Density Functional Theory-Based Indicators to Estimate the Corrosion Potentials of Zinc Alloys in Chlorine-, Oxidizing-, and Sulfur-Harsh Environments
Abstract
1. Introduction
2. Results
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wątroba, M.; Mech, K.; Bednarczyk, W.; Kawałko, J.; Marciszko-Wiąckowska, M.; Marzec, M.; Shepherd, D.E.T.; Bała, P. Long-term in vitro corrosion behavior of Zn-3Ag and Zn-3Ag-0.5Mg alloys considered for biodegradable implant applications. Mater. Des. 2022, 213, 110289. [Google Scholar] [CrossRef]
- Shao, L.; Xie, G.; Zhang, C.; Liu, X.; Lu, W.; He, G.; Huang, J. Combustion of metals in oxygen-enriched atmospheres. Metals 2020, 10, 128. [Google Scholar] [CrossRef]
- Langman, J.B.; Ali, J.D.; Child, A.W.; Wilhelm, F.M.; Moberly, J.G. Sulfur species, bonding environment, and metal mobilization in mining-impacted lake sediments: Column experiments replicating seasonal anoxia and deposition of algal detritus. Minerals 2020, 10, 849. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Carey, M.P.; Koch, J.C.; Baughman, C.; Hill, K.; Zimmerman, C.E.; Sullivan, P.F.; Dial, R.; Lyons, T.; Cooper, D.J.; et al. Metal mobilization from thawing permafrost to aquatic ecosystems is driving rusting of Arctic streams. Commun. Earth Environ. 2024, 5, 268. [Google Scholar] [CrossRef]
- Dong, H.; Lin, F.; Boccaccini, A.R.; Virtanen, S. Corrosion behavior of biodegradable metals in two different simulated physiological solutions: Comparison of Mg, Zn and Fe. Corr. Sci. 2021, 182, 109278. [Google Scholar] [CrossRef]
- Barzegari, M.; Mei, D.; Lamaka, S.V.; Geris, L. Computational modeling of degradation process of biodegradable magnesium biomaterials. Corr. Sci. 2021, 190, 109674. [Google Scholar] [CrossRef]
