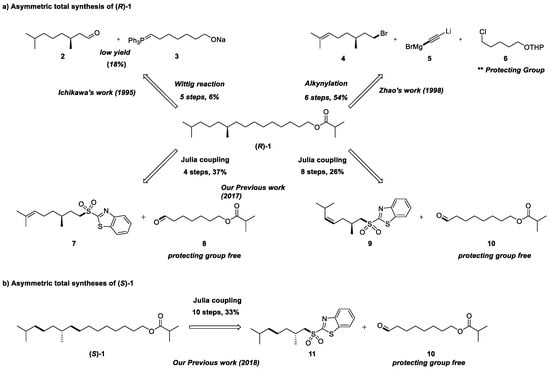
Scheme 1.
Structure and asymmetric total syntheses of (R)- and (S)-1 [6,8,11,12].
As for the Julia–Kocienski reaction [13], sulfone as a key compound can be more readily synthesized through the Mitsunobu reaction [14] and oxidation reactions from easily available simple alcohols. Therefore, the Julia–Kocienski reaction seems more convenient and environmentally friendly. Given this, as a continuation of our interest in organic synthesis, we still hope to develop another new route using Julia–Kocienski olefination to achieve the construction of the C-C skeleton using diverse starting materials. We herein report an asymmetric synthetic approach for the total synthesis of (R)-1.
2. Results and Discussion
The retrosynthesis of (R)-1 is shown in Scheme 2. The target molecule (R)-1 can be readily obtained from alkene 28 through catalytic hydrogenation reduction. The synthesis of alkene 28 can be achieved through Julia–Kocienski olefination between PT-sulfone 12 and chiral aldehyde 13. The synthesis of PT-sulfone 12 can be accomplished by isobutyl bromide 25 in a two-step process involving the Mitsunobu reaction [14] and subsequent oxidation. Chiral aldehyde 13 can be synthesized from olefin 14 via both catalytic hydrogenation and oxidation processes. Olefin 14 was synthesized via Julia–Kocienski olefination using BT-sulfone 15 and aldehyde 10 as starting materials. BT-sulfone 15 can still be synthesized from chiral alcohol 20 through a two-step process involving the Mitsunobu reaction and oxidation. The synthesis of chiral alcohol 20 can be accomplished via a four-step process starting from 4-benzyloxybutyric acid 16 [15], with the pivotal step being the asymmetric chiral induction using Evans’ template [16].
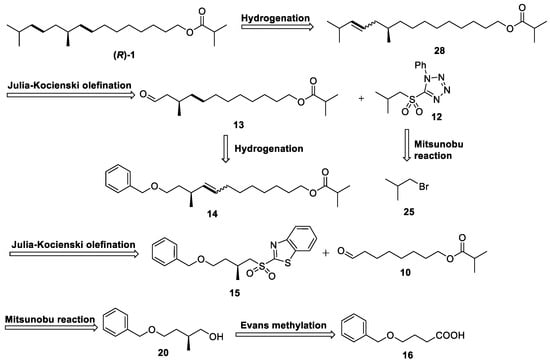
Scheme 2.
Retrosynthesis of sex pheromone of (R)-1.
The synthesis of the key intermediates of 13 is shown in Scheme 3. The compound 18 was synthesized by reacting Evans’ template 17 with acid 16 in the presence of piavloyl chloride [17], triethylamine, and LiCl [18], resulting in a yield of 89%. This suggests that LiCl exhibits commendable catalytic activity in the acylation reaction, and the role of LiCl is to form intermediates with a complex structure with the components participating in the reaction. The desired compound 19 was obtained in 75% yield through deprotonation of 18 with NaHMDS, followed by highly diastereoselective alkylation of the resulting chiral imide enolate with MeI at a low temperature. Due to the highly efficient alkali catalytic properties of NaHMDS, its reaction activity and deprotonation ability are enhanced at elevated temperatures. However, conducting the reaction at a low temperature is advantageous for achieving a higher yield of the target product 19 while minimizing the formation of undesired byproducts [19]. The enantiomeric excess (ee) of the product 19 was determined to be 99% using high-performance liquid chromatography (HPLC) with a chiral AD-H column. Subsequently, compound 19 was treated with LiAlH4 in dry THF to afford the desired primary alcohol 20, which possessed a chiral center, in a yield of 90%. Next, the chiral alcohol 20 was effectively converted into thioether 22 in the presence of PPh3 and DIAD via the Mitsunobu reaction with MBT 21 in a 90% yield [14]. The mechanism of the Mitsunobu reaction is illustrated in Scheme 3. Initially, triphenylphosphine (PPh3) undergoes a reaction with diisopropyl azodicarboxylate (DIAD), leading to the formation of an active intermediate. This intermediate subsequently abstracts a proton from mercaptan 21, while alcohol 20 attacks the phosphine cationic center to generate phosphine oxide, and simultaneously, DIAD dissociates as a byproduct of hydrazine. Subsequently, the anions of the nucleophilic mercaptan 21 initiate a reverse attack on the alkylphosphine oxide intermediate, resulting in the formation of the product 22 and the concomitant generation of triphenylphosphine oxide as a byproduct. The enantioselectivity of Evans methylation was determined to be 91:9 er for the derivative 20 through high-performance liquid chromatography (HPLC) analysis on a chiral OD-H column.