- Bryzgalov, V.; Kistanov, A.A.; Khafizova, E.; Polenok, M.; Izosimov, A.; Korznikova, E.A. Experimental study of corrosion rate supplied with an ab-initio elucidation of corrosion mechanism of biodegradable implants based on Ag-doped Zn alloys. Appl. Surf. Sci. 2024, 652, 159300. [Google Scholar] [CrossRef]
- Chiter, F.; Bonnet, M.L.; Lacaze-Dufaure, C.; Tang, H.; Pebere, N. DFT studies of the bonding mechanism of 8-hydroxyquinoline and derivatives on the (111) aluminum surface. Phys. Chem. Chem. Phys. 2018, 20, 21474–21486. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jin, Y.; Leygraf, C. A DFT-Study of Cl Iingress into α-Al2O3(0001) and Al(111) and its possible influence on localized corrosion of Al. J. Electrochem. Soc. 2019, 166, 3124–3130. [Google Scholar] [CrossRef]
- Pan, Y.; Guan, W. Origin of enhanced corrosion resistance of Ag and Au doped anatase TiO2. Int. J. Hydrogen Energy 2019, 44, 10407–10414. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Shao, H.; Zhang, Y.; Dong, H.; Xia, L.; Hu, S. Anticorrosion mechanism of Cr-doped nickel-base alloy in Br/O environment: A DFT study. Mol. Simul. 2019, 45, 1506–1514. [Google Scholar] [CrossRef]
- Gao, W.; Baker, T.A.; Zhou, L.; Pinnaduwage, D.S.; Kaxiras, E.; Friend, C.M. Chlorine adsorption on Au(111): Chlorine overlayer or surface chloride? J. Am. Chem. Soc. 2008, 130, 3560–3565. [Google Scholar] [CrossRef]
- Saraireh, S.A.; Altarawneh, M.; Tarawneh, M.A. Nanosystem’s density functional theory study of the chlorine adsorption on the Fe(100) surface. Nanotech. Rev. 2021, 10, 719–727. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, Y.; Ejtemaei, M.; Nguyen, A.V.; Wang, Y.; Wen, S. DFT simulation of S-species interaction with smithsonite (001) surface: Effect of water molecule adsorption position. Results Phys. 2019, 15, 102575. [Google Scholar] [CrossRef]
- Kang, M.; Huang, K.; Li, J.; Liu, H.; Lian, C. Theoretical design of dendrite-free zinc anode through intrinsic descriptors from symbolic regression. J. Mater. Inf. 2024, 4, 6. [Google Scholar] [CrossRef]
- Giulimondi, V.; Mitchell, S.; Pérez-Ramírez, J. Challenges and opportunities in engineering the electronic structure of single-atom catalysts. ACS Catal. 2023, 13, 2981–2997. [Google Scholar] [CrossRef]
- Xie, T.; Wang, P.; Tian, C.; Zhao, G.; Jia, J.; He, C.; Zhao, C.; Wu, H. Adsorption characteristics of gas molecules adsorbed on graphene doped with Mn: A first principle study. Molecules 2022, 27, 2315. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, M.; Xue, J.; Li, M.; Cheng, Y.; Lai, Z.; Hu, J.; Zhou, F.; Qu, N.; Liu, Y.; et al. A Study of the adsorption properties of individual atoms on the graphene surface: Density functional theory calculations assisted by machine learning techniques. Materials 2024, 17, 1428. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Ren, G.; Wu, Y.; Lu, S.; Huan, Y.; Peng, T.; Liu, P.; Sun, J.; Su, H.; Cui, H. Machine-learning-assisted hydrogen adsorption descriptor design for bilayer MXenes. J. Clean. Prod. 2024, 450, 141953. [Google Scholar] [CrossRef]
- Lubbers, N.; Smith, J.S.; Barros, K. Hierarchical modeling of molecular energies using a deep neural network. J. Chem. Phys. 2018, 148, 241715. [Google Scholar] [CrossRef]
- Malone, W.; Kara, A. Predicting adsorption energies and the physical properties of H, N, and O adsorbed on transition metal surfaces: A machine learning study. Surf. Sci. 2023, 731, 122252. [Google Scholar] [CrossRef]
- Dean, J.; Taylor, M.G.; Mpourmpakisu, G. Unfolding adsorption on metal nanoparticles: Connecting stability with catalysis. Sci. Adv. 2019, 5, eaax5101. [Google Scholar] [CrossRef]