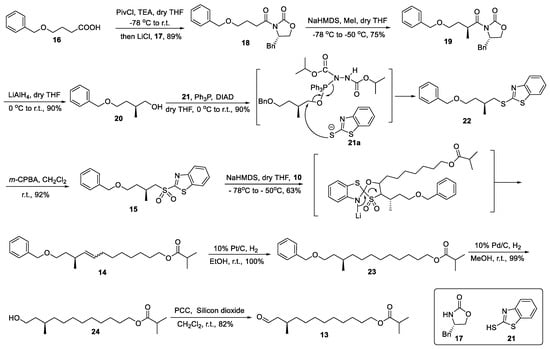
Scheme 3.
Synthesis of key intermediates of 13.
Thioether 22 was further oxidized with m-CPBA to afford sulfone 15 in a high yield of 92% [20]. Meanwhile, the aldehyde 10 was synthesized following the established protocol in [11] using 1, 8-octanediol through a two-step procedure. Subsequently, Julia–Kocienski olefination of aldehyde 10 with sulfone 15 deprotonated by NaHMDS resulted in the formation of alkene 14 as a mixture of Z/E (3:1, determined from 1H NMR) isomers, with a yield of 63%. Julia–Kocienski olefination [13] proceeds through a five-membered ring transition state, as depicted in Scheme 3. Then, alkene 14 was followed by hydrogenation to give compound 23 in a 100% yield using a Pt/C catalyst to avoid racemization of the allylic methyl group [21]. The compound 23 was subjected to Pd/C catalytic hydrogenation removal of the benzyl protecting group, giving 24 in a 99% yield. The compound 24 was oxidized by PCC dispersed with silicon dioxide to obtain the key intermediate aldehyde 13 with a yield of 82%. Because the addition of silicon dioxide can enhance the adsorption of the byproduct resulting from PCC, the oxidation of compound 24 was better promoted by PCC. This strategy not only enhances reaction efficiency but also mitigates environmental pollution through byproduct adsorption [22].
As outlined in Scheme 4, isobutyl bromide 25 was converted into sulfone 12 via S-alkylation and m-CPBA/NaHCO3 oxidation in dry CH2Cl2 in a 70% yield over two steps. For the total synthesis of (R)-1, compound 28 was first completed using sulfone 12 and chiral aldehyde 13 via Julia–Kocienski olefination in 98% yields, and then further subjected to Pd/C catalytic hydrogenation, giving the target molecule (R)-1 in a100% yield. All of the data are consistent with the literature values [4,5].
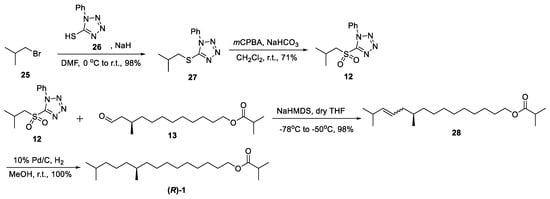
Scheme 4.
Synthesis of sex pheromone of (R)-1.