- Restuccia, P.; Ahmada, E.A.; Harrison, N.M. A transferable prediction model of molecular adsorption on metals based on adsorbate and substrate properties. Phys. Chem. Chem. Phys. 2022, 24, 16545–16555. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.-H.; Da Silva, J.L.F.; Deng, H.-Q.; Li, W.-X. Density functional theory study of the energetics, electronic structure, and core-level shifts of NO adsorption on the Pt(111) surface. Phys. Rev. B 2009, 79, 205413. [Google Scholar] [CrossRef]
- Gallego, S.; Sanchez, N.; Martin, S.; Muñoz, M.C.; Szunyogh, L. Reversible enhancement of the magnetism of ultrathin Co films by H adsorption. Phys. Rev. B 2010, 82, 085414. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Dostert, K.H.; O’Brien, C.P.; Riedel, W.; Savara, A.; Schauermann, S.; Tkatchenko, A. Catalysis beyond frontier molecular orbitals: Selectivity in partial hydrogenation of multi-unsaturated hydrocarbons on metal catalysts. Sci. Adv. 2017, 3, e1700939. [Google Scholar] [CrossRef] [PubMed]
- Korostelev, V.; Wagnera, J.; Klyukin, K. Simple local environment descriptors for accurate prediction of hydrogen absorption and migration in metal alloys. J. Mater. Chem. A 2023, 11, 23576–23588. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Wei, G.; Li, B.; Mao, Z.; Xu, S.-M.; Tang, S.; Jiang, J.; Li, Z.; Wang, X.; et al. Engineering spin polarization of the surface-adsorbed fe atom by intercalating a transition metal atom into the MoS2 bilayer for enhanced nitrogen reduction. JACS Au 2024, 4, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, M.J.; Santos-Carballal, D.; Cadi-Essadek, A.; van Sittert, C.G.C.E.; de Leeuw, N.H. Interaction of H2O with the platinum Pt (001), (011), and (111) surfaces: A density functional theory study with long-range dispersion corrections. J. Phys. Chem. C 2019, 123, 27465–27476. [Google Scholar] [CrossRef]