3. Experimental Section
3.1. General Method
All commercially available reagents were used without further purification. THF and diethyl ether were distilled from sodium under argon. Dichloromethane was distilled from calcium hydride under argon. Column chromatography was performed on silica gel (200–400 mesh). The optical rotations were measured using a polarimeter equipped with a sodium lamp. 1H NMR (500 MHz, TMS at δ 0.00 ppm or CDCl3 at δ 7.26 ppm) and 13C NMR (125 MHz, CDCl3 at δ 77.00 ppm as internal standard) spectra were recorded on a Bruker 500 MHz NMR spectrometer. HRMS data were recorded on Thermo Scientific LTQ Orbitrap XL (Waltham, MA, USA).
3.2. (S)-4-Benzyl-3-(4-(benzyloxy)butanoyl)oxazolidin-2-one (18)
A stirred solution of 16 (1.5 g, 7.7 mmol) in anhydrous THF (38 mL) under argon was cooled to −78 °C for 15 min, and then added with Et3N (2.1 mL, 15.4 mmol) followed by PivCl (1.1 mL, 9.3 mmol). After being stirred for 20 min at −78 °C, the mixture was warmed to room temperature and stirred for 1 h. Solid LiCl (0.66 g, 15.62 mmol) and (S)-4-benzyloxazolidin-2-one (17) (1.4 g, 7.7 mmol) were added at −78 °C. The reaction mixture was stirred for 1 h at −78 °C, slowly warmed to room temperature, and stirred overnight. The reaction was quenched with H2O (10 mL), and the mixture was extracted with ethyl acetate (2 × 10 mL). The combined organic layers were washed with brine (30 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:5) to give compound 18 as a colorless oil (2.4 g, 89%). 1H NMR (500 MHz, CDCl3) δ 7.34–7.31 (m, 6H), 7.28–7.25 (m, 2H), 7.18 (d, J = 7.1 Hz, 2H), 4.63–4.58 (m, 1H), 4.50 (s, 2H), 4.13–4.08 (m, 2H), 3.58 (t, J = 6.2 Hz, 2H), 3.26 (dd, J = 3.3, 13.4 Hz, 1H), 3.08–3.05 (m, 2H), 2.69 (dd, J = 9.7, 13.4 Hz, 1H), 2.06–2.01 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 173.04, 153.45, 138.44, 135.35, 129.37, 128.91, 128.33, 127.67, 127.53, 127.28, 72.88, 69.23, 66.12, 55.14, 37.87, 32.46, 24.48.
3.3. (S)-4-Benzyl-3-((S)-4-(benzyloxy)-2-methylbutanoyl)oxazolidin-2-one (19)
A stirred solution of 18 (2.4 g, 6.9 mmol) in anhydrous THF (30 mL) at −78 °C under argon was added to a solution of NaHMDS (2.0 M in THF, 6.9 mL, 13.7 mmol). After being stirred at −78 °C for 30 min, MeI (0.83 mL, 13.42 mmol) was added dropwise and then stirring was continued for another 2 h at −78 °C. Then, the reaction was allowed to warm to −50 °C and stirred overnight. The reaction mixture was quenched with saturated NH4Cl solution (10 mL), and the mixture was warmed to room temperature and then extracted with ethyl acetate (2 × 30 mL). The combined organic layers were washed with brine (30 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:5) to give compound 19 as a colorless oil (1.9 g, 75%). [α]23D = +45.531 (c = 2.6, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.32–7.20 (m, 8H), 7.14 (d, J = 7.0 Hz, 2H), 4.42 (d, J = 2.7 Hz, 2H), 4.0–3.92 (m, 2H), 3.73 (t, J = 8.6 Hz, 1H), 3.59–3.51 (m, 2H), 3.18 (dd, J = 3.3, 13.4 Hz, 1H), 2.7 (dd, J = 9.5, 13.4 Hz, 1H), 2.21–2.14 (m, 1H), 1.77–1.71 (m, 1H), 1.26–1.2 (d, J = 6.9 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 177.03, 153.20, 138.50, 135.40, 129.35, 128.78, 128.21, 127.58, 127.46, 127.17, 72.78, 68.43, 65.79, 55.15, 37.95, 35.11, 33.61, 18.01.
3.4. (S)-4-(Benzyloxy)-2-methylbutan-1-ol (20)
A stirred solution of LiAlH4 (2.7 g, 71.4 mmol) in anhydrous THF (55 mL) at 0 °C under argon was added dropwise to a mixture of 19 (5.2 g, 14.3 mmol) and anhydrous THF (40 mL). The reaction mixture was allowed to warm to room temperature, stirred overnight, and quenched with H2O, 10% NaOH solution, andH2O (1:2:3) at 0 °C. The mixture was filtered under reduced pressure and the resulting residue was washed with ethyl acetate. The aqueous layer was extracted with ethyl acetate (2 × 20 mL). The combined organic layers were dried with Na2SO4 and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:10) to afford compound 20 as a colorless oil (2.5 g, 90%). [α]23D = +4.113 (c = 2.3, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.36–7.27 (m, 5H), 4.52 (s, 2H), 3.61–3.57 (m, 1H), 3.55–3.48 (m, 2H), 3.43 (dd, J = 6.6, 10.9 Hz, 1H), 2.62 (s, 1H), 1.85–1.78 (m, 1H), 1.74–1.67 (m, 1H), 1.60–1.54 (m, 1H), 0.92 (d, J = 6.9 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 137.99, 128.40, 127.71, 127.67, 73.10, 68.64, 68.03, 34.06, 33.97, 29.65, 17.13.
3.5. (S)-2-(4-(Benzyloxy)-2-methylbutylthi-o) benzo[d]thiazole (22)
A solution of 20 (1.9 g, 10.0 mmol), PPh3 (3.1 g, 12.0 mmol), and 21 in THF (50 mL) was added to DIAD (2.4 mL, 12.0 mmol) at 0 °C. The reaction mixture was allowed to warm to room temperature, stirred for 4 h, and then concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:30) to afford compound 22 as a yellow oil (3.1 g, 90%). [α]23D = +7.305 (c = 3.0, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 8.1 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.41–7.83 (m, 1H), 7.32–7.27 (m, 6H), 4.51 (d, J = 3.1 Hz, 2H), 3.62–3.54 (m, 2H), 3.46 (dd, J = 5.6, 12.8 Hz, 1H), 3.25 (dd, J = 7.4, 12.9 Hz, 1H), 2.2–2.12 (m, 1H), 1.92–1.85 (m, 1H), 1.65–1.61 (m, 1H), 1.09 (d, J = 6.8 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.60, 153.32, 138.45, 135.20, 128.41, 127.68, 127.59, 126.02, 124.13, 121.46, 120.94,73.01, 68.13, 40.58, 35.74, 30.70, 19.39.
3.6. (S)-2-(4-(Benzyloxy)-2-methylbutylsulfonyl)benzo[d]thiazole (15)
A solution of 22 (2.7 g, 8.0 mmol) in CH2Cl2 (80 mL) at room temperature was added to m-CPBA (70% purity, 6.9 g, 40.0 mmol). The reaction mixture was stirred at room temperature overnight, quenched with the saturated Na2S2O3 aqueous solution (10 mL), stirred for 10 min, neutralized with the saturated NaHCO3 solution (10 mL), extracted with CH2Cl2 (3 × 10 mL), washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:10) to give compound 15 as a yellowish oil (1.9 g, 92%). 1H NMR (500 MHz, CDCl3) δ 8.2 (d, J = 8.3 Hz, 1H), 8.0 (d, J = 7.9 Hz, 1H), 7.65–7.57 (m, 2H), 7.43–7.23 (m, 5H), 4.41 (s, 2H), 3.72 (dd, J = 4.5, 14.4 Hz, 2H), 3.55–3.48 (m, 2H), 3.39 (dd, J = 8.2, 14.4 Hz, 1H), 2.53–2.47 (m, 1H), 1.87–1.8 (m, 1H), 1.69–1.62 (m, 1H), 1.18 (d, J = 6.8 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 166.72, 152.74, 138.18, 136.80, 128.40, 128.0, 127.66, 127.62, 125.46, 122.40, 73.0, 67.53, 60.67, 36.21, 26.53, 20.02. HRMS (ESI): m/z calculated for C16H24NO2S2+ (M + H)+: 326.12430; found: 326.12448.