- Romero, M.A.; Bonetto, F.; García, E.A. Influence of single adsorbed atoms on charge exchange during ion-surface collisions. Phys. Rev. A 2023, 107, 032803. [Google Scholar] [CrossRef]
- Hofmann, O.T.; Egger, D.A.; Zojer, E. Work-Function Modification beyond Pinning: When Do Molecular Dipoles Count? Nano Lett. 2010, 10, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Rusu, P.C.; Brocks, G. Surface dipoles and work functions of alkylthiolates and fluorinated alkylthiolates on Au(111). J. Phys. Chem. B 2006, 110, 22628–22634. [Google Scholar] [CrossRef] [PubMed]
- Kistanov, A.A. Atomic insights into the interaction of N2, CO2, NH3, NO, and NO2 gas molecules with Zn2(V, Nb, Ta)N3 ternary nitride monolayers. Phys. Chem. Chem. Phys. 2024, 26, 13719. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. J. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, S. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Kabalan, L.; Kowalec, I.; Catlow, C.R.A.; Logsdail, A.J. A computational study of the properties of low- and high-index Pd, Cu and Zn surfaces. Phys. Chem. Chem. Phys. 2021, 23, 14649–14661. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules—A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Python Software Foundation. Python Language Reference, Version 3.7. Available online: http://www.python.org (accessed on 25 January 2020).

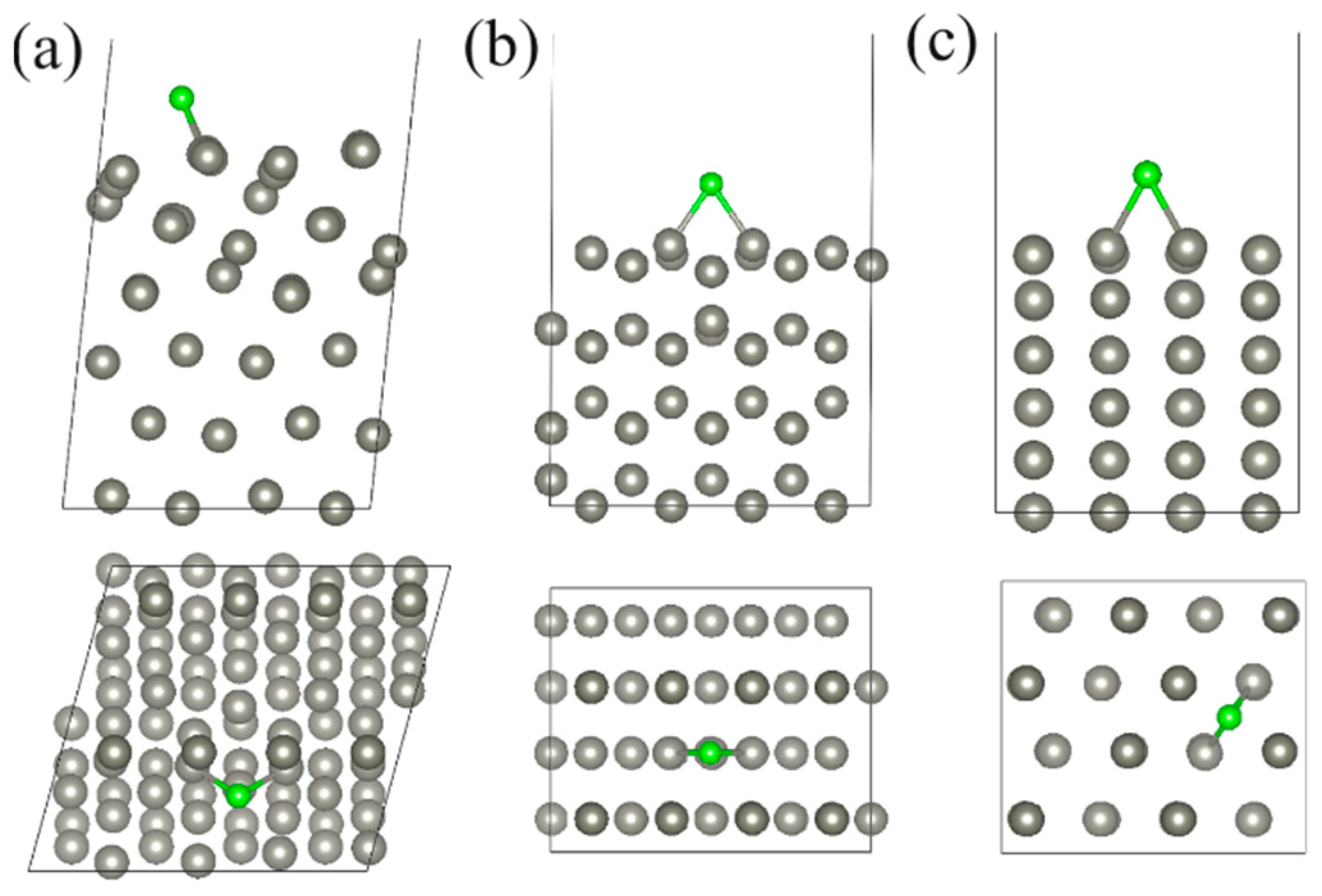
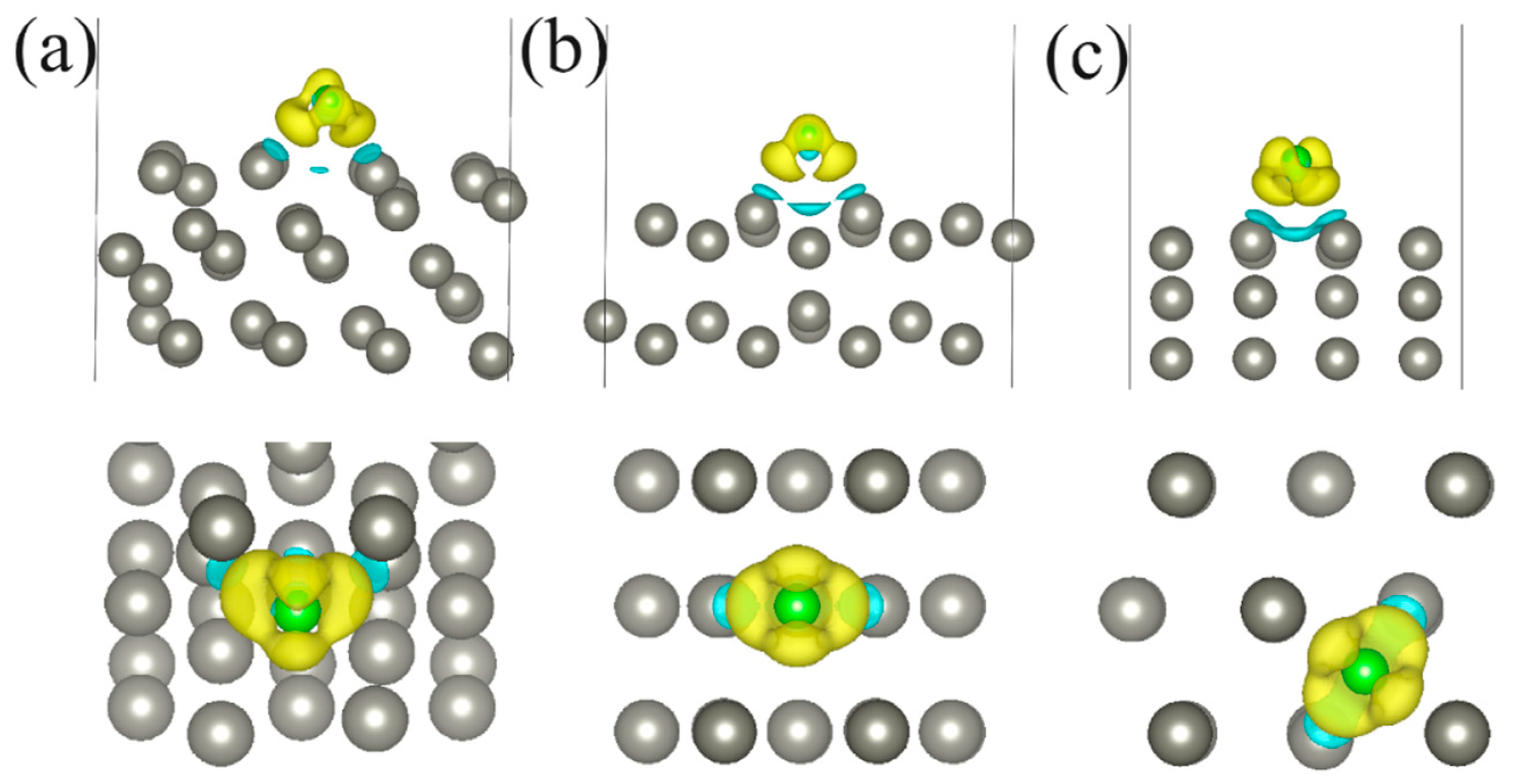
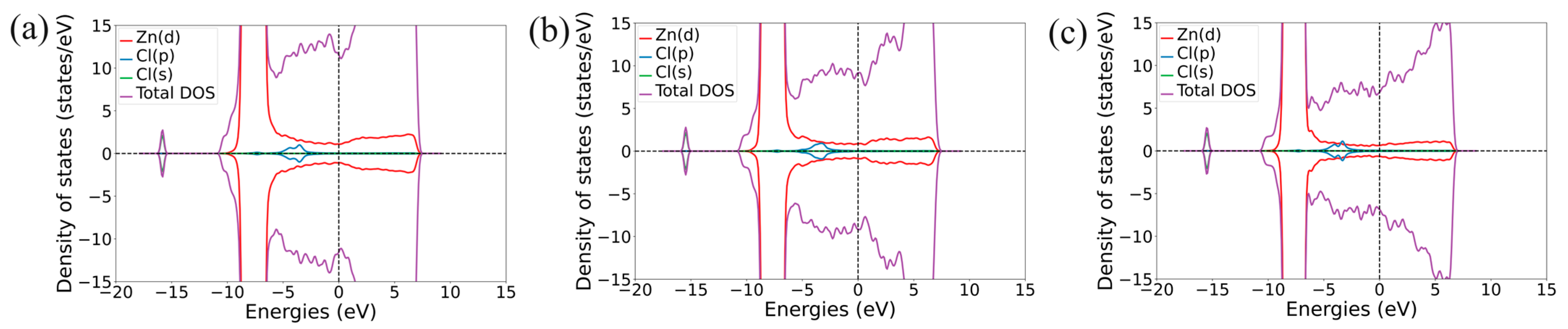
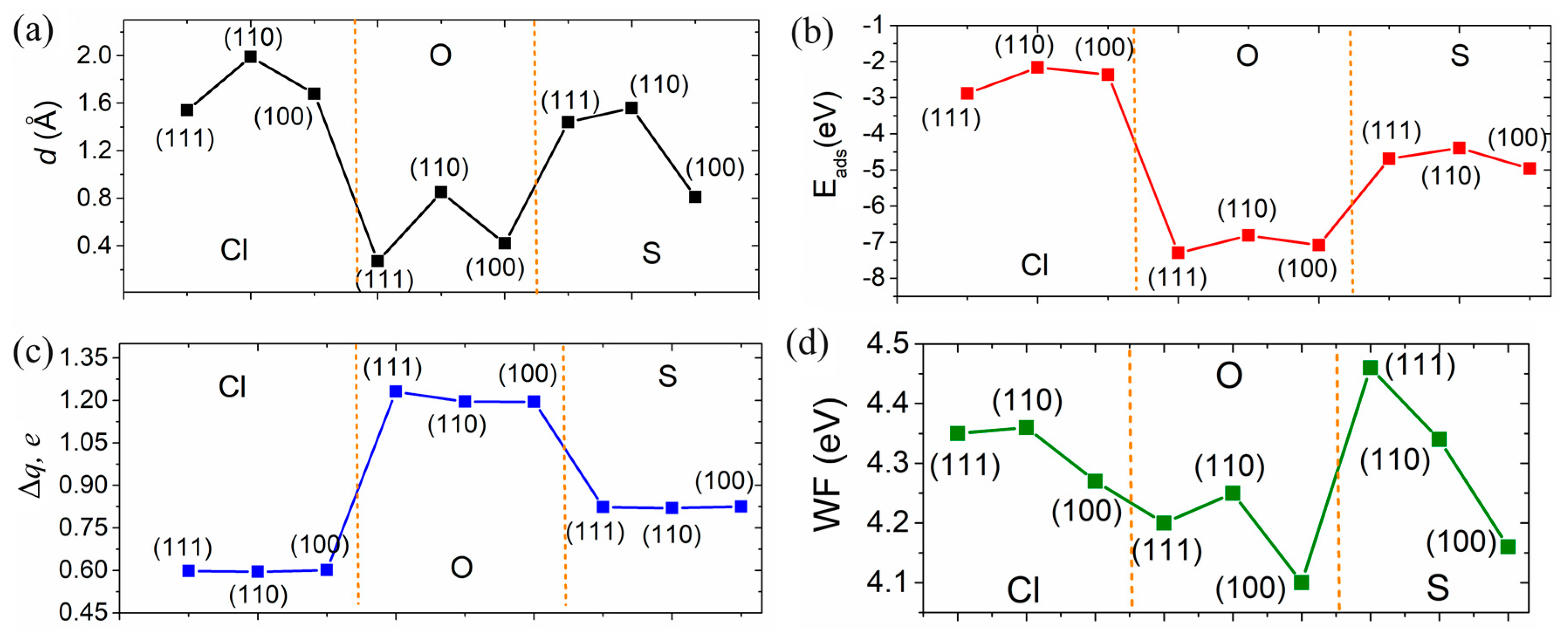
| Structure | Position | d, Å | Ea, eV | Doping Nature | Δq, e |
|---|---|---|---|---|---|
| Zn(111) + Cl | hcp | 1.54 | −2.88 | acceptor | 0.598 |
| Zn(110) + Cl | hcp | 1.99 | −2.16 | acceptor | 0.595 |