3.7. (S, E)-12-(Benzyloxy)-10-methyldodec-8-enyl isobutyrate (14)
A solution of 15 (0.8 g, 2.1 mmol) in anhydrous THF (25 mL) under argon was added to NaHMDS (2.0 M in THF, 1.6 mL, 3.2 mmol), the reaction mixture was stirred at −78 °C for 30 min, and a solution of 8-oxooctyl isobutyrate 10 (0.7 g, 3.2 mmol) in anhydrous THF (25 mL) was added dropwise. The reaction mixture was stirred at −78 °C for 3 h, slowly warmed to −50 °C, and stirred overnight. The reaction mixture was quenched with a saturated NH4Cl aqueous solution at −50 °C, the mixture was warmed to room temperature, and the aqueous layer was extracted with ethyl acetate (2 × 5 mL), washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:200) to give compound 14 as a colorless oil (0.5 g, 63%). (Z/E 3:1 mixture) [α]23D = +3.056 (c = 2.5, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.33–7.25 (m, 5H), 5.36–5.08 (m, 2H), 4.49–4.44 (m, 2H), 4.06–4.03 (m, 2H), 3.49–3.39 (m, 2H), 2.65–2.5 (m, 2H), 2.07–1.93 (m, 2H), 1.7–1.43 (m, 4H), 1.3 (s, 8H), 1.16 (d, J = 7 Hz, 6H), 0.98–0.95 (m, 3H); 13C NMR (125 MHz, CDCl3) δ 177.25, 138.76, 138.73, 135.53, 129.05, 128.34, 127.66, 127.61, 127.47, 72.99, 68.82, 64.4, 37.28, 34.08, 29.85, 29.25, 29.19, 28.68, 28.61, 27.43, 25.93, 25.9, 21.51, 19.05.
3.8. (R)-12-(Benzyloxy)-10-methyldodecyl isobutyrate (23)
A suspension of 10% Pt on carbon (104 mg, 0.5 mmol) and 14 (503 mg, 1.3 mmol) in ethanol (45 mL) were pretreated with hydrogen by five vacuum–hydrogen cycles. The mixture was stirred at room temperature under a hydrogen balloon for 12 h, the catalyst was filtered off, and the solvent was removed under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:50) to give compound 23 as a colorless oil (495 mg, 100%). [α]23D = +1.761 (c = 2.6, CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.34–7.26 (m, 5H), 4.5 (s, 2H), 4.05 (t, J = 6.7 Hz, 2H), 3.52–3.47 (m, 2H), 2.57–2.51 (m, 1H), 1.69–1.56 (m, 4H), 1.45–1.38 (m, 1H), 1.33–1.27 (m, 14H), 0.86 (d, J = 6.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 177.29, 138.76, 128.36, 127.63, 127.48, 72.92, 68.81, 64.43, 37.15, 36.82, 34.09, 29.94, 29.9, 29.61, 29.55, 29.28, 28.69, 26.97, 25.94, 21.51, 19.71, 19.06. HRMS (ESI): m/z calculated for C24H41O3+ (M + H)+: 377.30502; found: 377.30499.
3.9. (R)-12-Hydroxy-10-methyldodecyl isobutyrate (24)
A suspension of 10% Pd on carbon (104 mg, 0.5 mmol) and (R)-12-(benzyloxy)-10-methyldodecyl isobutyrate (23) (503 mg, 1.3 mmol) in ethanol (45 mL) were pretreated with hydrogen by five vacuum–hydrogen cycles. The mixture was stirred at room temperature under a hydrogen balloon for 12 h, the catalyst was filtered off, and the solvent was removed under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:10) to give compound 24 as a colorless oil (495 mg, 99%). 1H NMR (500 MHz, CDCl3) δ 4.06 (t, J = 6.7 Hz, 2H), 3.7–3.65 (m, 2H), 2.53 (td, J = 5, 10 Hz, 1H), 1.63–1.6 (m, 4H), 1.39–1.27 (m, 15H), 1.16 (d, J = 5.0 Hz, 6H), 0.89 (d, J = 5.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 177.31, 64.42, 61.26, 40.0, 37.14, 34.08, 29.88, 29.56, 29.51, 29.5, 29.24, 28.66, 26.94, 25.9, 19.66, 19.03. HRMS (ESI): m/z calculated for C17H35O3+ (M + H)+: 287.25807; found: 287.25818.
3.10. 5-(Isobutylthio)-1-phenyl-1H-tetrazole (27)
A solution of NaH (2.7 g, 83.3 mmol) in DMF (70 mL) at 0 °C was added to a solution of 25 (8.9 g, 83.3mmol) in DMF (30 mL). The reaction mixture was stirred at 0 °C for 10 min, and 26 (5.4 mL, 83.3 mmol) was added and stirred for 12 h. The reaction mixture was quenched with water, extracted with ethyl acetate, washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:5) to give compound 27 as a colorless oil (13.0 g, 98%). 1H NMR (500 MHz, CDCl3) δ 7.64–7.58 (m, 5H), 3.35 (d, J = 6.9 Hz, 2H), 2.18–2.1 (m, 1H), 1.1 (d, J = 6.7 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 154.75, 133.8, 130.1, 129.8, 123.93, 41.81, 28.35, 21.74.
3.11. 5-(Isobutylsulfonyl)-1-phenyl-1H-tetrazole (12)
A solution of 27 (12.8 g, 54.2 mmol) in CH2Cl2 (424 mL) was added to NaHCO3 (22.8 g, 270.9 mmol) at room temperature. The reaction mixture was stirred at room temperature for 5 min, and m-CPBA (22.3 g, 90.4 mmol) was added and stirred overnight. The reaction mixture was added to a Na2S2O3 solution (10 mL) and a saturated NaHCO3 aqueous solution (15 mL), stirred for 30 min, extracted with CH2Cl2, washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:5) to give compound 12 as a yellowish oil (10.3 g, 71%). 1H NMR (500 MHz, CDCl3) δ 7.68–7.6 (m, 5H), 3.68 (d, J = 6.6 Hz, 2H), 2.53–2.45 (m, 1H), 1.16 (d, J = 6.8 Hz, 6H); 13C NMR (125 MHz, CDCl3) δ 133.13, 131.47, 129.71, 125.18, 63.11, 23.97, 22.55.
3.12. (R, E)-10, 14-Dimethylpentadec-12-enyl isobutyrate (28)
A solution of 12 (269 mg, 1.0 mmol) in anhydrous THF (8 mL) under argon at −78 °C was slowly added to a solution of NaHMDS (2.0 M in THF, 0.5 mL, 1.0 mmol) dropwise. After stirring at −78 °C for 30 min, a solution of 13 (190 mg, 0.7 mmol) in anhydrous THF (8 mL) was added dropwise and stirred for another 2 h at −78 °C. The reaction mixture was allowed to warm to −50 °C and stirred overnight. The reaction mixture was quenched with a saturated NH4Cl solution (5 mL) at −50 °C, and the mixture was warmed to room temperature, extracted with ethyl acetate (2 × 10 mL), washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:5) to give compound 28 as a colorless oil (213 mg, 98%). [α]23D = +1.997 (c = 2.3, CHCl3). 1H NMR (500 MHz, CDCl3) δ 5.35–5.21 (m, 2H), 4.05 (d, J = 6.8 Hz, 2H), 2.59–2.5 (m, 1H), 2.26–2.22 (m, 1H), 2.04–1.76 (m, 2H), 1.63–1.59 (m, 2H), 1.31–1.25 (m, 15H), 1.16 (d, J = 7 Hz, 6H), 0.95 (dd, J = 6.8, 15 Hz, 6H), 0.84 (dd, J = 6.7, 12.6 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 177.3, 138.86, 138.17, 126.04, 125.69, 64.44, 40.04, 36.73, 34.64, 34.09, 33.22, 29.93, 29.61, 29.54, 29.27, 28.68, 27.18, 27.07, 26.48, 25,93, 23.18, 23.14, 22.78, 19.05.
3.13. (R)-10, 14-Dimethylpentadecyl isobutyrate ((R)-1)
A suspension of 10% Pd on carbon (13 mg, 0.04 mmol) and 28 (6.5 mg, 0.05 mmol) in ethanol (10 mL) were pretreated with hydrogen using five vacuum–hydrogen cycles. The mixture was stirred under a hydrogen balloon at room temperature for 12 h, the catalyst was filtered off, and the solvent was removed under reduced pressure. The crude product was purified using silica gel (EtOAc/hexane 1:5) to give compound (R)-1 as a colorless oil (13 mg, 100%). [α]23D = +0.581 (c = 2.3, CHCl3). 1H NMR (500 MHz, CDCl3) δ 4.05 (t, J = 6.8 Hz, 2H), 2.56–2.5 (m, 1H), 1.65–1.48 (m, 4H), 1.4–1.19 (m, 17H), 1.16 (d, J = 7 Hz, 6H), 1.15–1.04 (m, 3H), 0.85 (dd, J = 6.6, 13.2 Hz, 9H); 13C NMR δ (125 MHz, CDCl3) 176.08, 63.16, 38.37, 36.32, 36.09, 33.05, 31.76, 28.97, 28.59, 28.51, 28.24, 27.66, 26.97, 26.06, 24.9, 23.79, 21.7, 21.61, 18.7, 18.0. HRMS (ESI): m/z calculated for C21H43O2+ (M + H)+: 327.32576; found: 327.32599.