| Zn(100) + Cl | bridge | 1.68 | −2.38 | acceptor | 0.601 |
| Zn(111) + O | bridge | 0.27 | −7.30 | acceptor | 1.231 |
| Zn(110) + O | hcp | 0.85 | −6.81 | acceptor | 1.196 |
| Zn(100) + O | bridge | 0.42 | −7.08 | acceptor | 1.195 |
| Zn(111) + S | bridge | 1.44 | −4.69 | acceptor | 0.823 |
| Zn(110) + S | hcp | 1.56 | −4.39 | acceptor | 0.820 |
| Zn(100) + S | bridge | 0.81 | −4.96 | acceptor | 0.825 |
| Structure | Pure | Cl | O | S |
|---|---|---|---|---|
| Zn(111) | 4.18 | 4.35 | 4.20 | 4.46 |
| Zn(110) | 4.15 | 4.36 | 4.25 | 4.34 |
| Zn(100) | 4.03 | 4.27 | 4.10 | 4.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhametov, A.; Samikov, I.; Korznikova, E.A.; Kistanov, A.A. Density Functional Theory-Based Indicators to Estimate the Corrosion Potentials of Zinc Alloys in Chlorine-, Oxidizing-, and Sulfur-Harsh Environments. Molecules 2024, 29, 3790. https://doi.org/10.3390/molecules29163790
Mukhametov A, Samikov I, Korznikova EA, Kistanov AA. Density Functional Theory-Based Indicators to Estimate the Corrosion Potentials of Zinc Alloys in Chlorine-, Oxidizing-, and Sulfur-Harsh Environments. Molecules. 2024; 29(16):3790. https://doi.org/10.3390/molecules29163790
Chicago/Turabian StyleMukhametov, Azamat, Insaf Samikov, Elena A. Korznikova, and Andrey A. Kistanov. 2024. "Density Functional Theory-Based Indicators to Estimate the Corrosion Potentials of Zinc Alloys in Chlorine-, Oxidizing-, and Sulfur-Harsh Environments" Molecules 29, no. 16: 3790. https://doi.org/10.3390/molecules29163790
APA StyleMukhametov, A., Samikov, I., Korznikova, E. A., & Kistanov, A. A. (2024). Density Functional Theory-Based Indicators to Estimate the Corrosion Potentials of Zinc Alloys in Chlorine-, Oxidizing-, and Sulfur-Harsh Environments. Molecules, 29(16), 3790. https://doi.org/10.3390/molecules29163790