4. Conclusions
In summary, we have successfully achieved the asymmetric total synthesis of (R)-1 with a 25% overall yield in 11 steps, a major component of the sex pheromone of the tea tussock moth. The C-C skeletons were constructed by employing Julia–Kocienski coupling as the key step, while Evans’ template was utilized for asymmetric-induced methylation. The concise, efficient synthetic route to (R)-1 will facilitate the subsequent evaluation and application of pheromones as environmental benign tools for pest management.
Author Contributions
Z.-F.S. conducted the experiments, handled the data, and wrote and corrected the manuscript. H.L. and Y.-F.L. participated in the chemical synthesis. Y.-P.D. and L.-X.J. analyzed the data. X.-H.J. and H.-P.D. corrected the manuscript. J.-F.L. supervised and coordinated all studies and reviewed the manuscript. The manuscript was written through contributions of all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Shaanxi Province (2020JM-598, 2019JQ006, 2023-JC-QN-0126), Sanqin Talents, Shaanxi Provincial First-class Team—Contaminated Soil Remediation and Resource Utilization Innovation Team at Shaanxi University of Technology and the Talent Initiation Project of Shaanxi University of Technology (SLGRC10, SLGRC12).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this article are available in the text.
Acknowledgments
We thank the Natural Science Foundation of Shaanxi Province, Sanqin Talents, Shaanxi Provincial First-Class Team—Contaminated Soil Remediation and Resource Utilization Innovation Team at Shaanxi University of Technology and the Talent Initiation Project of Shaanxi University of Technology for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Z.-Q.; Yuan, T.-T.; Cui, S.-W.; Zhao, Y.-J.; Shao, Y.-H.; Shang, J.-N.; Luo, Z.-X.; Cai, X.-M.; Bian, L.; Chen, Z.-M. Development of a high-efficiency sex pheromone formula to control. J. Integr. Agric. 2023, 22, 195–201. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Gu, Q.-Y.; Zhang, W.; Jiang, H.-Y.; Chen, S.-C.; Smagghe, G.; Niu, J.-Z.; Wang, J.-J. Prevalence of a novel bunyavirus in tea tussock moth Euproctis pseudoconspersa (Lepidoptera: Lymantriidae). J. Insect Sci. 2021, 21, 5. [Google Scholar] [CrossRef]
- Wang, T.-K.; Liu, X.-F.; Luo, Z.-X.; Cai, X.-M.; Li, Z.-Q.; Bian, L.; Xiu, C.-L.; Chen, Z.-M.; Li, Q.-R.; Fu, N.-X. Transcriptome-wide identification of cytochrome P450s in tea black tussock moth (Dasychira baibarana) and candidate genes involved in Type-II sex pheromone biosynthesis. Insects 2024, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Wakamura, S.; Yasuda, T.; Ichikawa, A.; Fukumoto, T.; Mochizuki, F. Sex attractant pheromone of the tea tussock moth, Euproctis pseudoconspersa (Strand) (Lepidoptera: Lymantriidae): Identification and field attraction. Appl. Entomol. Zool. 1994, 29, 403–411. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Millar, J.G.; Wen, Z.; Wang, S.; Wang, X.; Zhu, Y. Isolation, identification, and synthesis of the female sex pheromone of the tea tussock moth, Euproctis pseudoconspersa (Lepidoptera: Lymantridae). Entomol. Sin. 1996, 3, 58–69. [Google Scholar] [CrossRef]
- Ichikawa, A.; Yasuda, T.; Wakamura, S. Absolute configuration of sex pheromone for tea tussock moth, Euproctis pseudoconspersa (Strand) via synthesis of (R)- and (S)-10, 14- dimethyl-1-pentadecyl isobutyrates. J. Chem. Ecol. 1995, 21, 627–634. [Google Scholar] [CrossRef]
- Wakamura, S.; Ichikawa, A.; Yasuda, T.; Arakaki, N.; Fukumoto, T. EAG and field responses of the male tea tussock moth, Euproctis pseudoconspersa (Strand) (Lepidoptera: Lymantriidae) to (R)- and (S)-enantiomers and racemic mixture of 10, 14-dimethylpentadecyl isobutyrate. Appl. Entomol. Zool. 1996, 31, 623–625. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Millar, J.G.; Pan, K.-H.; Xu, C.-S. Responses of tea tussock moth, Euproctis pseudoconspersa, to its pheromone, (R)-10, 14-dimethylpentadecyl isobutyrates, and to the S-enantiomer of its pheromone. J. Chem. Ecol. 1998, 24, 1347–1353. [Google Scholar] [CrossRef]
- Müller, M.; Buchbauer, G. Essential oil components as pheromones. A review. Flavour Fragr. J. 2011, 26, 357–377. [Google Scholar] [CrossRef]
- Zarbin, P.H.G.; Villar, J.A.F.P.; Corrêa, A.G. Insect Pheromone Synthesis in Brazil: An Overview. J. Braz. Chem. Soc. 2007, 18, 1100–1124. [Google Scholar] [CrossRef]
- Sun, Z.-F.; Zhou, L.-N.; Meng, Y.; Zhang, T.; Du, Z.-T.; Zheng, H. Concise asymmetric synthesis of the sex pheromone of the tea tussock moth. Tetrahedron Asymmetry 2017, 28, 1562–1567. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Sun, Z.-F.; Zhou, L.-N.; Liu, L.; Zhang, T.; Du, Z.-T. Synthesis of the sex pheromone of the tea tussock moth based on a resource chemistry strategy. Molecules 2018, 23, 1374. [Google Scholar] [CrossRef]
- Blakemore, P.R. The modified Julia olefination: Alkene synthesis via the condensation of metallated heteroarylalkylsulfones with carbonyl compounds. J. Chem. Soc. Perkin Trans. 2002, 1, 2563–2585. [Google Scholar] [CrossRef]
- Mitsunobu, O. The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. Synthesis 1981, 1981, 1–28. [Google Scholar] [CrossRef]
- Braun, M.; Atalick, S.; Guldi, D.M.; Lanig, H.; Brettreich, M.; Burghardt, S.; Hatzimarinaki, M.; Ravanelli, E.; Prato, M.; Eldik, R.V.; et al. Electrostatic complexation and photo induced electron transfer between Zn-Cytochrome c and polyanionic fullerene dendrimers. Chem. Eur. J. 2003, 9, 3867–3875. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Ennis, M.D.; Mathre, D.J. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of α -substituted carboxylic acid derivatives. J. Am. Chem. Soc. 1982, 104, 1737–1739. [Google Scholar] [CrossRef]
- Yadav, J.S.; Thirupathaiah, B.; Ghamdi, A.A.K.A. Protecting group free formal total synthesis of the antitubercular agent erogorgiaene. Eur. J. Org. Chem. 2012, 2012, 2072–2076. [Google Scholar] [CrossRef]
- Liu, D.B.; Qiu, Z.M.; Liu, Z.W. Research progress on catalysis of Lithium chloride. Inorg. Chem. Ind. 2008, 40, 5–8. [Google Scholar]
- Yang, Z.C.; Jin, X.M.; Guaciaro, M.; Molino, B.F. Asymmetric Synthesis and Absolute Configuration of Streptophenazine G. J. Org. Chem. 2012, 77, 3191–3196. [Google Scholar] [CrossRef]
- Summeren, R.P.V.; Moody, D.B.; Feringa, B.L.; Minnaard, A.J. Total synthesis of enantiopure β-D-mannosyl phosphomycoketides from Mycobacterium tuberculosis. J. Am. Chem. Soc. 2006, 128, 4546–4547. [Google Scholar] [CrossRef]
- Li, N.-S.; Scharf, L.; Adams, E.J.; Piccirilli, J.A. Highly stereocontrolled total synthesis of β-D-mannosyl phosphomycoketide: A natural product from Mycobacterium tuberculosis. J. Org. Chem. 2013, 78, 5970–5986. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Suggs, J.W. Pyridinium chlorochromate. Efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Lett. 1975, 6, 2647–2650. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).